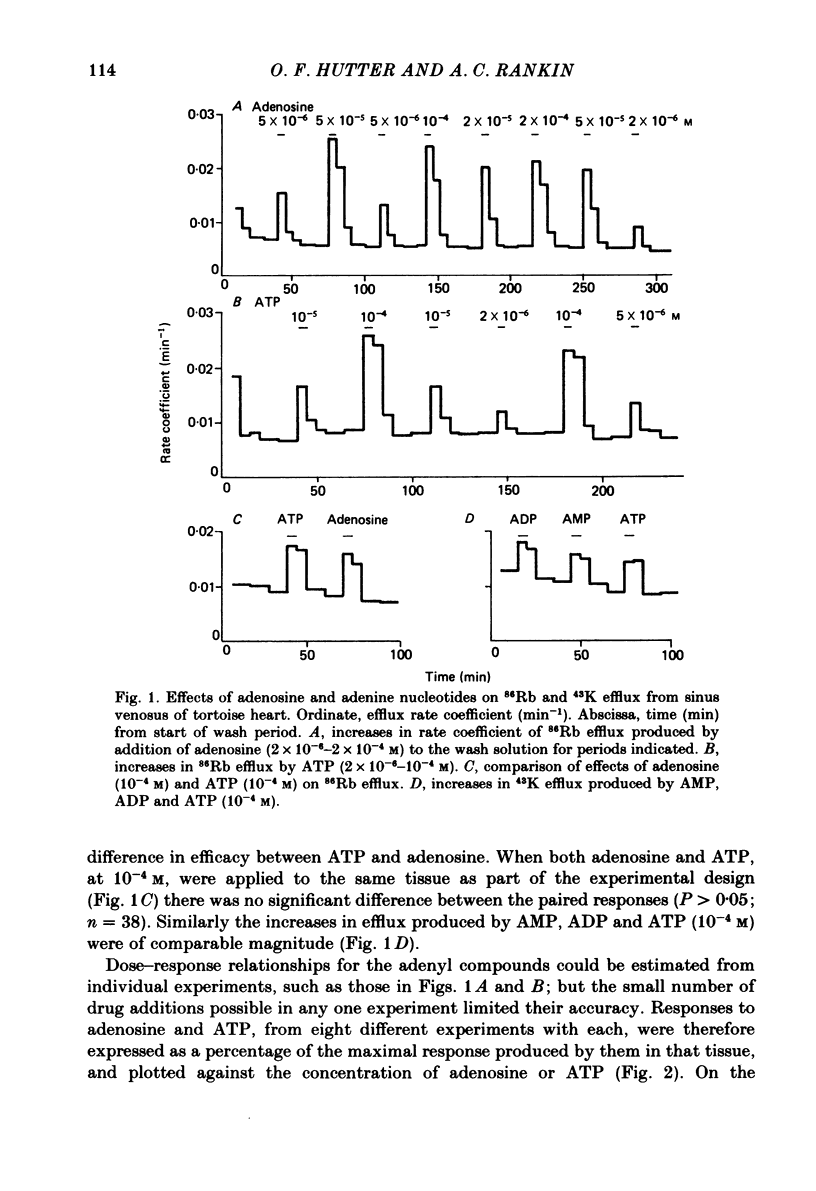
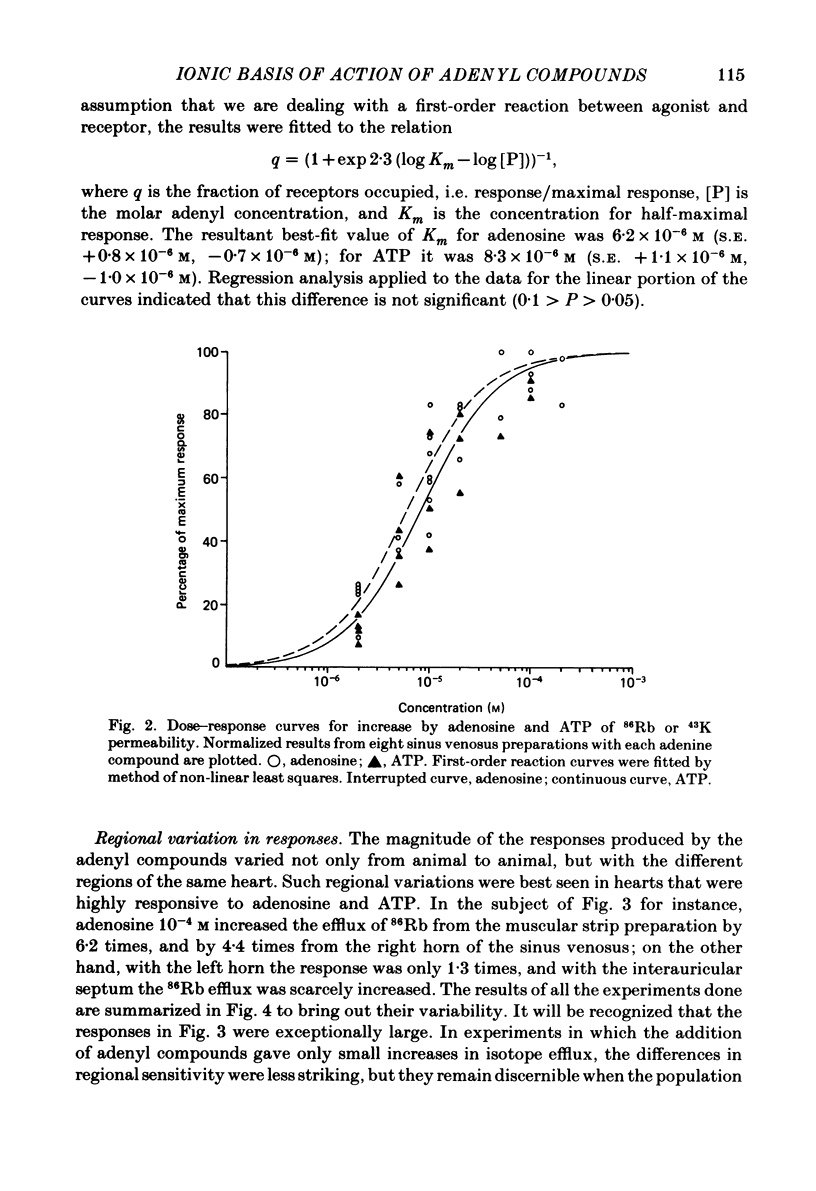
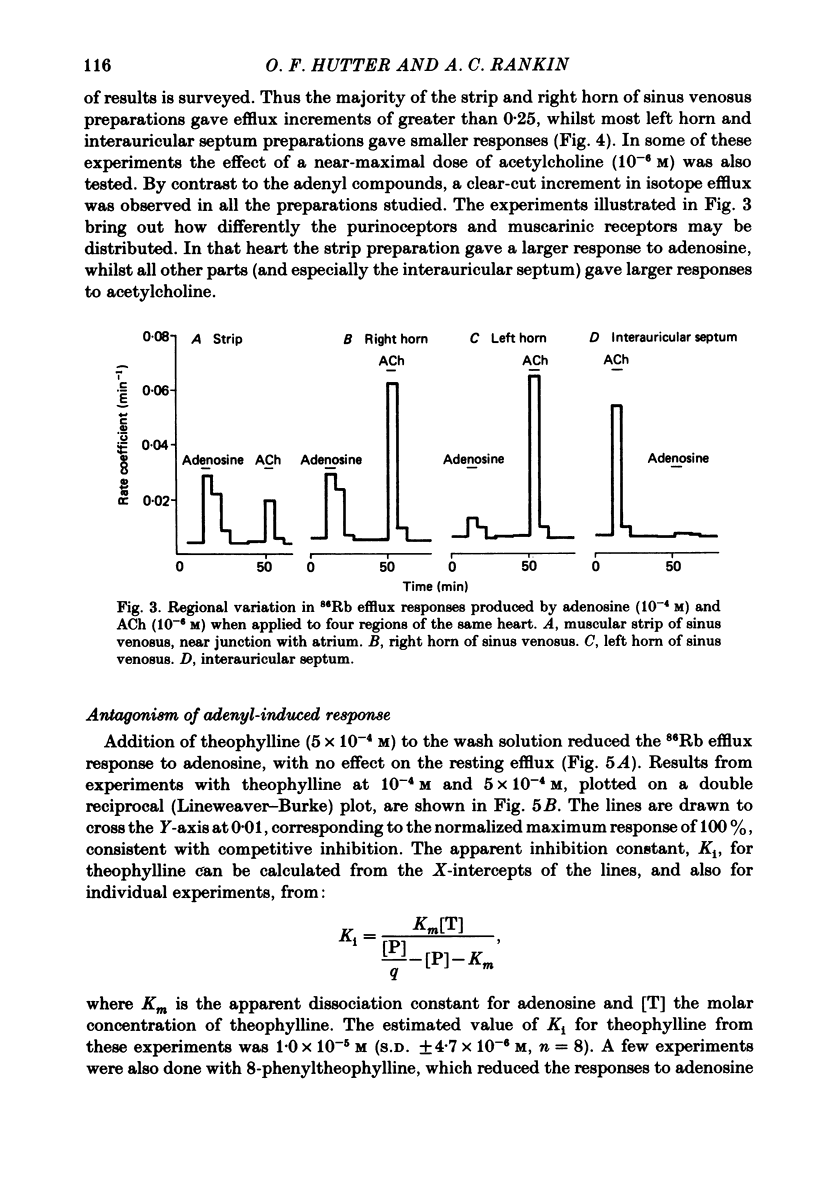
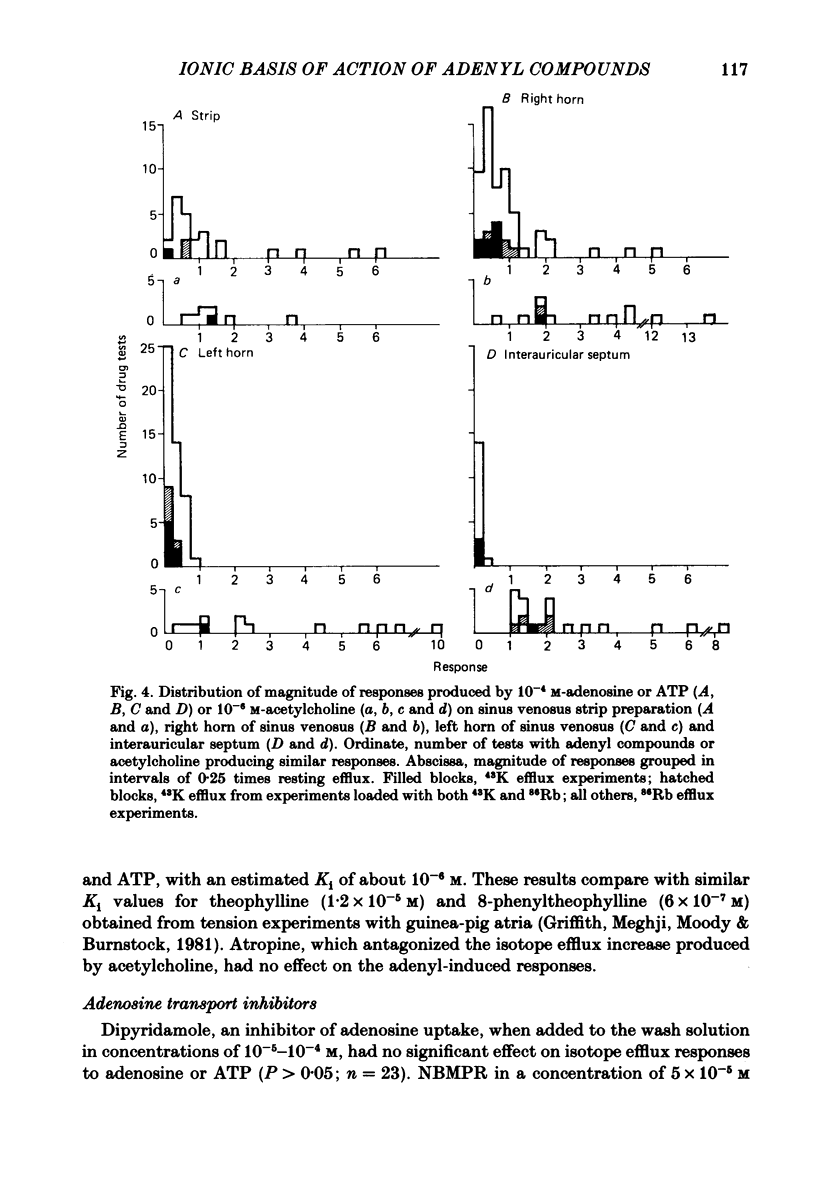
Abstract
The ionic mechanism underlying the hyperpolarizing action of adenosine and adenine nucleotides was studied by measuring the efflux of 43K or 86Rb from sinus venosus and interauricular septum of tortoise heart. Preparations rendered quiescent by high-K (27 mM) Ringer solution were used. Adenosine and ATP increased the efflux of 43K and 86Rb from sinus venosus. The magnitude of the responses varied from preparation to preparation, but in the same tissue adenosine and ATP were of equal efficacy. When dose-response relationships could be determined, the adenyl compounds were found to be of similar potency. Km for adenosine was 6.2 X 10(-6) M, for ATP 8.3 X 10(-6) M. Regional variations in the magnitude of the responses were observed. The largest responses were obtained from the muscular strip of sinus venosus near its junction with atrium, and from the right horn of the sinus venosus. In interauricular septum the adenyl compounds caused only a slight increase in isotope efflux. Acetylcholine, by contrast, produced large increases in 86Rb efflux from all these preparations. Thus the distribution of the purinoceptors in the tortoise heart is more confined than that of the muscarinic receptors. Antagonism of the response to adenyl compounds by theophylline and 8-phenyltheophylline was studied. The apparent Ki for theophylline was 10(-5) M; that for 8-phenyltheophylline about 10(-6) M. Atropine did not inhibit the responses to the adenyl compounds. These results indicate that the changes in K permeability produced by adenosine and ATP are mediated by P1-purinoceptors. The adenosine transport inhibitors, dipyridamole and nitrobenzylthioinosine (NBMPR), had no effect on the adenyl-induced responses, indicating that adenosine uptake is of little importance in tortoise sinus venosus. The effects of phosphate-modified ATP analogues were studied. Adenylimidodiphosphate (APPNP) produced increases in 86Rb efflux similar to those found with ATP, confirming that breakdown of ATP to adenosine is not obligatory for its action at P1-purinoceptors. Alpha-beta methylene ATP (APCPP) and beta-gamma methylene ATP (APPCP) produced much smaller effects, which may be explained by their structural and chemical differences from ATP. The use of 86Rb as a tracer (Rb: K less than 0.01 in load solution) gives qualitatively similar results to those obtained when 43K is used to study the permeability increases produced by the adenyl compounds or acetylcholine. Quantitative differences in the measures obtained with the two isotopes, however, become apparent when the efflux of both is studied simultaneously.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adgey A. A., Geddes J. S., Mulholland H. C., Keegan D. A., Pantridge J. F. Incidence, significance, and management of early bradyarrhythmia complicating acute myocardial infarction. Lancet. 1968 Nov 23;2(7578):1097–1101. doi: 10.1016/s0140-6736(68)91577-8. [DOI] [PubMed] [Google Scholar]
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- BURGEN A. S., TERROUX K. G. On the negative inotropic effect in the cat's auricle. J Physiol. 1953 Jun 29;120(4):449–464. doi: 10.1113/jphysiol.1953.sp004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billette J., Elharrar V., Porlier G., Nadeau R. A. Sinus slowing produced by experimental ischemia of the sinus node in dogs. Am J Cardiol. 1973 Mar;31(3):331–335. doi: 10.1016/0002-9149(73)90264-6. [DOI] [PubMed] [Google Scholar]
- Boyden P. A., Cranefield P. F., Gadsby D. C. Noradrenaline hyperpolarizes cells of the canine coronary sinus by increasing their permeability to potassium ions. J Physiol. 1983 Jun;339:185–206. doi: 10.1113/jphysiol.1983.sp014711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Meghji P. Distribution of P1- and P2-purinoceptors in the guinea-pig and frog heart. Br J Pharmacol. 1981 Aug;73(4):879–885. doi: 10.1111/j.1476-5381.1981.tb08741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis M. G., Pettinger S. J. Can ATP stimulate P1-receptors in guinea-pig atrium without conversion to adenosine? Eur J Pharmacol. 1982 Jul 30;81(4):521–529. doi: 10.1016/0014-2999(82)90341-7. [DOI] [PubMed] [Google Scholar]
- Dauchot P., Gravenstein J. S. Bradycardia after myocardial ischemia and its treatment with atropine. Anesthesiology. 1976 Jun;44(6):501–518. doi: 10.1097/00000542-197606000-00008. [DOI] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C. Beta-adrenoceptor agonists increase membrane K+ conductance in cardiac Purkinje fibres. Nature. 1983 Dec 15;306(5944):691–693. doi: 10.1038/306691a0. [DOI] [PubMed] [Google Scholar]
- Garnier D., Nargeot J., Ojeda C., Rougier O. The action of acetylcholine on background conductance in frog atrial trabeculae. J Physiol. 1978 Jan;274:381–396. doi: 10.1113/jphysiol.1978.sp012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco R., Musto B., Arienzo V., Alborino A., Garofalo S., Marsico F. Treatment of paroxysmal supraventricular tachycardia in infancy with digitalis, adenosine-5'-triphosphate, and verapamil: a comparative study. Circulation. 1982 Sep;66(3):504–508. doi: 10.1161/01.cir.66.3.504. [DOI] [PubMed] [Google Scholar]
- Griffith S. G., Meghji P., Moody C. J., Burnstock G. 8-phenyltheophylline: a potent P1-purinoceptor antagonist. Eur J Pharmacol. 1981 Oct 15;75(1):61–64. doi: 10.1016/0014-2999(81)90346-0. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F. Mode of action of autonomic transmitters on the heart. Br Med Bull. 1957 Sep;13(3):176–180. doi: 10.1093/oxfordjournals.bmb.a069609. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. Rectifying properties of heart muscle. Nature. 1960 Nov 5;188:495–495. doi: 10.1038/188495a0. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., TRAUTWEIN W. Vagal and sympathetic effects on the pacemaker fibers in the sinus venosus of the heart. J Gen Physiol. 1956 May 20;39(5):715–733. doi: 10.1085/jgp.39.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C. Adenosine receptors in frog sinus venosus: slow inhibitory potentials produced by adenine compounds and acetylcholine. J Physiol. 1979 Aug;293:23–49. doi: 10.1113/jphysiol.1979.sp012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER P. POTASSIUM AND RUBIDIUM EXCHANGE ACROSS THE SURFACE MEMBRANE OF CARDIAC PURKINJE FIBRES. J Physiol. 1965 Apr;177:453–462. doi: 10.1113/jphysiol.1965.sp007604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C. J., Burnstock G. Evidence for the presence of P1-purinoceptors on cholinergic nerve terminals in the guinea-pig ileum. Eur J Pharmacol. 1982 Jan 8;77(1):1–9. doi: 10.1016/0014-2999(82)90527-1. [DOI] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- SJODIN R. A. Rubidium and cesium fluxes in muscle as related to the membrane potential. J Gen Physiol. 1959 May 20;42(5):983–1003. doi: 10.1085/jgp.42.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentmiklósi A. J., Németh M., Szegi J., Papp J. G., Szekeres L. Effect of adenosine on sinoatrial and ventricular automaticity of the guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1980 Mar;311(2):147–149. doi: 10.1007/BF00510253. [DOI] [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]


