Abstract
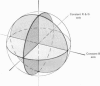
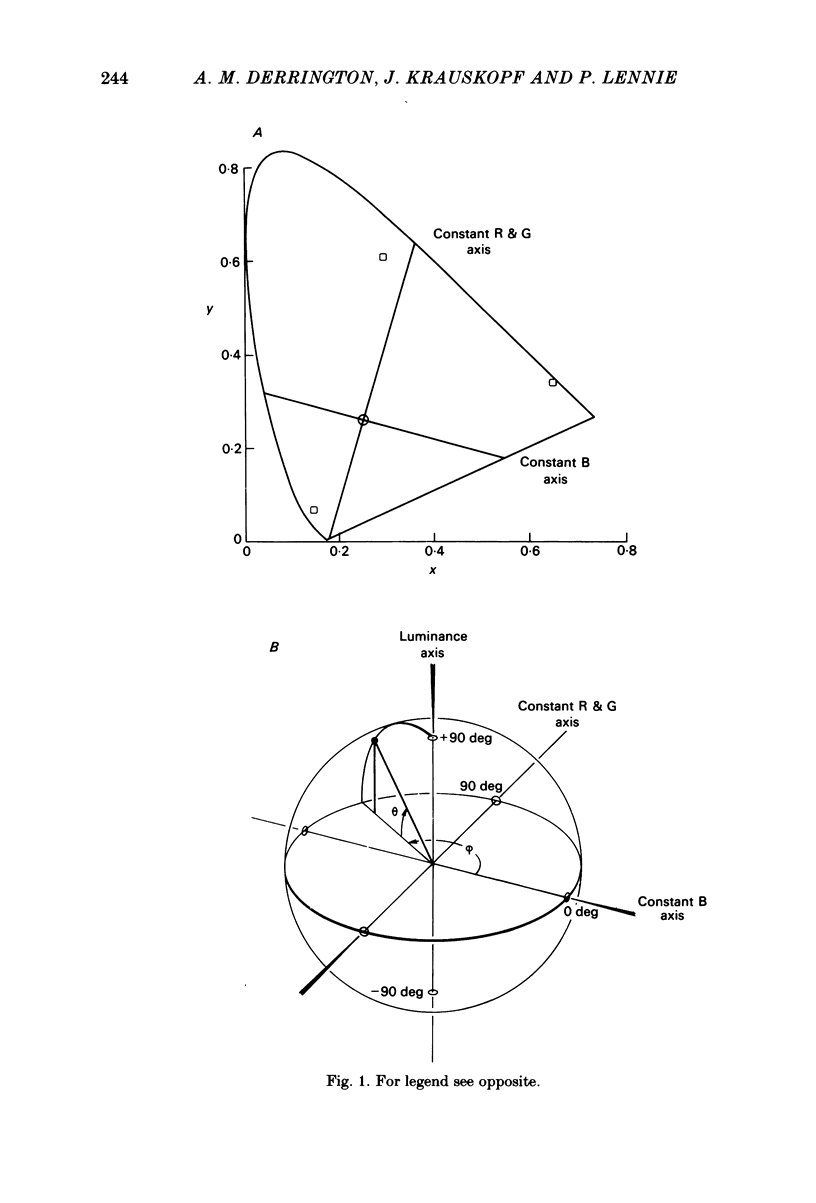
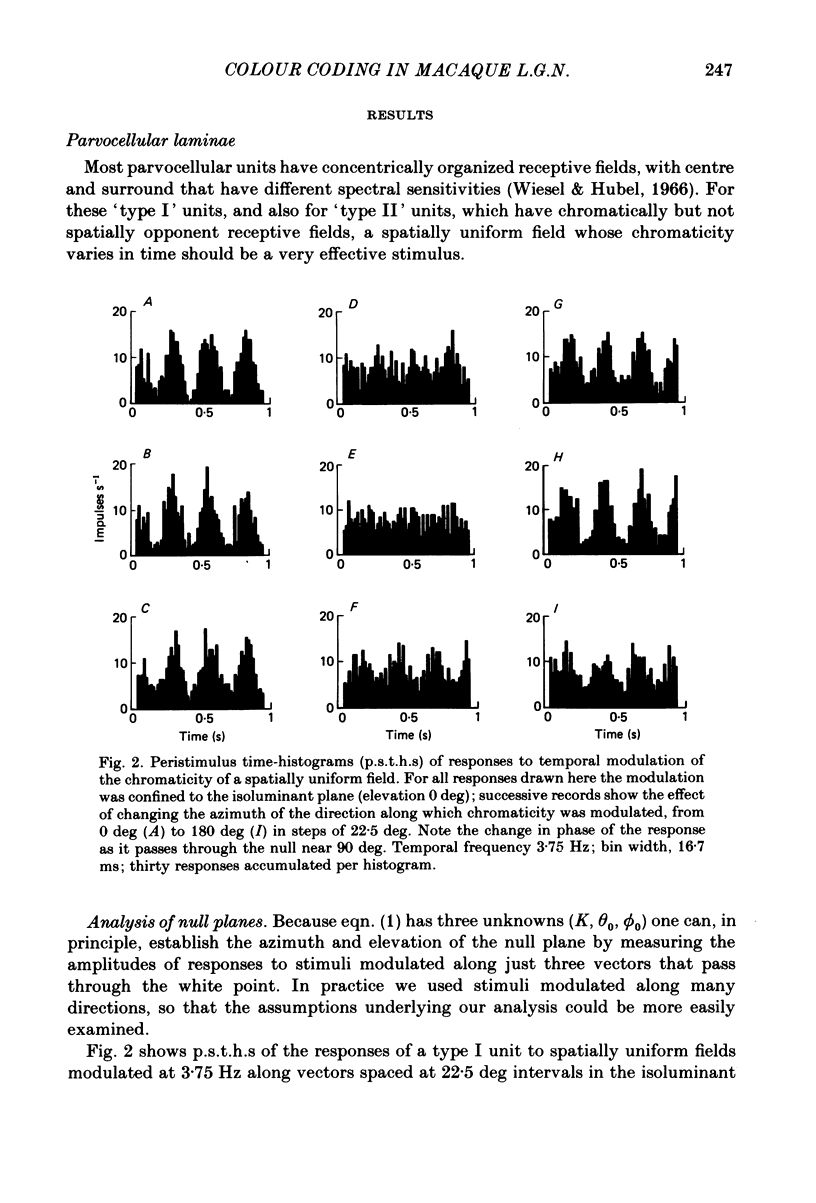
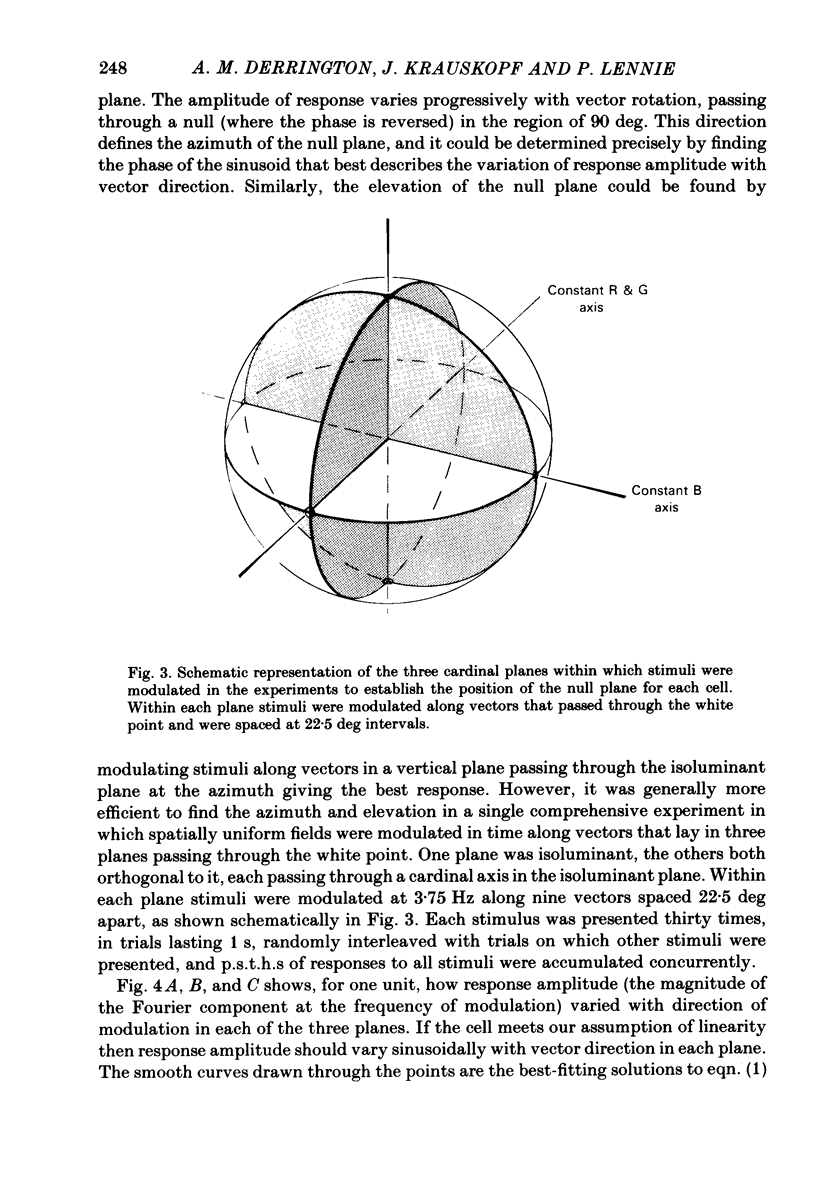
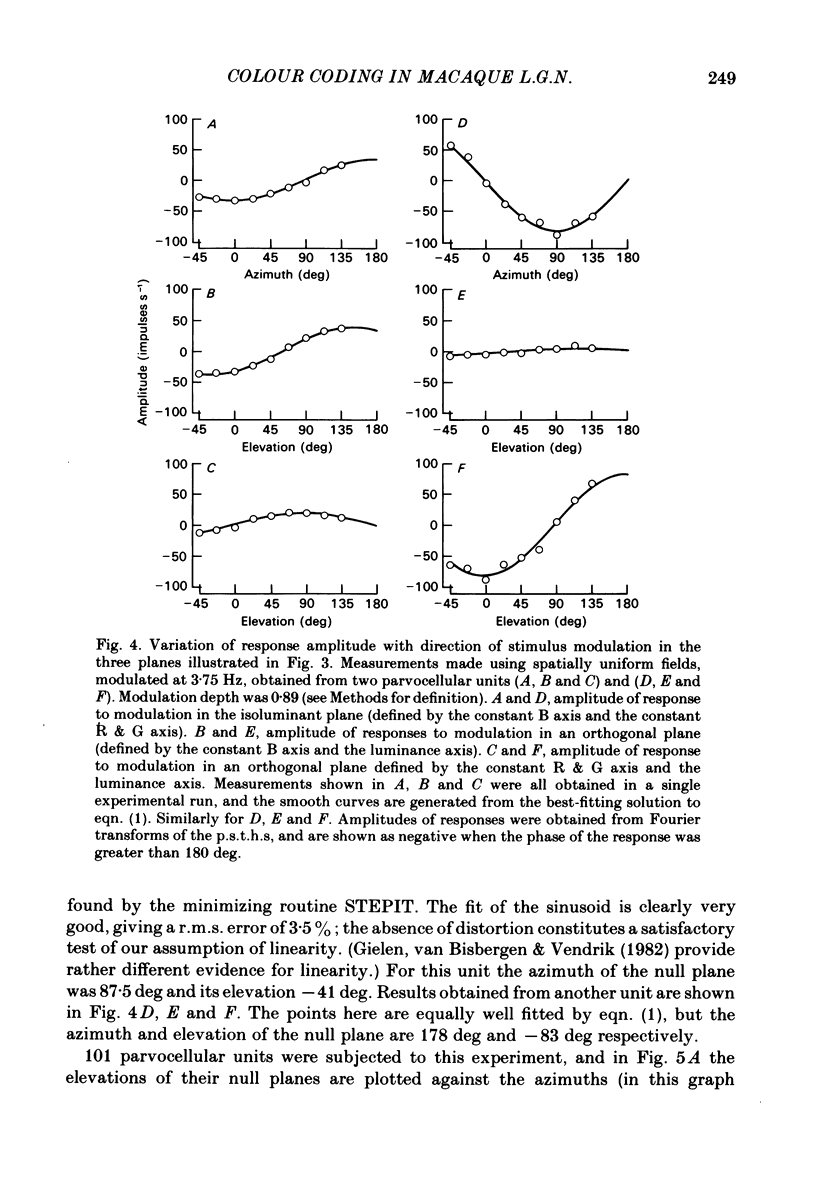
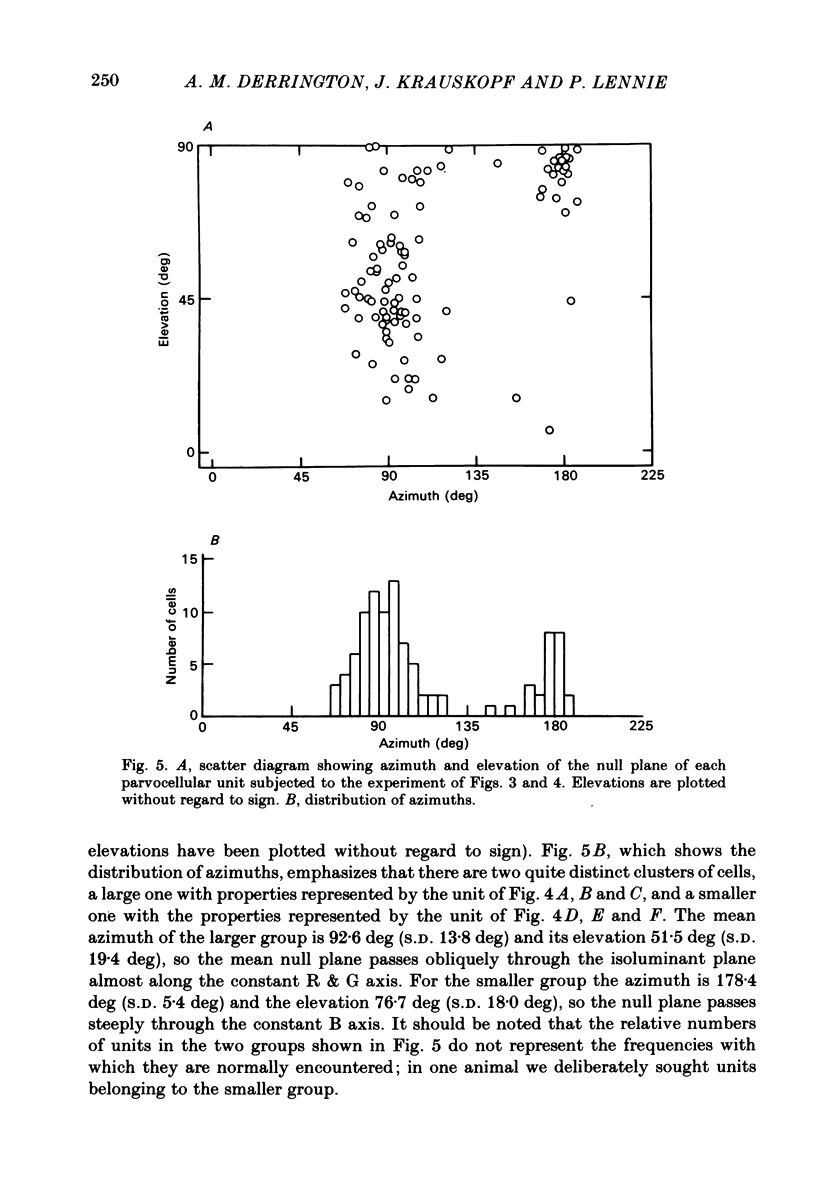
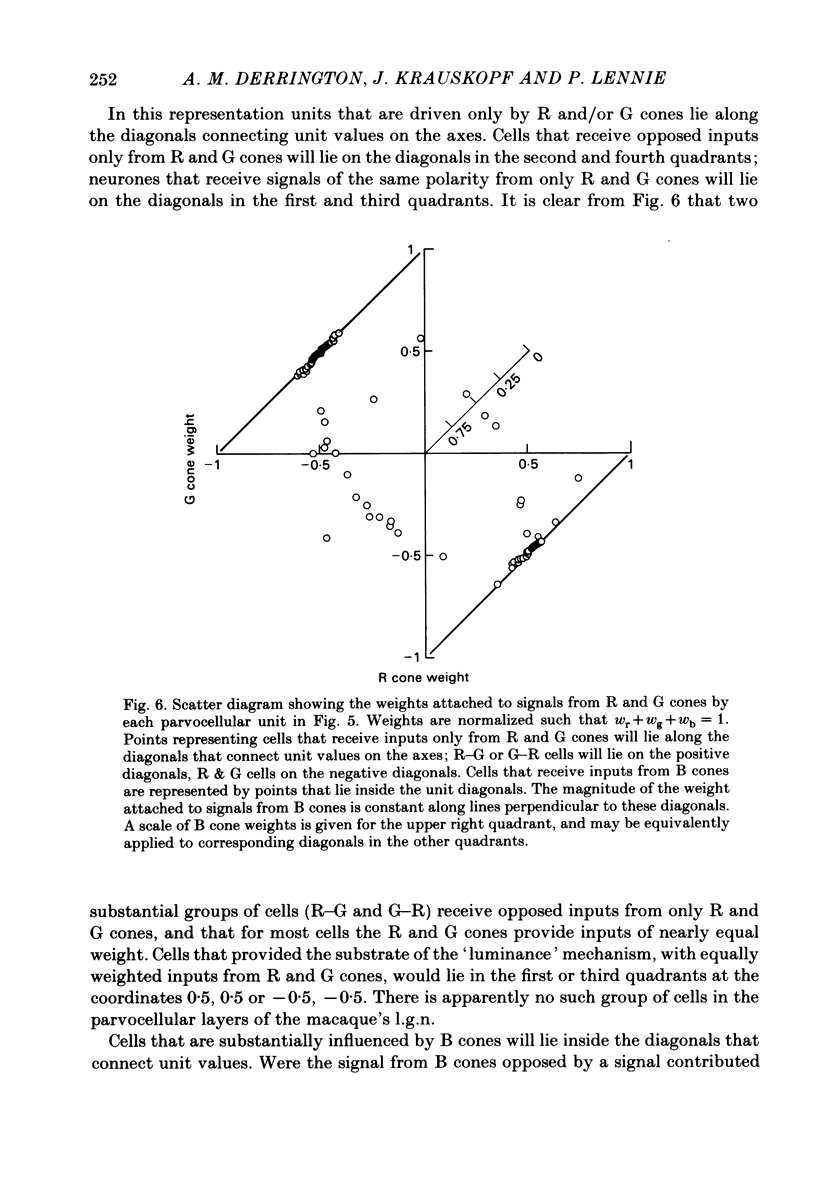
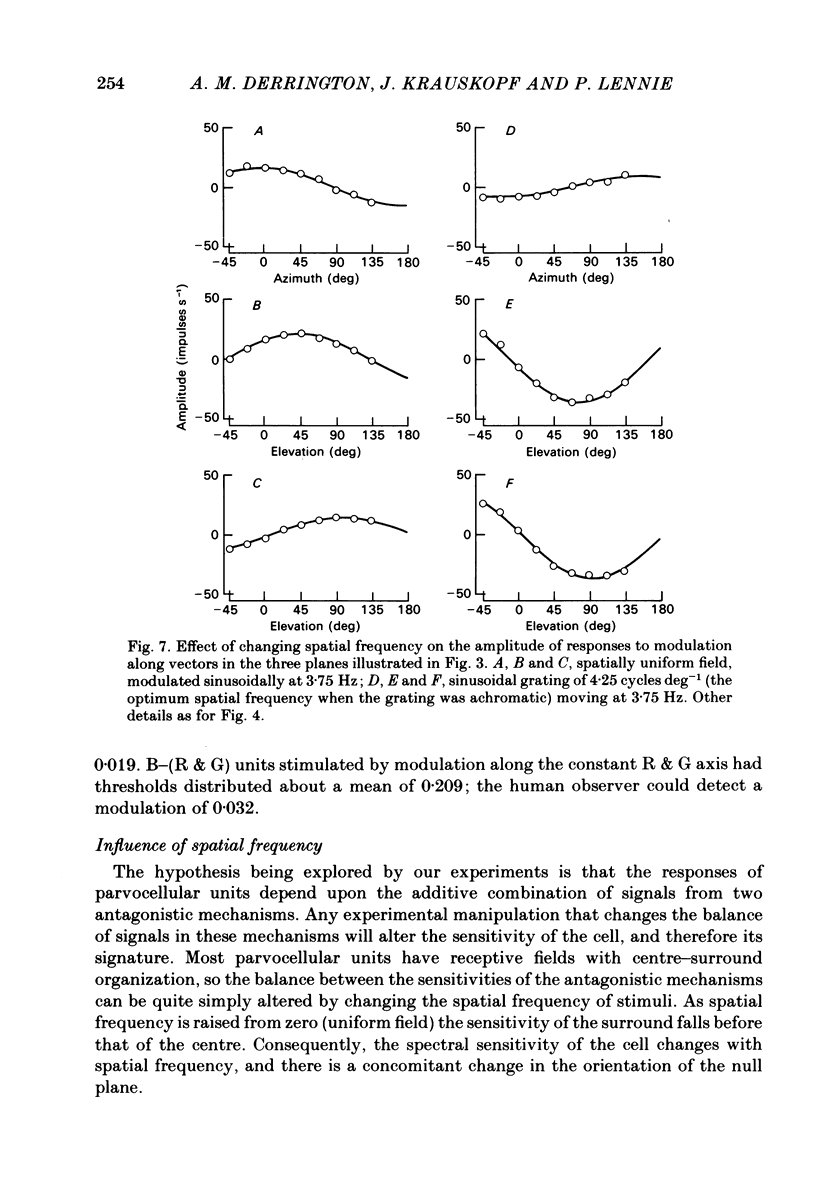
This paper introduces a new technique for the analysis of the chromatic properties of neurones, and applies it to cells in the lateral geniculate nucleus (l.g.n.) of macaque. The method exploits the fact that for any cell that combines linearly the signals from cones there is a restricted set of lights to which it is equally sensitive, and whose members can be exchanged for one another without evoking a response. Stimuli are represented in a three-dimensional space defined by an axis along which only luminance varies, without change in chromaticity, a 'constant B' axis along which chromaticity varies without changing the excitation of blue-sensitive (B) cones, a 'constant R & G' axis along which chromaticity varies without change in the excitation of red-sensitive (R) or green-sensitive (G) cones. The orthogonal axes intersect at a white point. The isoluminant plane defined by the intersection of the 'constant B' and 'constant R & G' axes contains lights that vary only in chromaticity. In polar coordinates the constant B axis is assigned the azimuth 0-180 deg, and the constant R & G axis the azimuth 90-270 deg. Luminance is expressed as elevation above or below the isoluminant plane (-90 to +90 deg). For any cell that combines cone signals linearly, there is one plane in this space, passing through the white point, that contains all lights that can be exchanged silently. The position of this 'null plane' provides the 'signature' of the cell, and is specified by its azimuth (the direction in which it intersects the isoluminant plane of the stimulus space) and its elevation (its angle of inclination to the isoluminant plane). A colour television receiver was used to produce sinusoidal gratings whose chromaticity and luminance could be modulated along any vector passing through the white point in the space described. The spatial and temporal frequencies of modulation could be varied over a large range. When stimulated by patterns of low spatial and low temporal frequency, two groups of cells in the parvocellular laminae of the l.g.n. were distinguished by the locations of their null planes. The null planes of the larger group were narrowly distributed about an azimuth of 92.6 deg and more broadly about an elevation of 51.5 deg, which suggests that they receive opposed, but not equally balanced, inputs from only R and G cones. These we call R-G cells.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






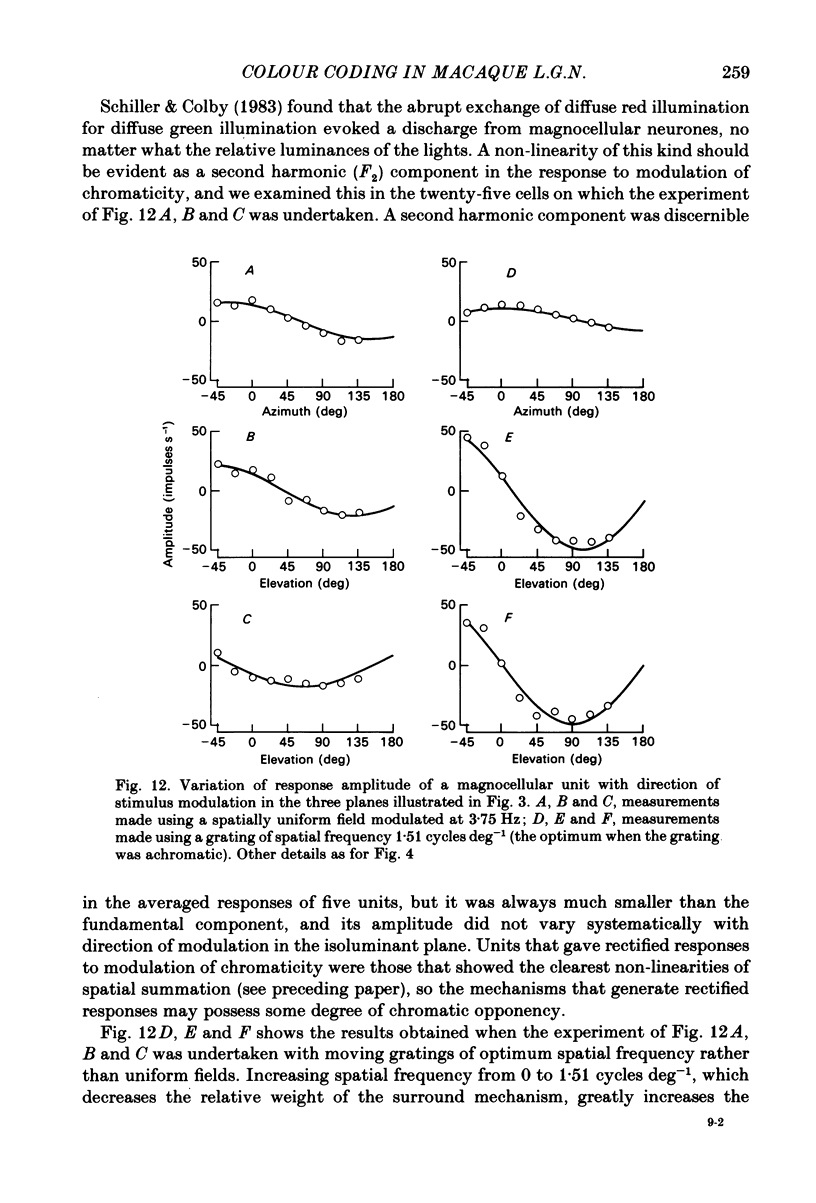
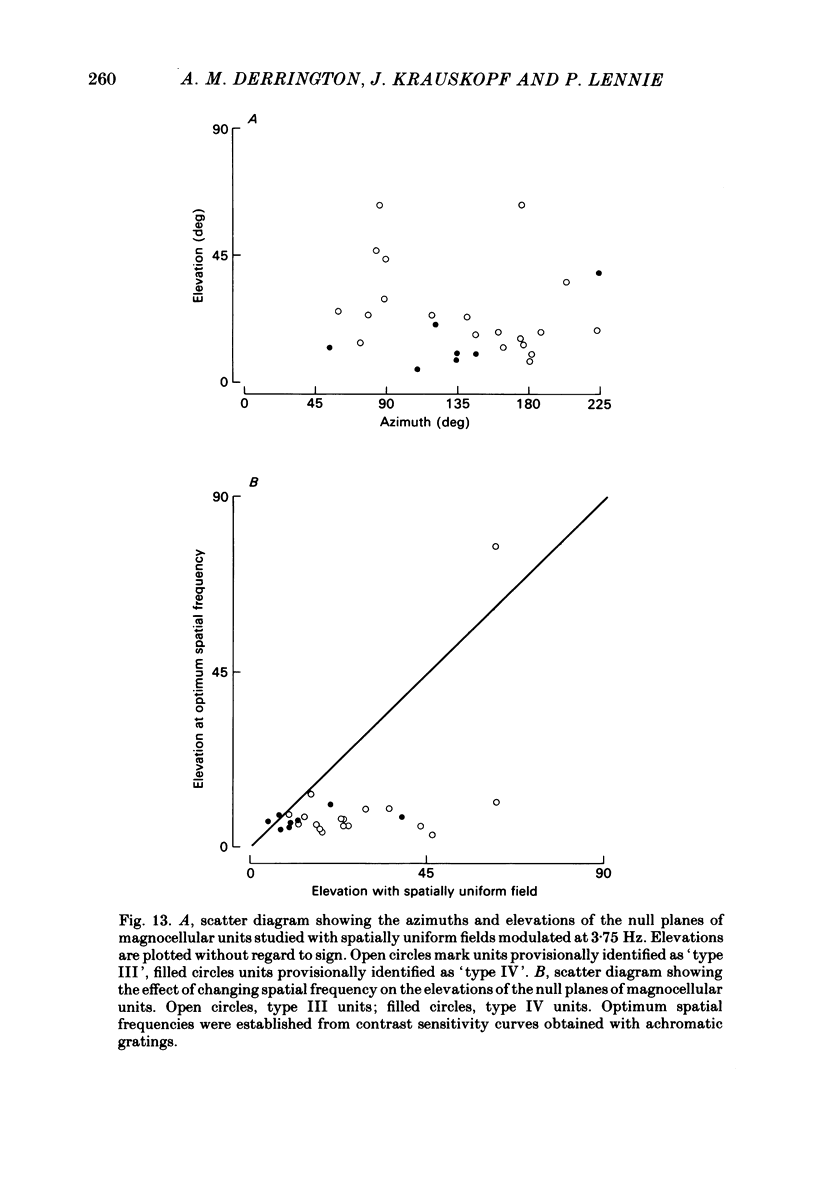
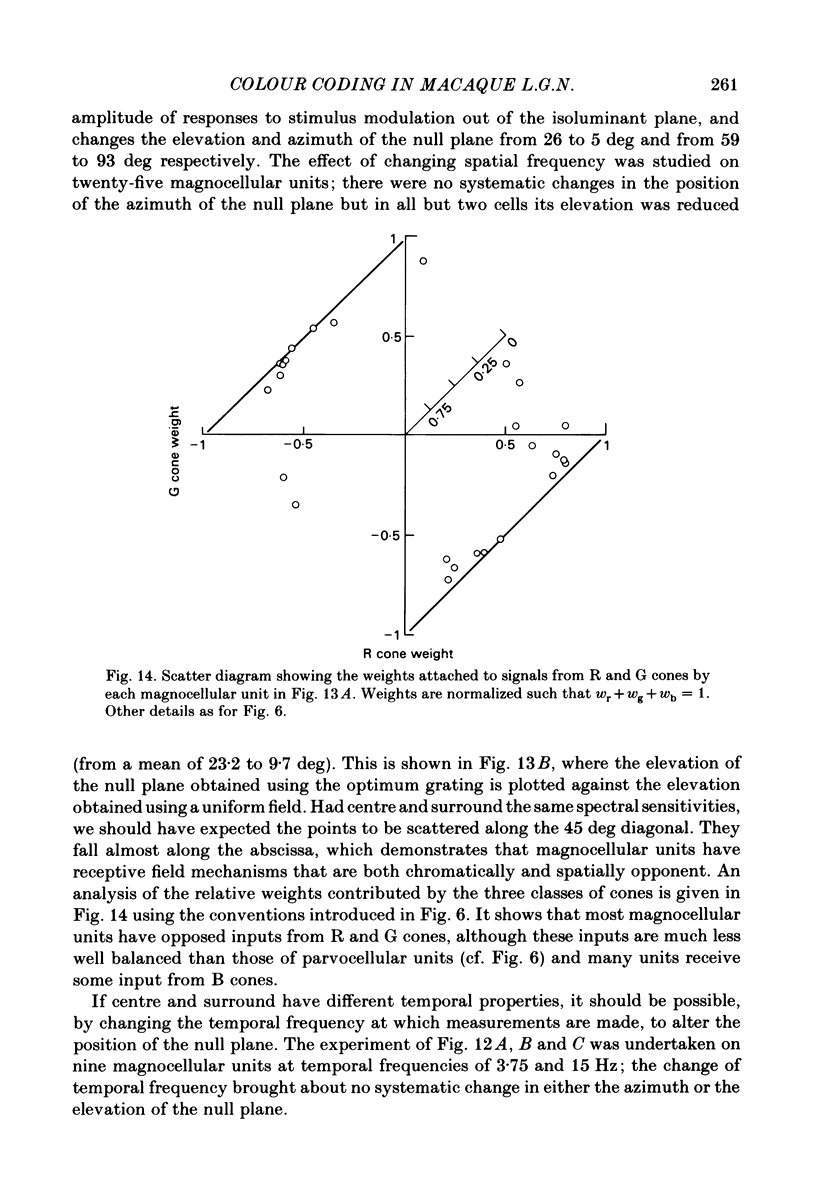

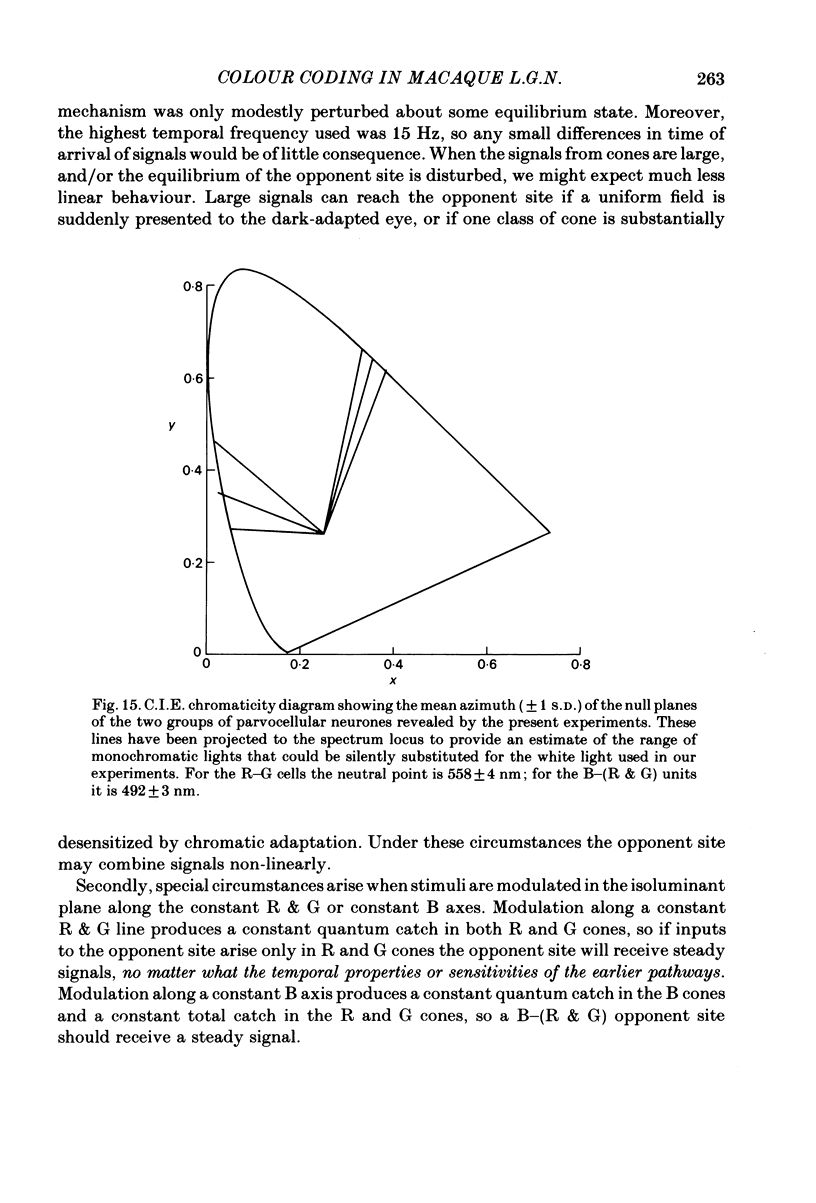


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowmaker J. K., Dartnall H. J., Mollon J. D. Microspectrophotometric demonstration of four classes of photoreceptor in an old world primate, Macaca fascicularis. J Physiol. 1980 Jan;298:131–143. doi: 10.1113/jphysiol.1980.sp013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Abramov I., Jacobs G. H. Analysis of response patterns of LGN cells. J Opt Soc Am. 1966 Jul;56(7):966–977. doi: 10.1364/josa.56.000966. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielen C. C., van Gisbergen J. A., Vendrik A. J. Reconstruction of cone-system contributions to responses of colour-opponent neurones in monkey lateral geniculate. Biol Cybern. 1982;44(3):211–221. doi: 10.1007/BF00344277. [DOI] [PubMed] [Google Scholar]
- Gouras P. Identification of cone mechanisms in monkey ganglion cells. J Physiol. 1968 Dec;199(3):533–547. doi: 10.1113/jphysiol.1968.sp008667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras P., Zrenner E. Enchancement of luminance flicker by color-opponent mechanisms. Science. 1979 Aug 10;205(4406):587–589. doi: 10.1126/science.109925. [DOI] [PubMed] [Google Scholar]
- HURVICH L. M., JAMESON D. An opponent-process theory of color vision. Psychol Rev. 1957 Nov;64, Part 1(6):384–404. doi: 10.1037/h0041403. [DOI] [PubMed] [Google Scholar]
- Krauskopf J., Williams D. R., Heeley D. W. Cardinal directions of color space. Vision Res. 1982;22(9):1123–1131. doi: 10.1016/0042-6989(82)90077-3. [DOI] [PubMed] [Google Scholar]
- MacLeod D. I., Boynton R. M. Chromaticity diagram showing cone excitation by stimuli of equal luminance. J Opt Soc Am. 1979 Aug;69(8):1183–1186. doi: 10.1364/josa.69.001183. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Colby C. L. The responses of single cells in the lateral geniculate nucleus of the rhesus monkey to color and luminance contrast. Vision Res. 1983;23(12):1631–1641. doi: 10.1016/0042-6989(83)90177-3. [DOI] [PubMed] [Google Scholar]
- Smith V. C., Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 1975 Feb;15(2):161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- Walraven P. L. A closer look at the tritanopic convergence point. Vision Res. 1974 Dec;14(12):1339–1343. doi: 10.1016/0042-6989(74)90007-8. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M., Schein S. J. Protan-like spectral sensitivity of foveal Y ganglion cells of the retina of macaque monkeys. J Physiol. 1980 Feb;299:385–396. doi: 10.1113/jphysiol.1980.sp013131. [DOI] [PMC free article] [PubMed] [Google Scholar]