Abstract
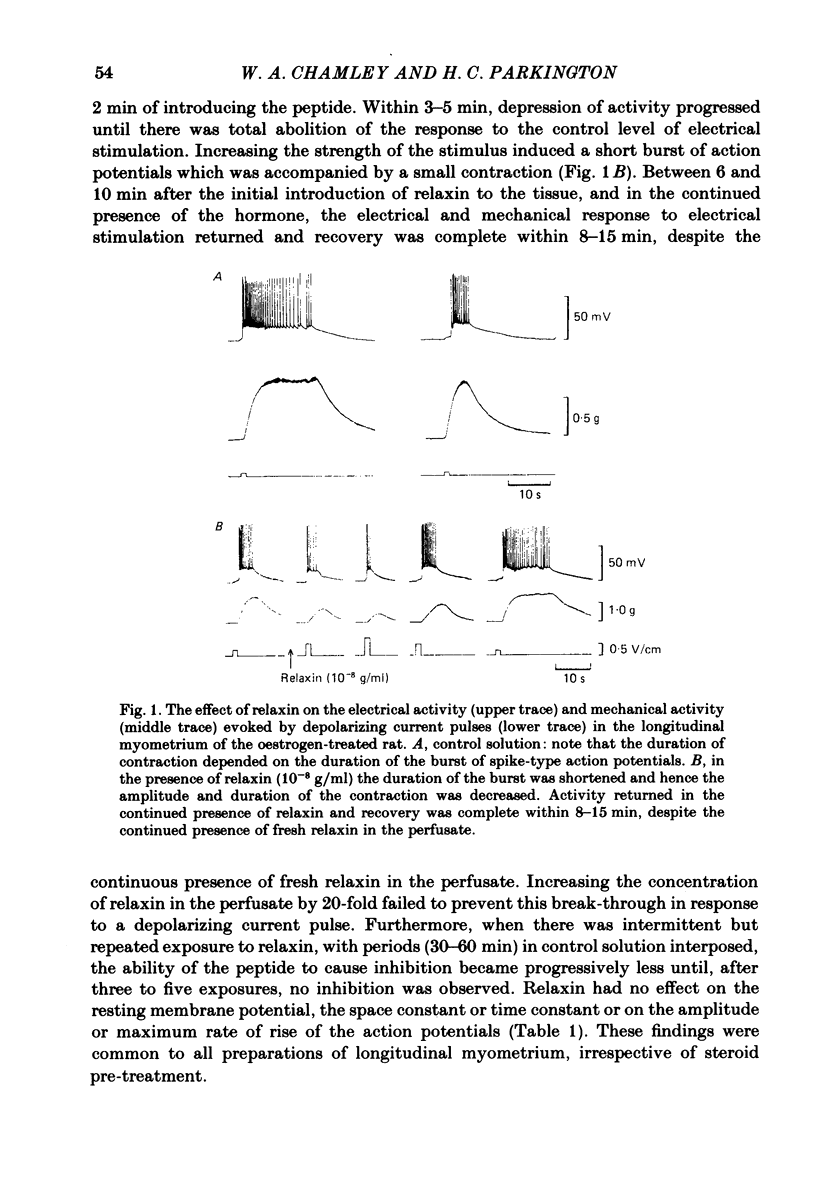
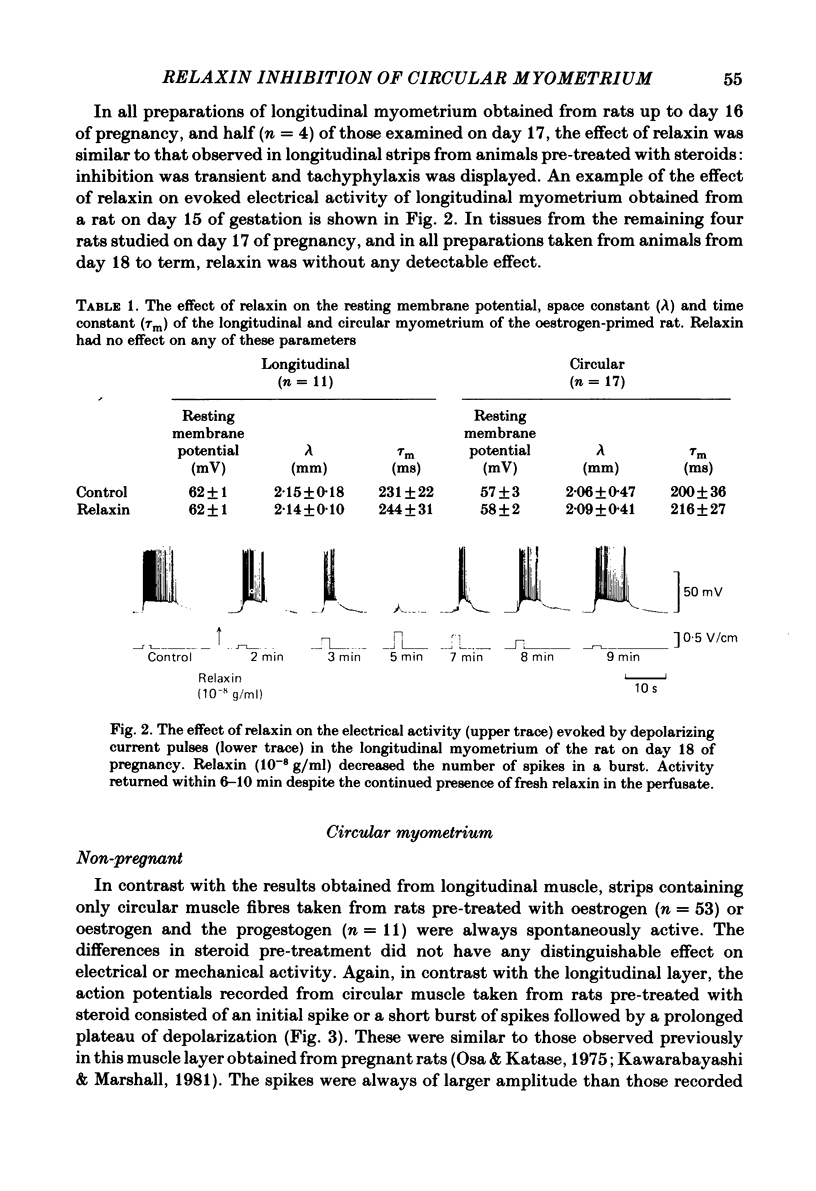
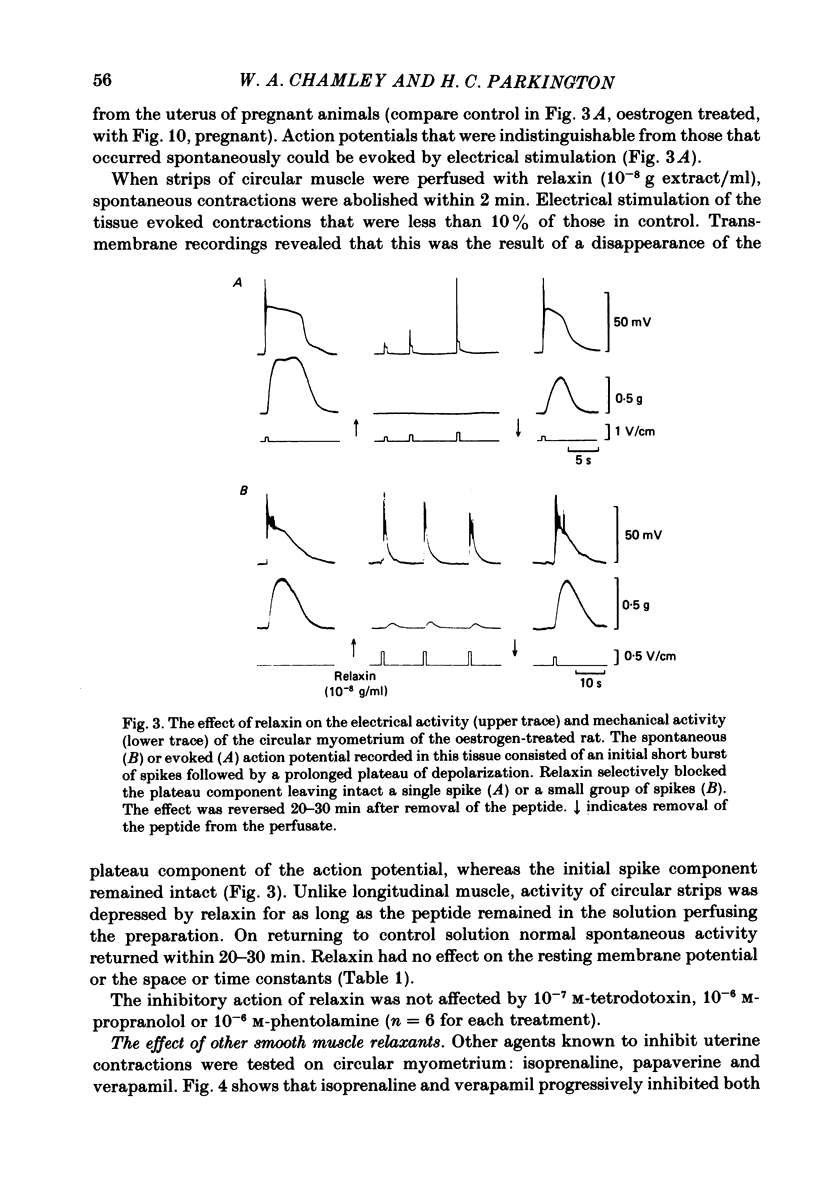
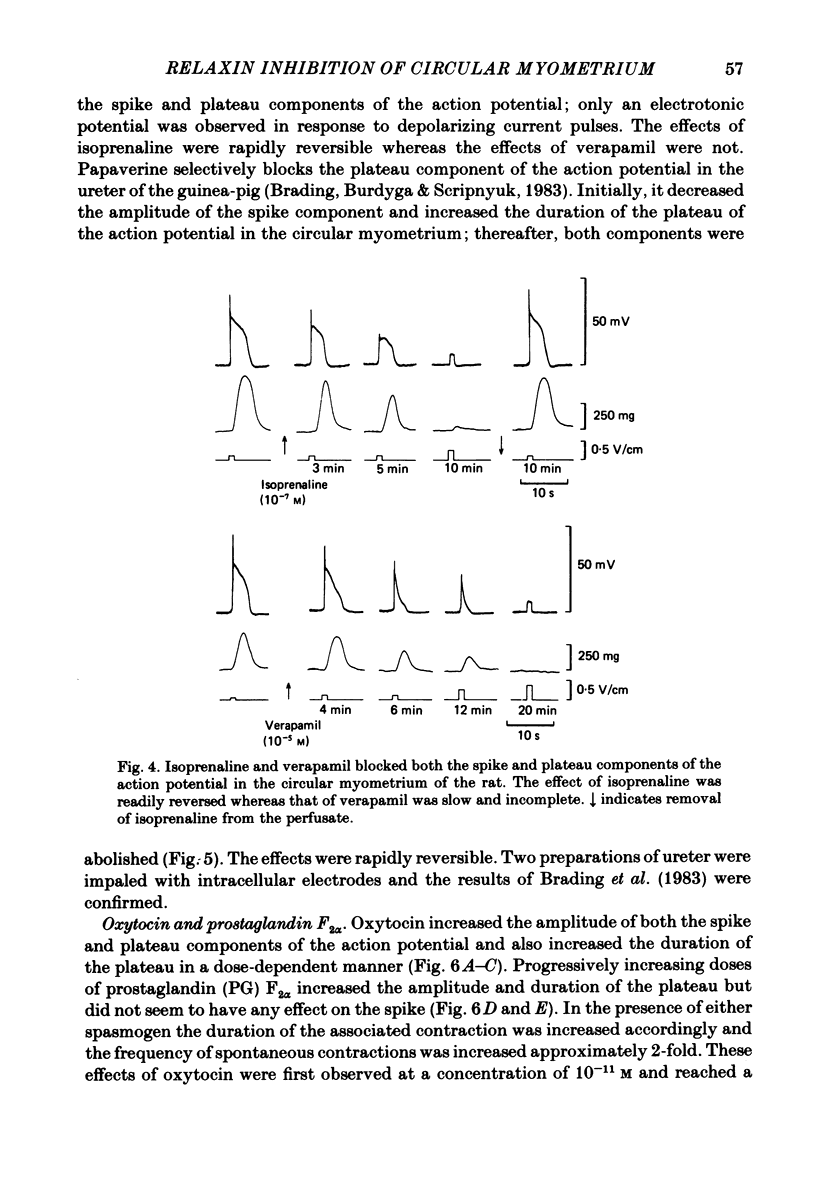
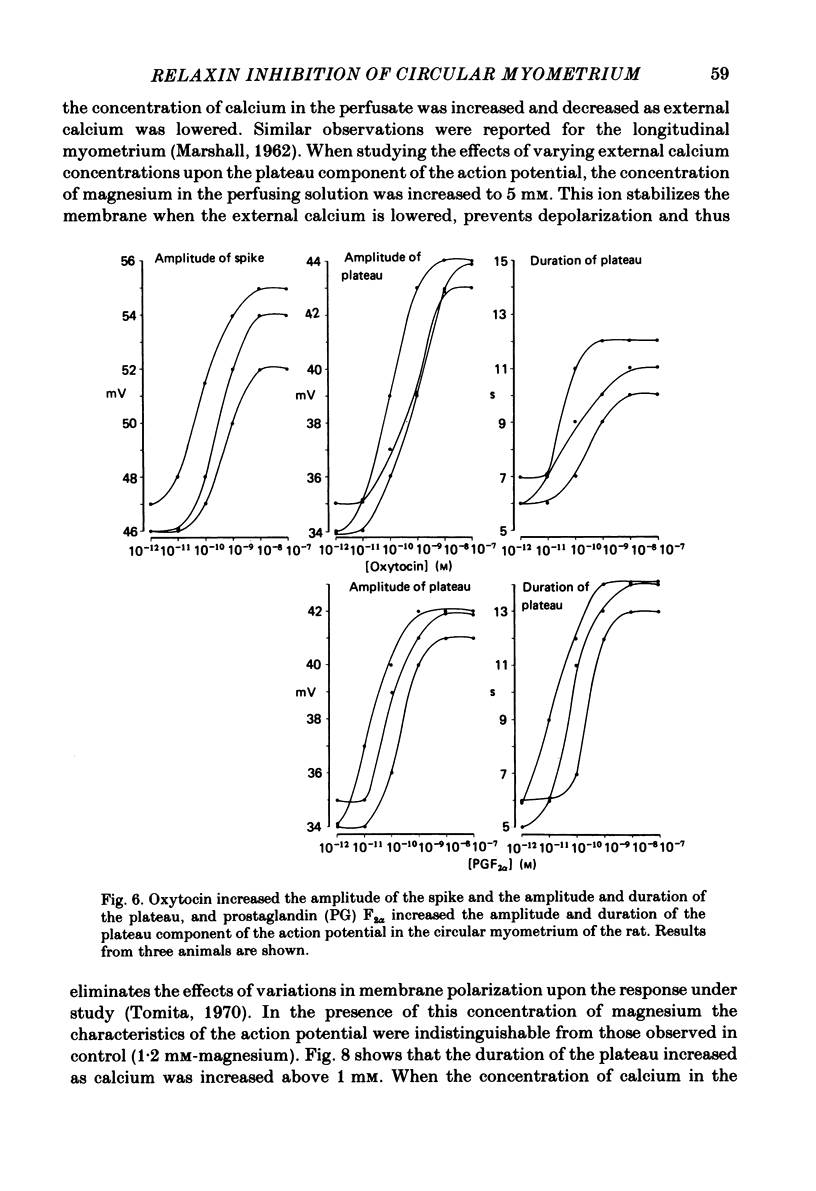
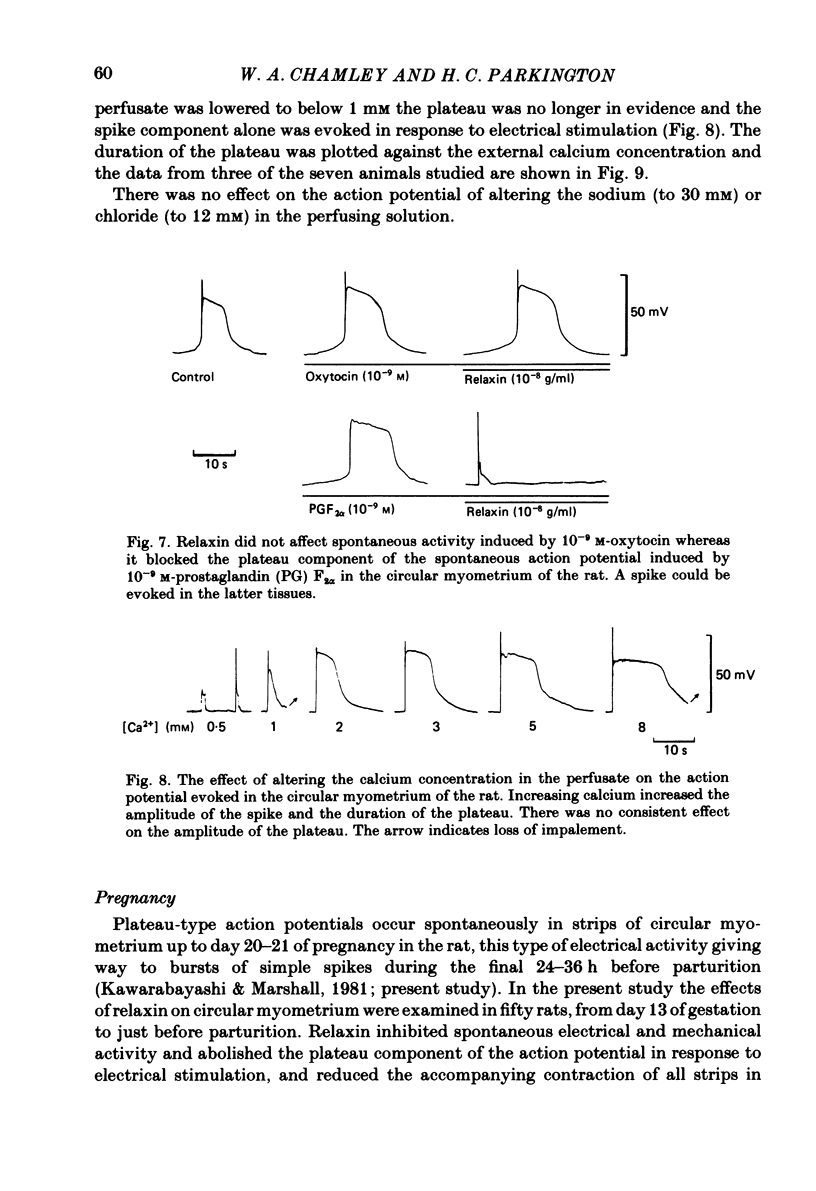
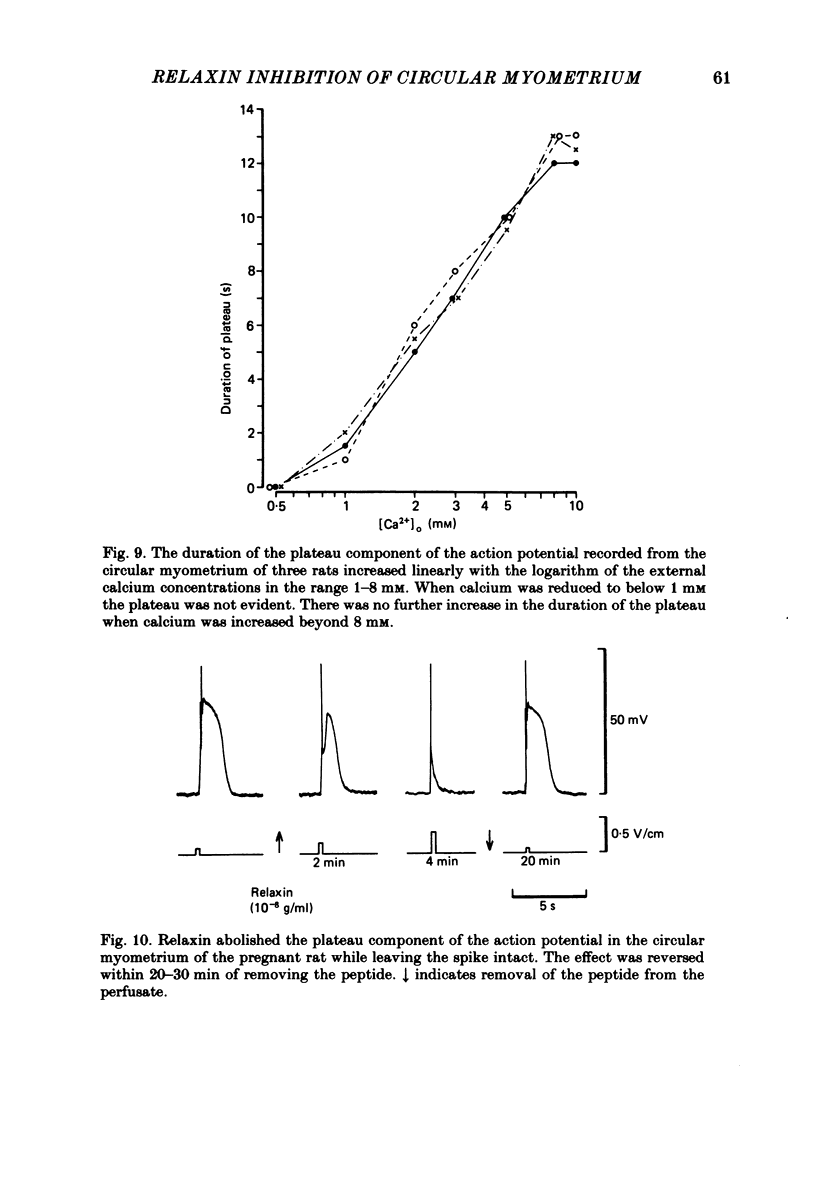
The effects of relaxin on contractility and membrane potential of the longitudinal and circular smooth muscle layers of the uterus have been studied in vitro using oestrogen-treated, non-pregnant rats and pregnant rats. Relaxin decreased the amplitude of contractions induced by electrical stimulation of longitudinal myometrium by decreasing the duration of the bursts of action potentials. This effect was transient and tachyphylaxis always developed and was observed following injection of steroids and up to day 17 of pregnancy. There was no inhibition of tissues from rats from day 18 of pregnancy to term. The peptide had no effect on resting membrane potential, space constant or time constant. Action potentials recorded from circular myometrium of non-pregnant rats pre-treated with oestrogen consisted of an initial spike or short burst of spikes followed by a prolonged plateau of depolarization. Spontaneous action potentials and associated contractions were abolished within 2 min of exposure to relaxin (10(-8) g/ml) while contractions of much smaller amplitude could be evoked with depolarizing current pulses. This effect was associated with depression of the plateau component of the action potential whereas the spike component was left intact. Relaxin had no effect on passive membrane properties. The action potentials of circular myometrium of rats up to day 21 of pregnancy were qualitatively similar to those recorded in the same muscle layer from oestrogen-treated, non-pregnant rats and the plateau component was also blocked by relaxin in these tissues. Bursts of spikes were observed in circular strips 24-36 h before parturition, and the effect of the peptide on these was a transient inhibition similar to that observed in longitudinal myometrium. Oxytocin increased the amplitude of the spike and the amplitude and duration of the plateau. Relaxin abolished the plateau in the presence of 10(-11) and 10(-10) M-oxytocin but was ineffective when the concentration of the spasmogen was increased further. Prostaglandin F2 alpha increased the amplitude and duration of the plateau. Relaxin abolished the responses to 10(-10) and 10(-9) M-prostaglandin F2 alpha. The results of this study demonstrate that relaxin specifically inhibits contractions in the circular layer of the myometrium by abolishing the plateau component of the action potential. This action appears to be different from that of other smooth muscle relaxants tested in these experiments (isoprenaline, papaverine and verapamil). All of these abolished simultaneously both the spike and plateau components of the action potential.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Burdyga T. V., Scripnyuk Z. D. The effects of papaverine on the electrical and mechanical activity of the guinea-pig ureter. J Physiol. 1983 Jan;334:79–89. doi: 10.1113/jphysiol.1983.sp014481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J. M., Downing S. J., Moffatt A., Hinton J. C., Porter D. G. Demonstration of some of the physiological properties of rat relaxin. J Reprod Fertil. 1981 Sep;63(1):145–153. doi: 10.1530/jrf.0.0630145. [DOI] [PubMed] [Google Scholar]
- Chamley W. A., Bagoyo M. M., Bryant-Greenwood G. D. In vitro response of relaxin-treated rat uterus to prostaglandins and oxytocin. Prostaglandins. 1977 Oct;14(4):763–769. doi: 10.1016/0090-6980(77)90204-0. [DOI] [PubMed] [Google Scholar]
- Chamley W. A., Bagoyo M. M., Bryant-Greenwood G. D. Potencies of porcine relaxins using two bioassays. J Endocrinol. 1981 Jan;88(1):89–96. doi: 10.1677/joe.0.0880089. [DOI] [PubMed] [Google Scholar]
- Downing S. J., Lye S. J., Bradshaw J. M., Porter D. G. Rat myometrial activity in vivo: effects of oestradiol-17 beta and progesterone in relation to the concentrations of cytoplasmic progesterone receptors. J Endocrinol. 1978 Jul;78(1):103–117. doi: 10.1677/joe.0.0780103. [DOI] [PubMed] [Google Scholar]
- Fuchs A. R. Myometrial response to prostaglandins enhanced by progesterone. Am J Obstet Gynecol. 1974 Apr 15;118(8):1093–1098. doi: 10.1016/0002-9378(74)90688-7. [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Poblete V. F., Jr Oxytocin and uterine function in pregnant and parturient rats. Biol Reprod. 1970 Jun;2(3):387–400. doi: 10.1095/biolreprod2.3.387. [DOI] [PubMed] [Google Scholar]
- Hudson P., Haley J., John M., Cronk M., Crawford R., Haralambidis J., Tregear G., Shine J., Niall H. Structure of a genomic clone encoding biologically active human relaxin. Nature. 1983 Feb 17;301(5901):628–631. doi: 10.1038/301628a0. [DOI] [PubMed] [Google Scholar]
- Isaacs N., James R., Niall H., Bryant-Greenwood G., Dodson G., Evans A., North A. C. Relaxin and its structural relationship to insulin. Nature. 1978 Jan 19;271(5642):278–281. doi: 10.1038/271278a0. [DOI] [PubMed] [Google Scholar]
- James R., Niall H., Kwok S., Bryand-Greenwood G. Primary structure of porcine relaxin: homology with insulin and related growth factors. Nature. 1977 Jun 9;267(5611):544–546. doi: 10.1038/267544a0. [DOI] [PubMed] [Google Scholar]
- KRANTZ J. C., Jr, BRYANT H. H., CARR C. J. The action of aqueous corpus luteum extract upon uterine activity. Surg Gynecol Obstet. 1950 Mar;90(3):372–375. [PubMed] [Google Scholar]
- Kawarabayashi T., Marshall J. M. Factors influencing circular muscle activity in the pregnant rat uterus. Biol Reprod. 1981 Mar;24(2):373–379. doi: 10.1095/biolreprod24.2.373. [DOI] [PubMed] [Google Scholar]
- Khaligh H. S. Inhibition by relaxin of spontaneous contractions of the uterus of the hamster in vitro. J Endocrinol. 1968 Jan;40(1):125–126. doi: 10.1677/joe.0.0400125. [DOI] [PubMed] [Google Scholar]
- MARSHALL J. M. Regulation of activity in uterine smooth muscle. Physiol Rev Suppl. 1962 Jul;5:213–227. [PubMed] [Google Scholar]
- Mironneau J. Effects of oxytocin on ionic currents underlying rhythmic activity and contraction in uterine smooth muscle. Pflugers Arch. 1976 May 12;363(2):113–118. doi: 10.1007/BF01062278. [DOI] [PubMed] [Google Scholar]
- Osa T., Katase T. Physiological comparison of the longitudinal and circular muscles of the pregnant rat uterus. Jpn J Physiol. 1975;25(2):153–164. doi: 10.2170/jjphysiol.25.153. [DOI] [PubMed] [Google Scholar]
- PATERSON G. THE NATURE OF THE INHIBITION OF THE RAT UTERUS BY RELAXIN. J Pharm Pharmacol. 1965 Apr;17:262–264. doi: 10.1111/j.2042-7158.1965.tb07666.x. [DOI] [PubMed] [Google Scholar]
- Parkington H. C. Electrical properties of the costo-uterine muscle of the guinea-pig. J Physiol. 1983 Feb;335:15–27. doi: 10.1113/jphysiol.1983.sp014515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. G., Downing S. J., Bradshaw J. M. Relaxin inhibits spontaneous and prostaglandin-driven myometrial activity in anaesthetized rats. J Endocrinol. 1979 Nov;83(2):183–192. doi: 10.1677/joe.0.0830183. [DOI] [PubMed] [Google Scholar]
- Porter D. G., Lye S. J., Bradshaw J. M., Kendall J. Z. Relaxin inhibits myometrial activity in the ovariectomized non-pregnant ewe. J Reprod Fertil. 1981 Mar;61(2):409–414. doi: 10.1530/jrf.0.0610409. [DOI] [PubMed] [Google Scholar]
- Porter D. G. Myometrium of the pregnant guinea pig: the probable importance of relaxin. Biol Reprod. 1972 Dec;7(3):458–464. doi: 10.1093/biolreprod/7.3.458. [DOI] [PubMed] [Google Scholar]
- Porter D. G. Unsolved problems of relaxin's physiological role. Ann N Y Acad Sci. 1982;380:151–162. doi: 10.1111/j.1749-6632.1982.tb18037.x. [DOI] [PubMed] [Google Scholar]
- RUDZIK A. D., MILLER J. W. The effect of altering the catecholamine content of the uterus on the rate of contractions and the sensitivity of the myometrium to relaxin. J Pharmacol Exp Ther. 1962 Oct;138:88–95. [PubMed] [Google Scholar]
- RUDZIK A. D., MILLER J. W. The mechanism of uterine inhibitory action of relaxin-containing ovarian extracts. J Pharmacol Exp Ther. 1962 Oct;138:82–87. [PubMed] [Google Scholar]
- Reiner O., Marshall J. M. Action of prostaglandin, PGF2alpha, on the uterus of the pregnant rat. Naunyn Schmiedebergs Arch Pharmacol. 1976;292(3):243–250. doi: 10.1007/BF00517384. [DOI] [PubMed] [Google Scholar]
- SAWYER W. H., FRIEDEN E. H., MARTIN A. C. In vitro inhibition of spontaneous contractions of the rat uterus by relaxin-containing extracts of sow ovaries. Am J Physiol. 1953 Mar;172(3):547–552. doi: 10.1152/ajplegacy.1953.172.3.547. [DOI] [PubMed] [Google Scholar]
- Sanborn B. M., Weisbrodt N. W., Sherwood O. D. Evidence against an obligatory role for catecholamine release or prostacyclin synthesis in the effects of relaxin on the rat uterus. Biol Reprod. 1981 Jun;24(5):987–992. doi: 10.1093/biolreprod/24.5.987. [DOI] [PubMed] [Google Scholar]
- Sherwood O. D., Crnekovic V. E., Gordon W. L., Rutherford J. E. Radioimmunoassay of relaxin throughout pregnancy and during parturition in the rat. Endocrinology. 1980 Sep;107(3):691–698. doi: 10.1210/endo-107-3-691. [DOI] [PubMed] [Google Scholar]
- Szlachter N., O'Byrne E., Goldsmith L., Steinetz B. G., Weiss G. Myometrial inhibiting activity of relaxin-containing extracts of human corpora lutea of pregnancy. Am J Obstet Gynecol. 1980 Mar 1;136(5):584–586. doi: 10.1016/0002-9378(80)91007-8. [DOI] [PubMed] [Google Scholar]
- WIQVIST N., PAUL K. G. Inhibition of the spontaneous uterine motility in vitro as a bioassay of relaxin. Acta Endocrinol (Copenh) 1958 Sep;29(1):135–146. doi: 10.1530/acta.0.0290135. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K., Hawkins R. A., Stocker J. F. Estrogen secretion by the rat ovary in vivo during the estrous cycle and pregnancy. Endocrinology. 1969 Jul;85(1):103–112. doi: 10.1210/endo-85-1-103. [DOI] [PubMed] [Google Scholar]


