Abstract
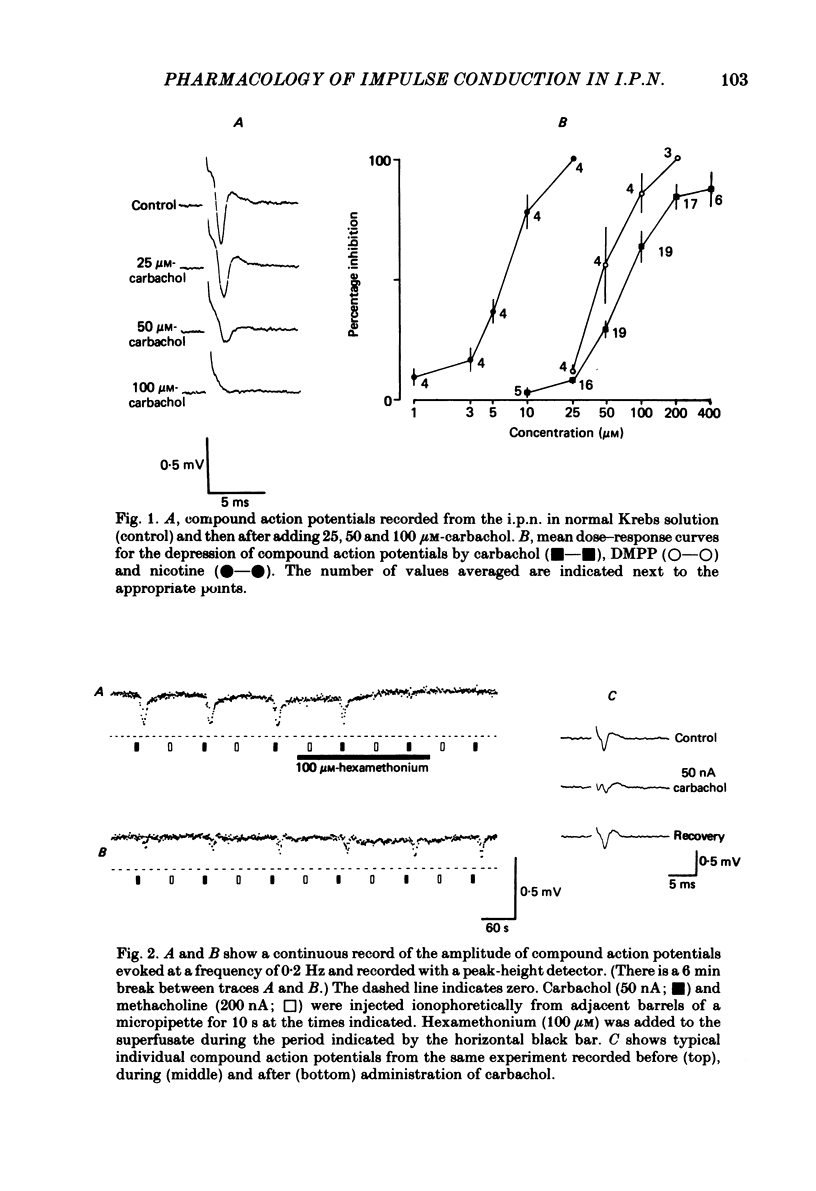
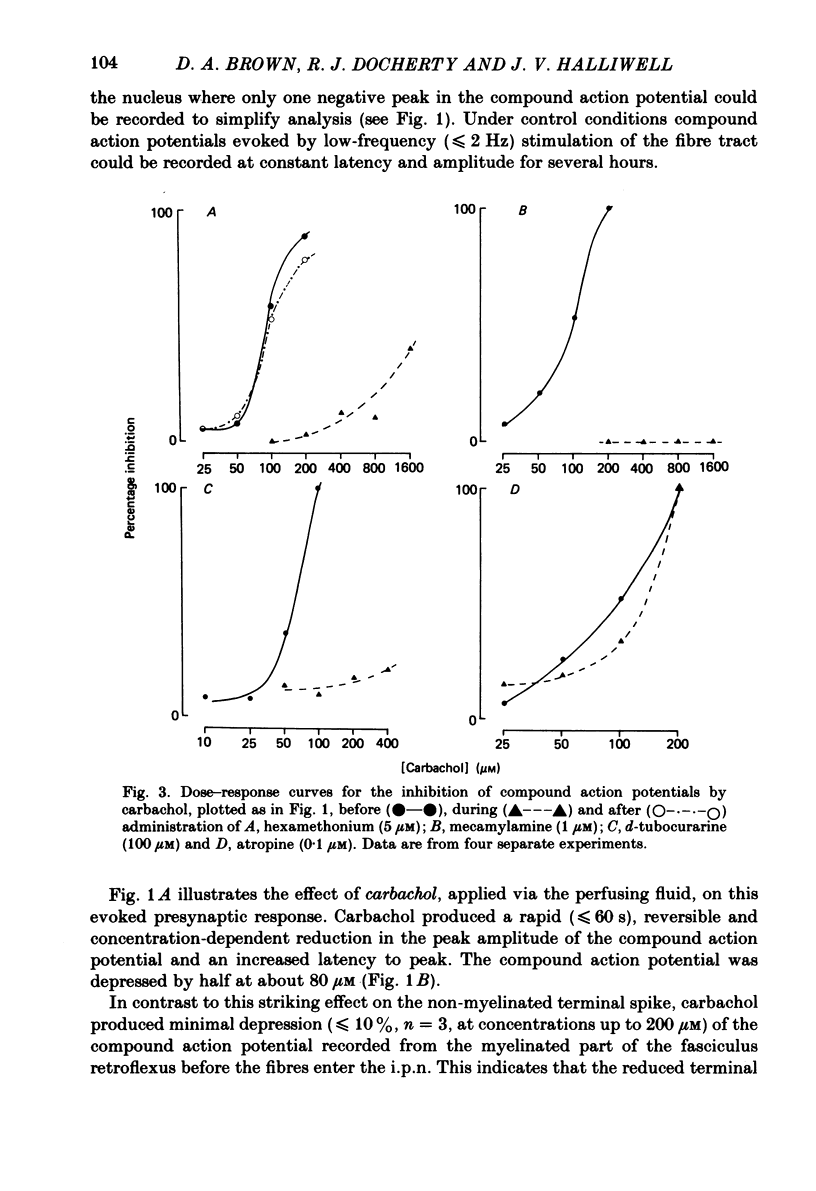
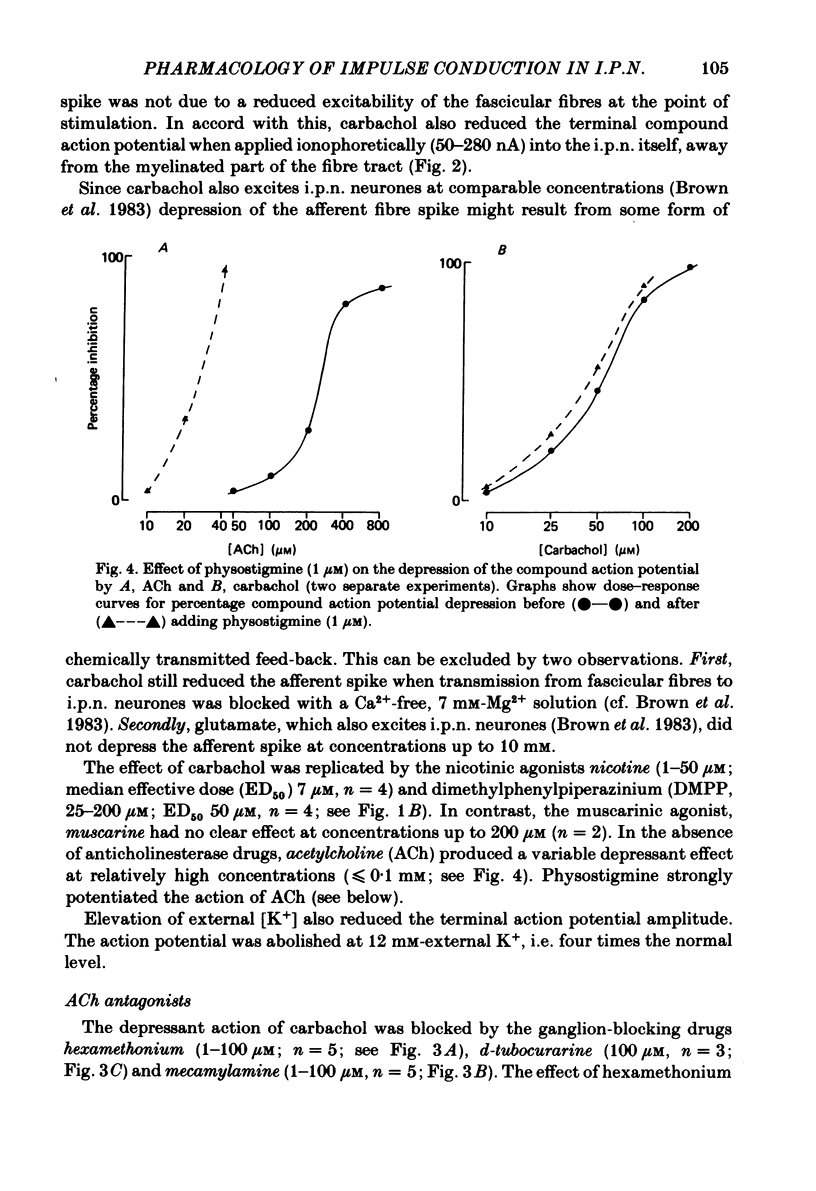
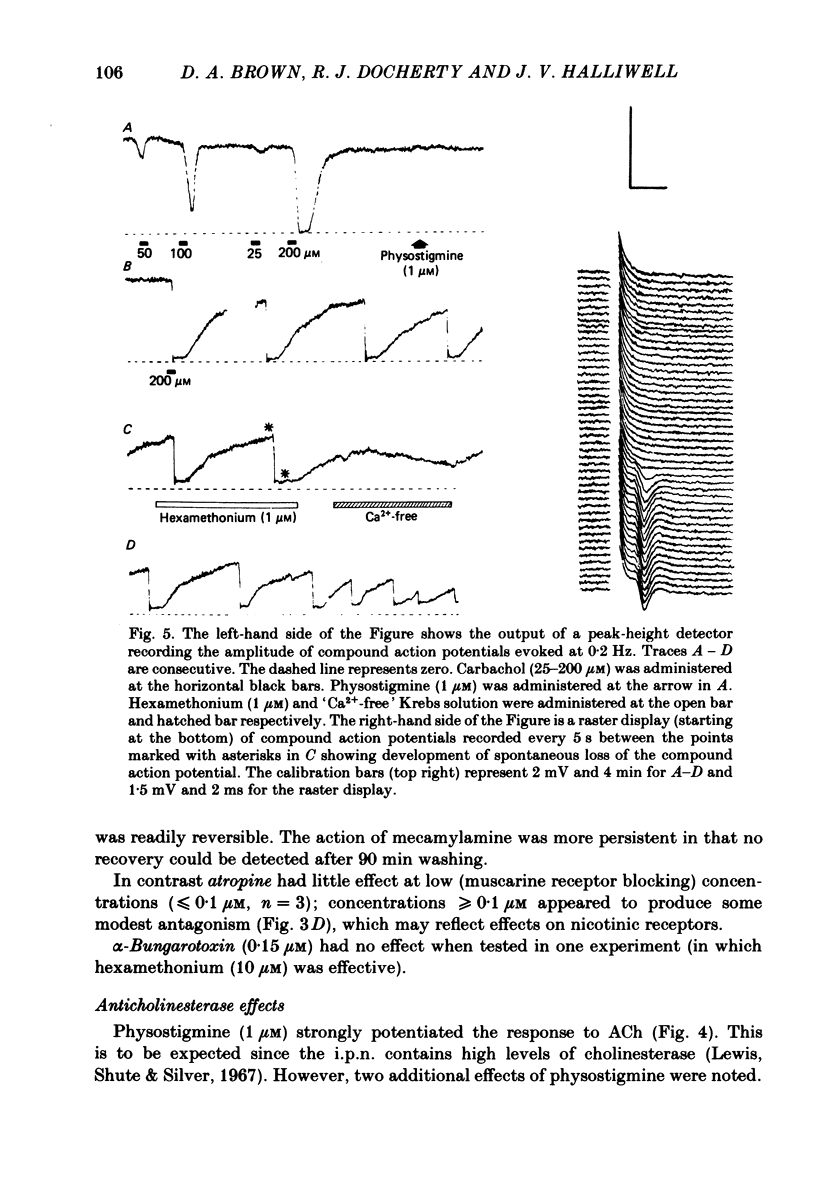
The effects of some cholinomimetic substances and their antagonists on the peak height of compound action potentials recorded from the terminal region of the habenulointerpeduncular pathway have been studied using a rat brain slice preparation. Carbachol and acetylcholine (ACh) depressed the peak height of the compound action potential and increased the latency to peak. The nicotinic agonists nicotine and dimethylphenylpiperazinium depressed the peak height of the compound action potential while muscarine and glutamate had no effect. The depressant effect of carbachol was blocked by the nicotinic antagonists hexamethonium, mecamylamine and d-tubocurarine but not by atropine. Physostigmine enhanced the effects of ACh and, to a lesser extent, carbachol. In the presence of physostigmine, carbachol or ACh initiated a spontaneous oscillation of the amplitude of the compound action potential which was Ca2+ dependent and was blocked by mecamylamine. It is concluded that depression of the amplitude of the compound action potential is due to activation of presynaptic nicotinic receptors. The results are discussed with reference to possible cholinergic mechanisms in the habenulointerpeduncular pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMETT C. J., RITCHIE J. M. The action of acetylcholine and some related substances on conduction in mammalian non-myelinated nerve fibres. J Physiol. 1961 Feb;155:372–384. doi: 10.1113/jphysiol.1961.sp006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMETT C. J., RITCHIE J. M. The action of acetylcholine on conduction in mammalian non-myelinated fibres and its prevention by an anticholinesterase. J Physiol. 1960 Jun;152:141–158. doi: 10.1113/jphysiol.1960.sp006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Docherty R. J., Halliwell J. V. Chemical transmission in the rat interpeduncular nucleus in vitro. J Physiol. 1983 Aug;341:655–670. doi: 10.1113/jphysiol.1983.sp014831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Marsh S. Axonal GABA-receptors in mammalian peripheral nerve trunks. Brain Res. 1978 Nov 3;156(1):187–191. doi: 10.1016/0006-8993(78)90098-7. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Gray J. A. Some effects of nicotine-like substances and their relation to sensory nerve endings. J Physiol. 1948 Jun 25;107(3):306–317. doi: 10.1113/jphysiol.1948.sp004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtice C. J. A circuit for recording evoked action potential amplitudes [proceedings]. J Physiol. 1977 Jun;268(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. Observations on the excitation by acetylcholine and by pressure of sensory receptors in the cat's carotid sinus. J Physiol. 1955 Dec 29;130(3):513–532. doi: 10.1113/jphysiol.1955.sp005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W. The effect of a ganglion-blocking drug, hexamethonium, on the response of the cat's carotid body to various stimuli. J Physiol. 1952 Nov;118(3):373–383. doi: 10.1113/jphysiol.1952.sp004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. A., Scholfield C. N., Brown D. A. Evoked surface-positive potentials in isolated mammalian olfactory cortex. Brain Res. 1974 Aug 16;76(2):235–245. doi: 10.1016/0006-8993(74)90457-0. [DOI] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C., Silver A. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol. 1967 Jul;191(1):215–224. doi: 10.1113/jphysiol.1967.sp008246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry B. R., Zialkowski S. E., Hansen L. M., Kavanagh J. P., Evoy E. M. Acetylcholine release in interpeduncular nucleus following the stimulation of habenula. Brain Res. 1979 Mar 23;164:334–337. doi: 10.1016/0006-8993(79)90032-5. [DOI] [PubMed] [Google Scholar]



