Abstract
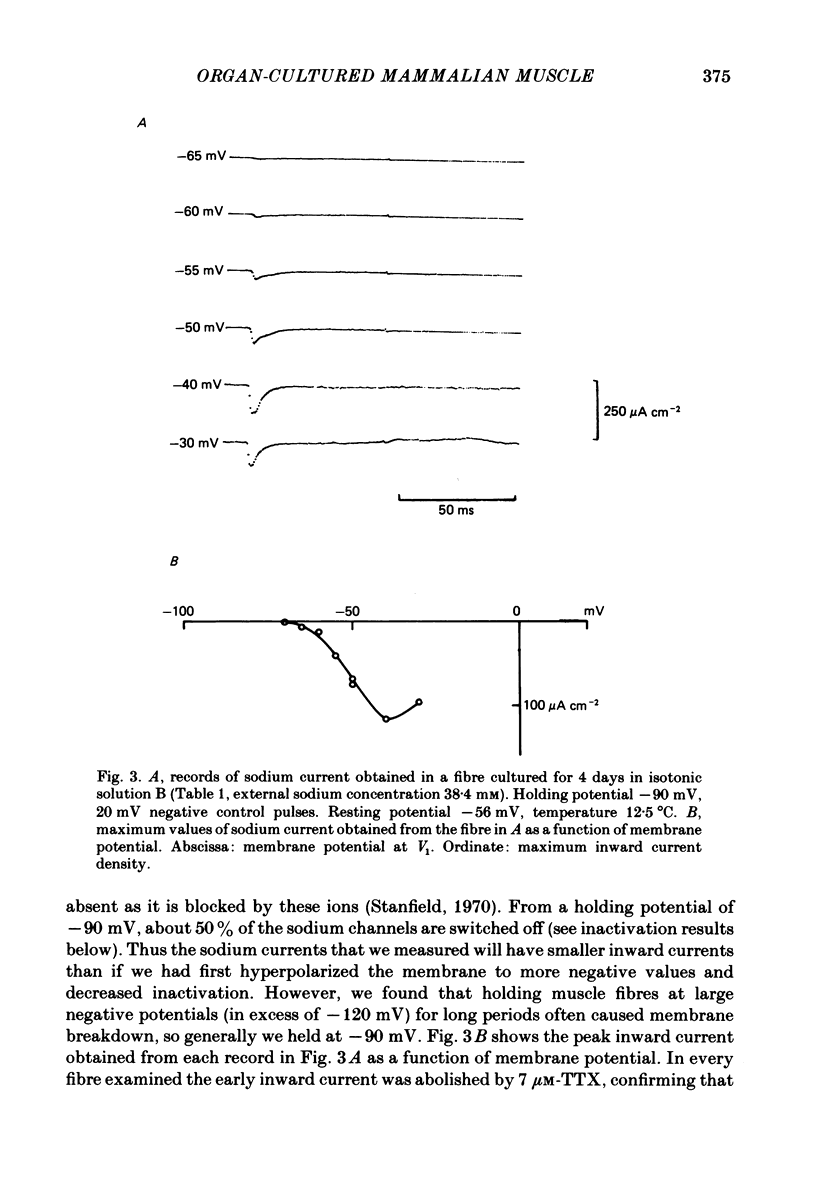
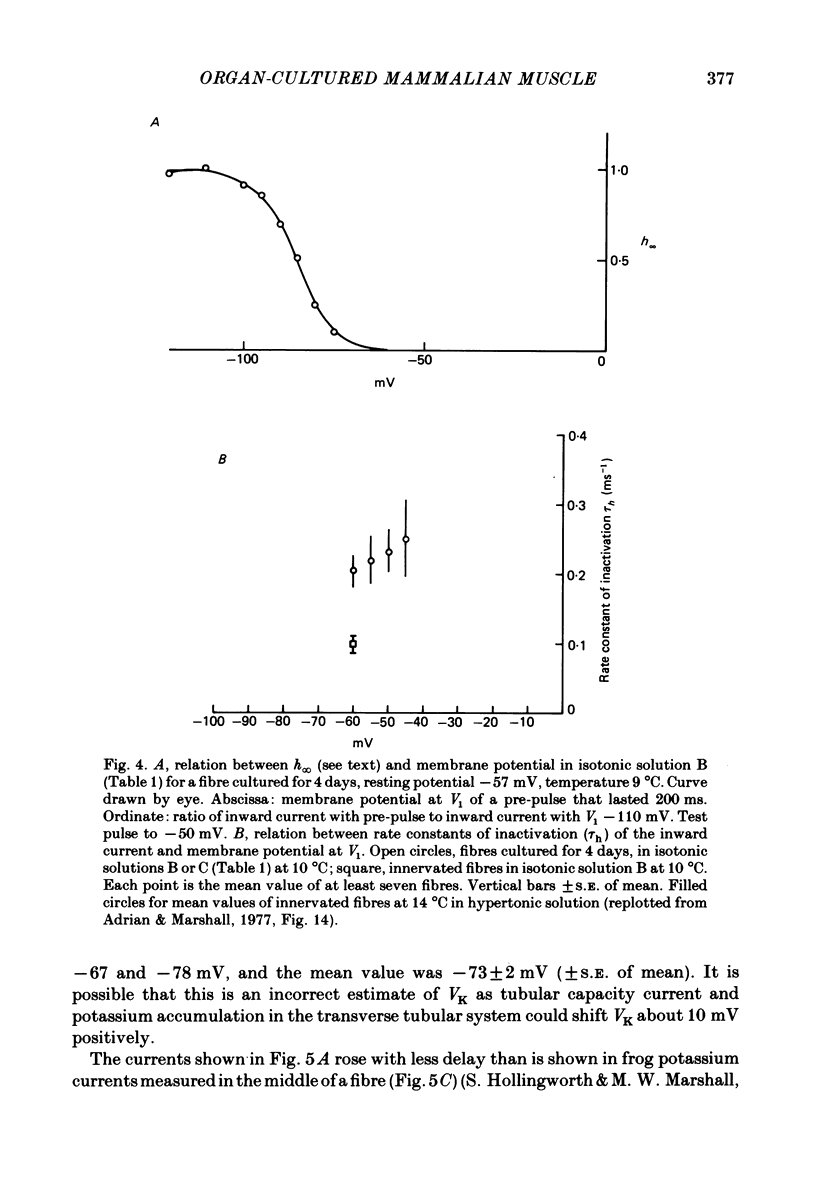
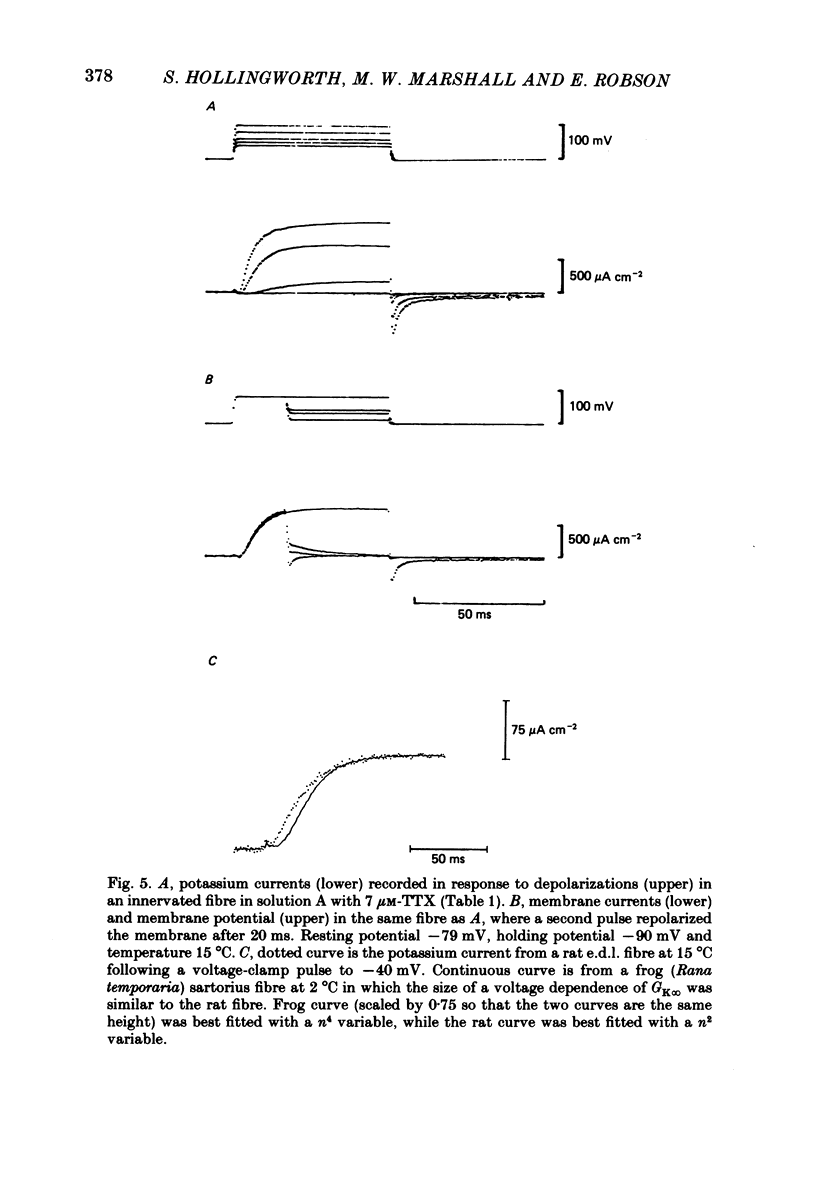
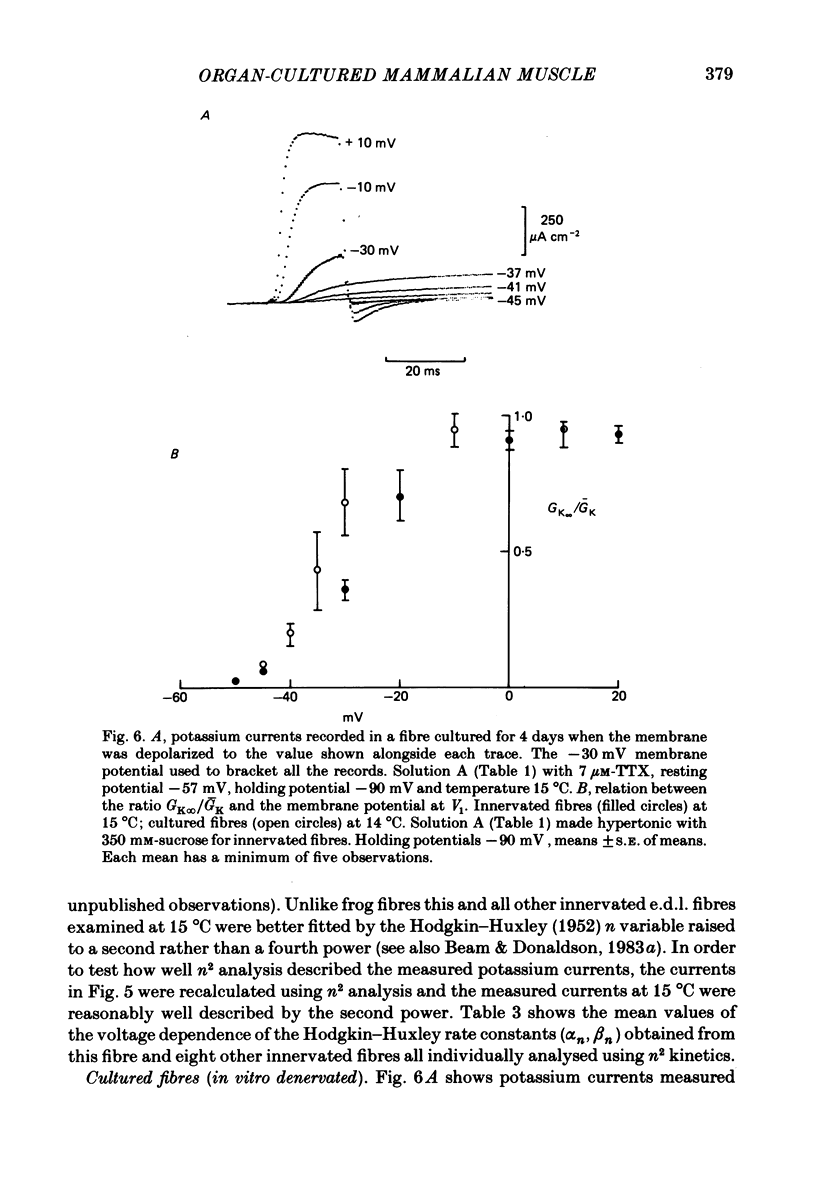
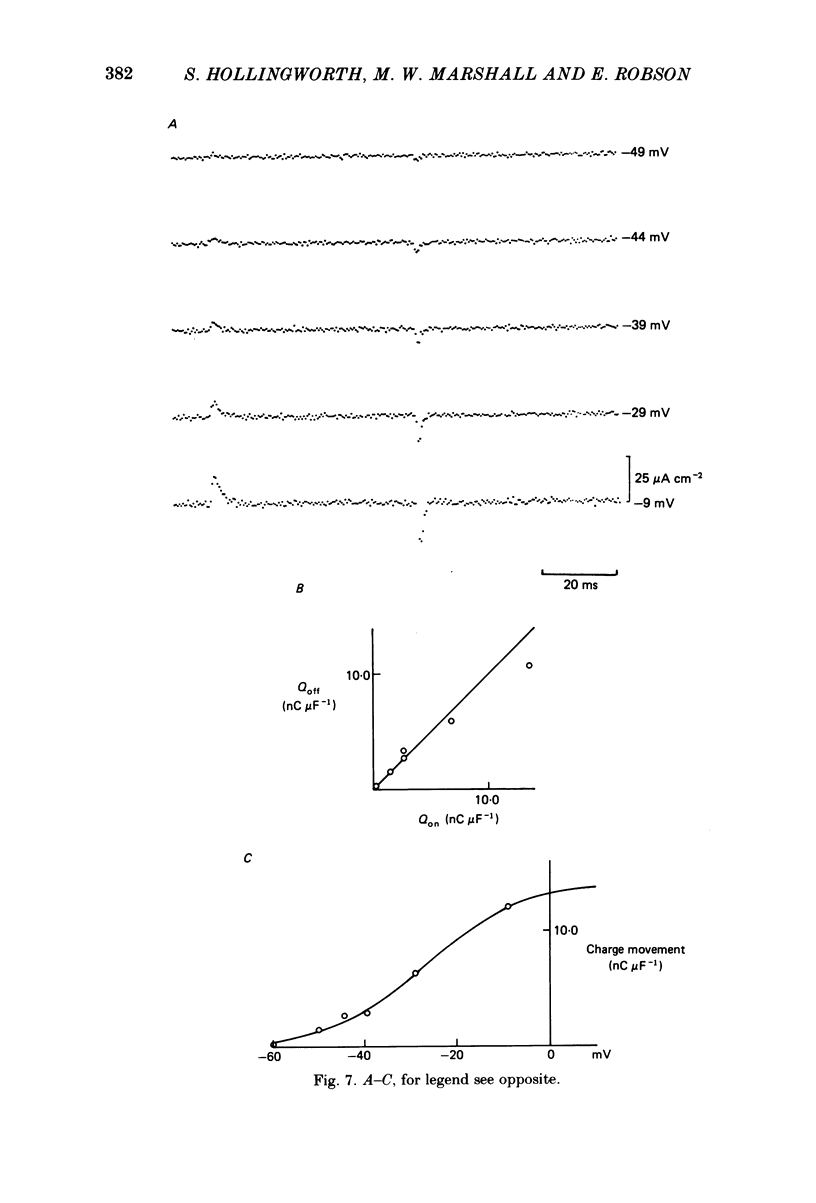
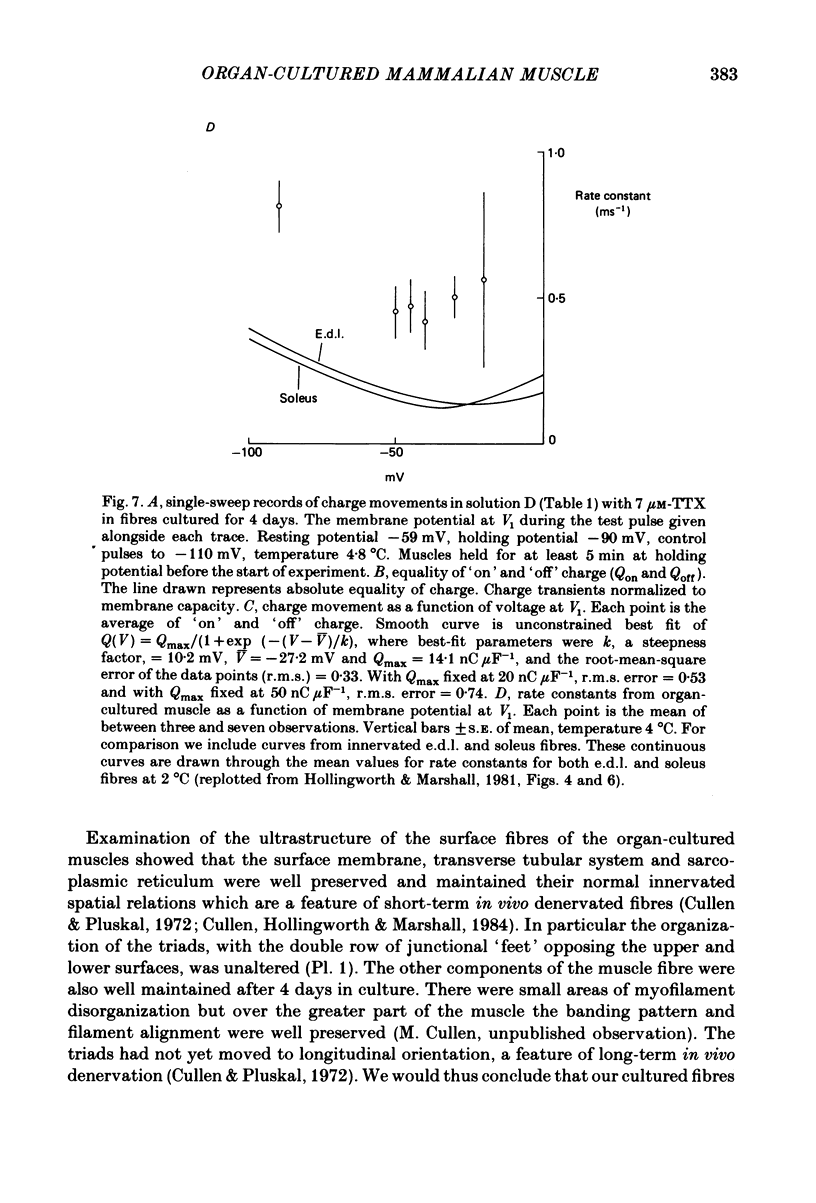
The middle of the fibre voltage-clamp technique was used to measure ionic currents and non-linear charge movements in intact, organ-cultured (in vitro denervated) mammalian fast-twitch (rat extensor digitorum longus) muscle fibres. Muscle fibres organ cultured for 4 days can be used as electrophysiological and morphological models for muscles in vivo denervated for the same length of time. Sodium currents in organ-cultured muscle fibres are similar to innervated fibres except that in the temperature range 0-20 degrees C (a) in the steady state, the voltage distribution of inactivation in cultured fibres is shifted negatively some 20 mV; (b) at the same temperature and membrane potential, the time constant of inactivation in cultured fibres is about twice that of innervated fibres. Potassium currents in innervated and cultured fibres at 15 degrees C can be fitted with the Hodgkin-Huxley n variable raised to the second power. Despite the large range we would estimate that the maximum value of the steady-state potassium conductance of cultured fibres is about one-half that of innervated fibres. The estimated maximum amount of charge moved in cultured fibre is about one-third that in innervated fibres. Compared to innervated fibres, culturing doubles the kinetics of the decay phase of charge movement. The possibility of a negative shift of the voltage distribution of charge movements in cultured fibres is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian R. H., Almers W. The voltage dependence of membrane capacity. J Physiol. 1976 Jan;254(2):317–338. doi: 10.1113/jphysiol.1976.sp011234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Marshall M. W. Sodium currents in mammalian muscle. J Physiol. 1977 Jun;268(1):223–250. doi: 10.1113/jphysiol.1977.sp011855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Schuh F. T., Kauffman F. C. Early membrane depolarization of the fast mammalian muscle after denervation. Pflugers Arch. 1971;328(1):36–50. doi: 10.1007/BF00587359. [DOI] [PubMed] [Google Scholar]
- Beam K. G., Donaldson P. L. A quantitative study of potassium channel kinetics in rat skeletal muscle from 1 to 37 degrees C. J Gen Physiol. 1983 Apr;81(4):485–512. doi: 10.1085/jgp.81.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Donaldson P. L. Slow components of potassium tail currents in rat skeletal muscle. J Gen Physiol. 1983 Apr;81(4):513–530. doi: 10.1085/jgp.81.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekoff A., Betz W. Properties of isolated adult rat muscle fibres maintained in tissue culture. J Physiol. 1977 Oct;271(2):537–547. doi: 10.1113/jphysiol.1977.sp012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. J., Hollingworth S., Marshall M. W. A comparative study of the transverse tubular system of the rat extensor digitorum longus and soleus muscles. J Anat. 1984 Mar;138(Pt 2):297–308. [PMC free article] [PubMed] [Google Scholar]
- Cullen M. J., Pluskal M. G. Early changes in the ultrastructure of denervated rat skeletal muscle. Exp Neurol. 1977 Jul;56(1):115–131. doi: 10.1016/0014-4886(77)90143-1. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Asymmetrical charge movement in slow- and fast-twitch mammalian muscle fibres in normal and paraplegic rats. J Physiol. 1983 Aug;341:213–231. doi: 10.1113/jphysiol.1983.sp014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval A., Léoty C. Comparison between the delayed outward current in slow and fast twitch skeletal muscle in the rat. J Physiol. 1980 Oct;307:43–57. doi: 10.1113/jphysiol.1980.sp013422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval A., Léoty C. Ionic currents in mammalian fast skeletal muscle. J Physiol. 1978 May;278:403–423. doi: 10.1113/jphysiol.1978.sp012312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval A., Léoty C. Ionic currents in slow twitch skeletal muscle in the rat. J Physiol. 1980 Oct;307:23–41. doi: 10.1113/jphysiol.1980.sp013421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finol H. J., Lewis D. M., Owens R. The effects of denervation on contractile properties or rat skeletal muscle. J Physiol. 1981;319:81–92. doi: 10.1113/jphysiol.1981.sp013893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E. Some effects of hypertonic solutions on contraction and excitation-contraction coupling in frog skeletal muscles. J Gen Physiol. 1970 Feb;55(2):254–275. doi: 10.1085/jgp.55.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann E., Melichna J., Syrový I. Independence of changes in contraction properties and myofibrillar ATPase activity of denervated mammalian muscle on length of nerve stump. Physiol Bohemoslov. 1976;25(1):43–50. [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. A study of frog muscle maintained in organ culture. J Physiol. 1972 Feb;221(1):207–226. doi: 10.1113/jphysiol.1972.sp009749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Marshall M. W. Tetrodotoxin-resistant action potentials in newborn rat muscle. Nat New Biol. 1973 Jun 6;243(127):191–192. doi: 10.1038/newbio243191a0. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Resting tension and the form of the twitch of rat skeletal muscle at low temperature. J Physiol. 1972 Feb;221(1):161–171. doi: 10.1113/jphysiol.1972.sp009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth S., Marshall M. W. A comparative study of charge movement in rat and frog skeletal muscle fibres. J Physiol. 1981 Dec;321:583–602. doi: 10.1113/jphysiol.1981.sp014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. W., Ward M. R. Anode break excitation in denervated rat skeletal muscle fibres. J Physiol. 1974 Jan;236(2):413–420. doi: 10.1113/jphysiol.1974.sp010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappone P. A. Voltage-clamp experiments in normal and denervated mammalian skeletal muscle fibres. J Physiol. 1980 Sep;306:377–410. doi: 10.1113/jphysiol.1980.sp013403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Sakmann B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. J Physiol. 1974 Feb;237(1):157–182. doi: 10.1113/jphysiol.1974.sp010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P., Thesleff S. Action potential generation in denervated rat skeletal muscle. II. The action of tetrodotoxin. Acta Physiol Scand. 1971 May;82(1):70–78. doi: 10.1111/j.1748-1716.1971.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Robbins N. Cation movements in normal and short-term denervated rat fast twitch muscle. J Physiol. 1977 Oct;271(3):605–624. doi: 10.1113/jphysiol.1977.sp012017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWELL O. A. The culture of mature organs in a synthetic medium. Exp Cell Res. 1959 Jan;16(1):118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]




