Abstract
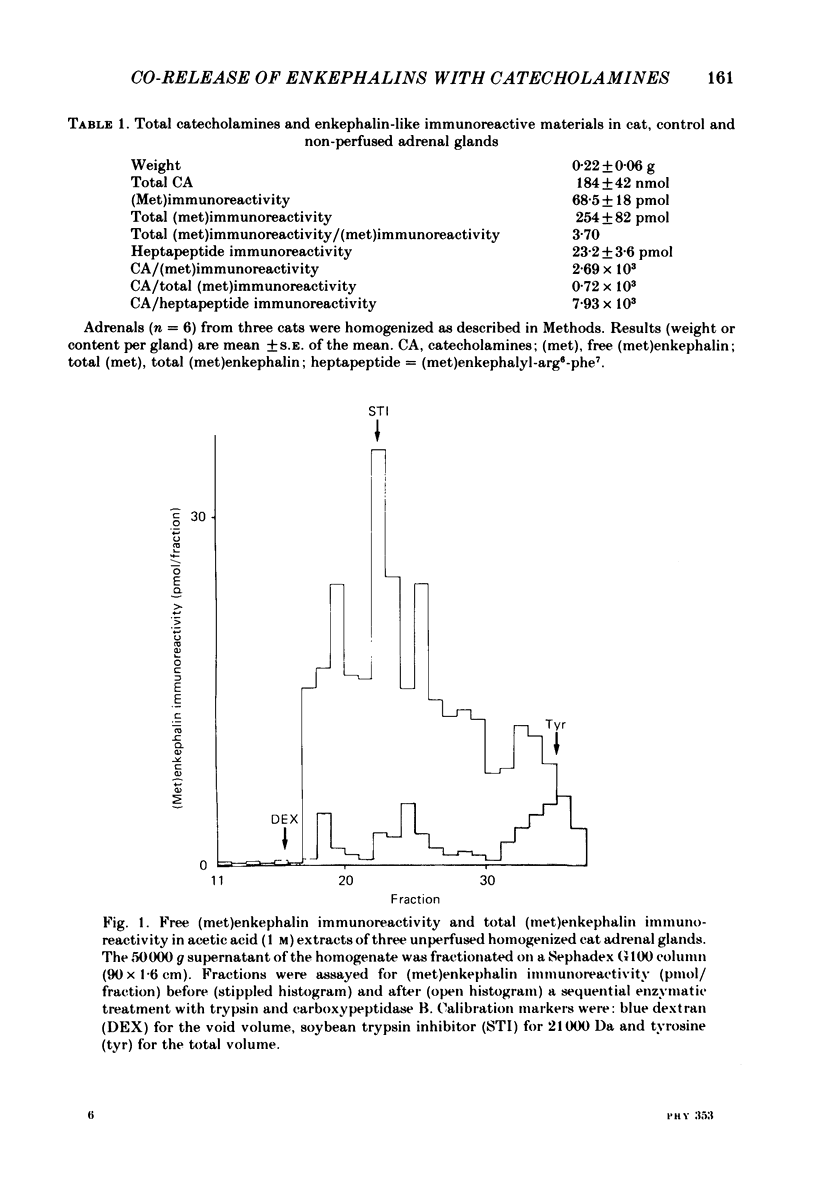
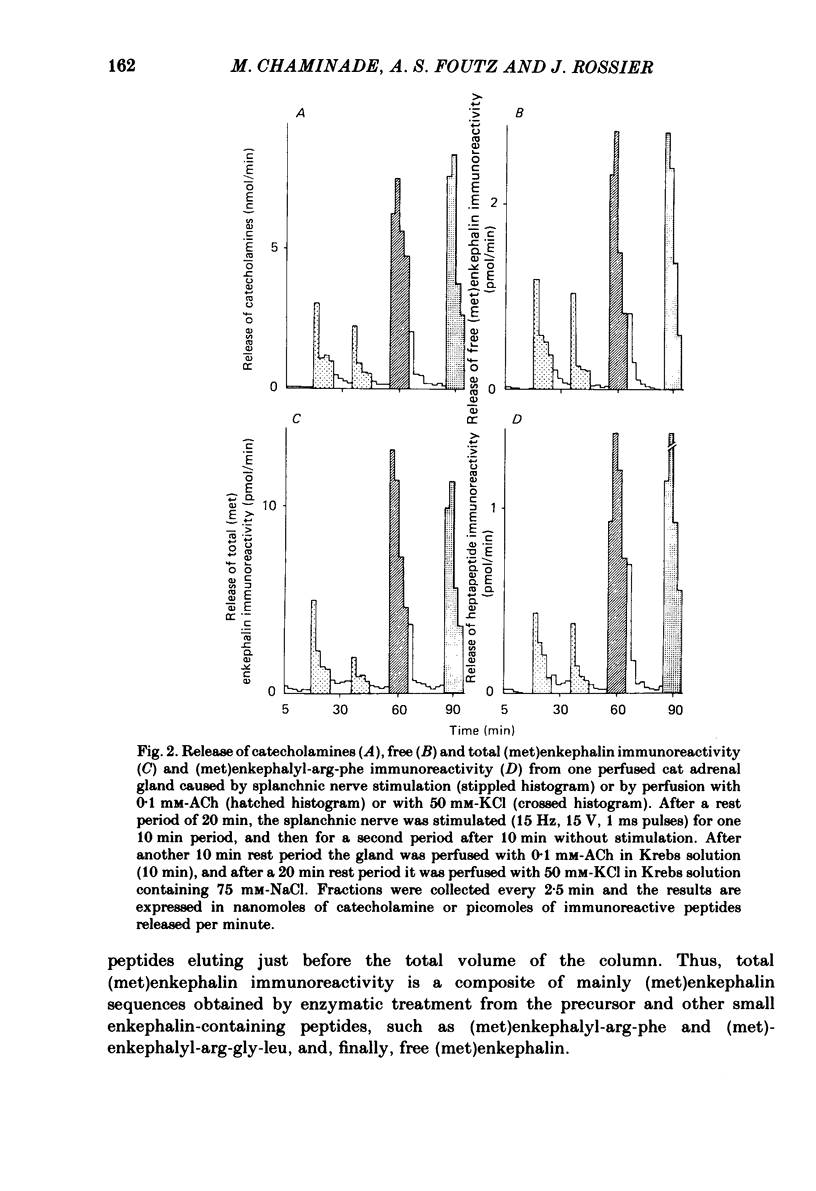
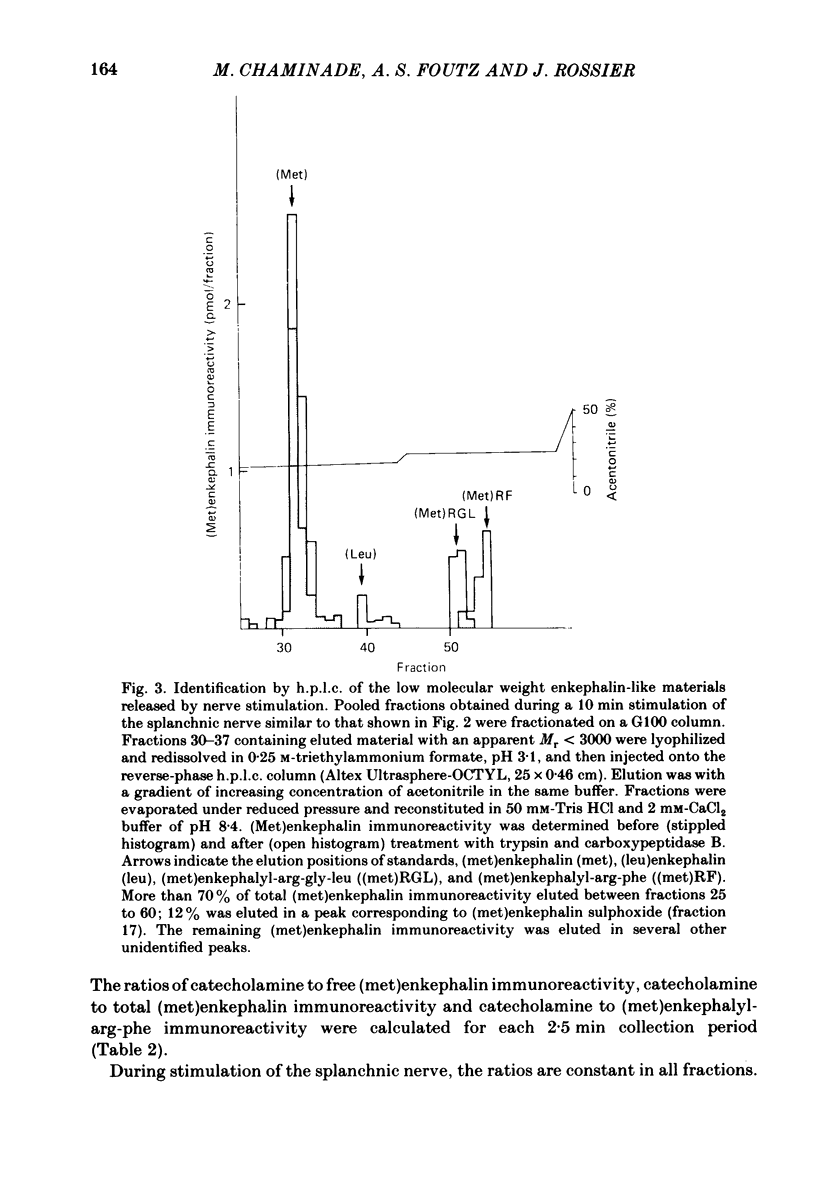
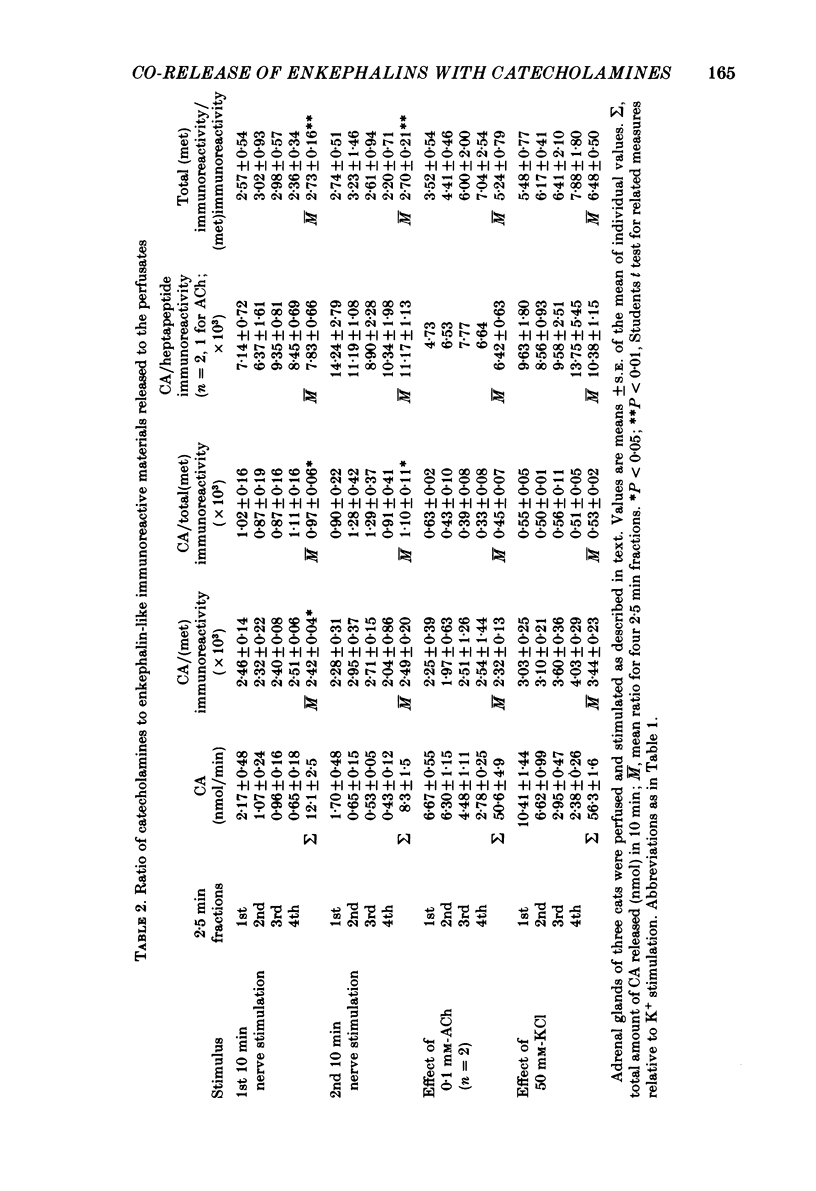
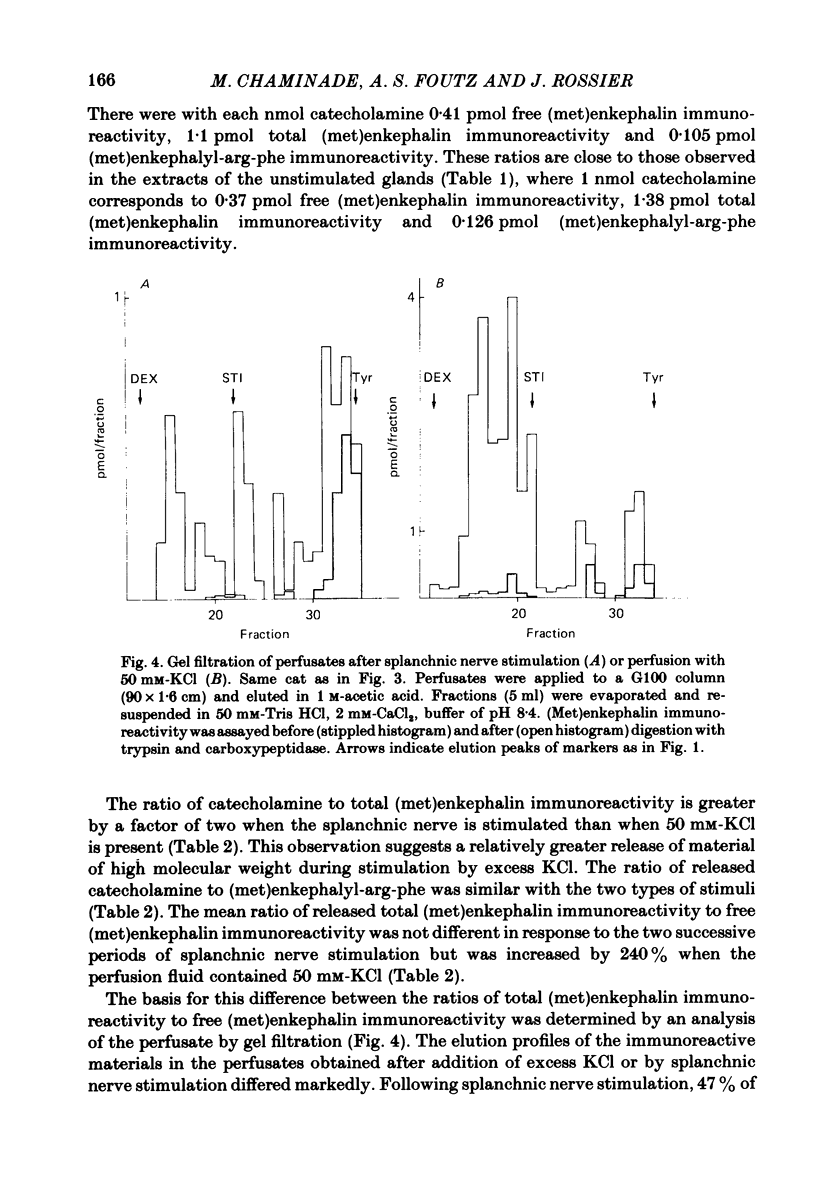
We have compared the nature of the enkephalin-like material derived from proenkephalin present in the intact cat adrenal gland with the material co-released with catecholamines from the perfused adrenal in response to splanchnic nerve stimulation and to perfusions with solutions containing acetylcholine (ACh) or high potassium chloride (KCl). In cat adrenals most of the enkephalin-like material was in the form of large enkephalin-containing peptides. Free (met)enkephalin immunoreactivity represented only 25% of the total (met)enkephalin immunoreactivity as determined by enzymatic digestion of large enkephalin-containing fragments. Electrical stimulation (15 Hz) of the splanchnic nerve or perfusion of the gland with ACh (0.1 mM) or KCl (50 mM), applied for 10 min, induced an immediate release of free (met)enkephalin immunoreactivity, (met)enkephalyl-arg-phe immunoreactivity, and of large (met)enkephalin-containing peptides. The release by all three modes of stimulation followed a pattern that paralleled the output of catecholamines. A rapid fatigue of all secretory processes developed during the stimulation periods, similar to that observed for catecholamines. During splanchnic nerve stimulation, each nanomole of catecholamine output was accompanied by the output of 0.4 pmol free (met)enkephalin immunoreactivity, of 1.1 pmol total (met)enkephalin immunoreactivity and of 0.1 pmol (met)enkephalyl-arg-phe immunoreactivity. Analysis of the perfusate by high-pressure liquid chromatography revealed that (met)enkephalin, (met)enkephalyl-arg-phe and (met)enkephalyl-arg-gly-leu were released in molar ratios of 4 to 1 to 1 which is similar to the ratio found in the precursor, proenkephalin. The ratio of total (met)enkephalin immunoreactivity to free (met)enkephalin immunoreactivity in the perfusate was the same (approximately 2.7) during two successive periods of splanchnic nerve stimulation separated by 10 min. When release was evoked by increasing the K+ concentration to 50 mM-KCl, this ratio was increased more than twofold compared with that obtained by electrical stimulation of the splanchnic nerve. Analysis of the perfusate by gel filtration showed that, during splanchnic nerve stimulation, 47% of the total (met)enkephalin immunoreactivity eluted in fractions containing fragments of low molecular weight. When KCl was used as stimulus only 12% of total (met)enkephalin immunoreactivity eluted in these fractions. The results indicate that the nature of the released peptides depends on the type of stimulus used to evoke release.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. M., Young J. G. Applications of liquid chromatographic-fluorometric systems in neurochemistry. Life Sci. 1981 Feb 2;28(5):507–517. doi: 10.1016/0024-3205(81)90144-2. [DOI] [PubMed] [Google Scholar]
- Chaminade M., Foutz A. S., Rossier J. Co-release of enkephalins and precursors with catecholamines by the perfused cat adrenal in-situ. Life Sci. 1983;33 (Suppl 1):21–24. doi: 10.1016/0024-3205(83)90434-4. [DOI] [PubMed] [Google Scholar]
- Corder R., Mason D. F., Perrett D., Lowry P. J., Clement-Jones V., Linton E. A., Besser G. M., Rees L. H. Simultaneous release of neurotensin, somatostatin, enkephalins and catecholamines from perfused cat adrenal glands. Neuropeptides. 1982 Oct;3(1):9–17. doi: 10.1016/0143-4179(82)90061-0. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud P., Castanas E., Patey G., Oliver C., Rossier J. Regional distribution of methionine-enkephalin-Arg6-Phe7 in the rat brain: comparative study with the distribution of other opioid peptides. J Neurochem. 1983 Jul;41(1):154–160. doi: 10.1111/j.1471-4159.1983.tb11827.x. [DOI] [PubMed] [Google Scholar]
- Govoni S., Hanbauer I., Hexum T. D., Yang H. Y., Kelly G. D., Costa E. In vivo characterization of the mechanisms that secrete enkephalin-like peptides stored in dog adrenal medulla. Neuropharmacology. 1981 Jul;20(7):639–645. doi: 10.1016/0028-3908(81)90110-6. [DOI] [PubMed] [Google Scholar]
- Hambrook J. M., Morgan B. A., Rance M. J., Smith C. F. Mode of deactivation of the enkephalins by rat and human plasma and rat brain homogenates. Nature. 1976 Aug 26;262(5571):782–783. doi: 10.1038/262782a0. [DOI] [PubMed] [Google Scholar]
- Hanbauer I., Govoni S., Majane E. A., Yang H. Y., Costa E. In vivo regulation of the release of met-enkephalin-like peptides from dog adrenal medulla. Adv Biochem Psychopharmacol. 1982;33:209–215. [PubMed] [Google Scholar]
- Ikeda Y., Nakao K., Yoshimasa T., Yanaihara N., Numa S., Imura H. Existence of Met-enkephalin-Arg6-Gly7-Leu8 with Met-enkephalin, Leu-enkephalin and Met-enkephalin-Arg6-Phe7 in the brain of guinea pig, rat and golden hamster. Biochem Biophys Res Commun. 1982 Jul 30;107(2):656–662. doi: 10.1016/0006-291x(82)91541-8. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Jones B. N., Kojima K., Udenfriend S. Identification of the octapeptide [Met]enkephalin -Arg6-Gly7-Leu8 in extracts of bovine adrenal medulla. Biochem Biophys Res Commun. 1981 Nov 30;103(2):698–705. doi: 10.1016/0006-291x(81)90506-4. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Lewis R. V., Stein S., Udenfriend S. Release of enkephalins and enkephalin-containing polypeptides from perfused beef adrenal glands. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7473–7475. doi: 10.1073/pnas.77.12.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston D. R., Vanderhaeghen J. J., Rossier J. Presence in brain of synenkephalin, a proenkephalin-immunoreactive protein which does not contain enkephalin. Nature. 1983 Mar 3;302(5903):62–65. doi: 10.1038/302062a0. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Day R., Elde R. P., Howe P. R. Co-storage of enkephalins and adrenaline in the bovine adrenal medulla. Neuroscience. 1982 May;7(5):1323–1332. doi: 10.1016/0306-4522(82)91138-1. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Dean D. M., Whelan L. G., Udenfriend S., Rossier J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature. 1981 Jan 22;289(5795):317–319. doi: 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- Patey G., Cupo A., Giraud P., Chaminade M., Rossier J. The heptapeptide Met-enkephalin-Arg6, Phe7 is released from rat striatum in vitro by high potassium. Neurosci Lett. 1983 Jun 30;37(3):267–271. doi: 10.1016/0304-3940(83)90442-1. [DOI] [PubMed] [Google Scholar]
- Pelto-Huikko M., Salminen T., Hervonen A. Enkephalin-like immunoreactivity is restricted to the adrenaline cells in the hamster adrenal medulla. Histochemistry. 1982;73(4):493–497. doi: 10.1007/BF00493363. [DOI] [PubMed] [Google Scholar]
- Roisin M. P., Artola A., Henry J. P., Rossier J. Enkephalins are associated with adrenergic granules in bovine adrenal medulla. Neuroscience. 1983 Sep;10(1):83–88. doi: 10.1016/0306-4522(83)90082-9. [DOI] [PubMed] [Google Scholar]
- Rossier J., Audigier Y., Ling N., Cros J., Udenfriend S. Met-enkephalin-Arg6-Phe7, present in high amounts in brain of rat, cattle and man, is an opioid agonist. Nature. 1980 Nov 6;288(5786):88–90. doi: 10.1038/288088a0. [DOI] [PubMed] [Google Scholar]
- Rossier J., Dean D. M., Livett B. G., Udenfriend S. Enkephalin congeners and precursors are synthesized and released by primary cultures of adrenal chromaffin cells. Life Sci. 1981 Feb 16;28(7):781–789. doi: 10.1016/0024-3205(81)90161-2. [DOI] [PubMed] [Google Scholar]
- Rossier J., Liston D., Patey G., Chaminade M., Foutz A. S., Cupo A., Giraud P., Roisin M. P., Henry J. P., Verbanck P. The enkephalinergic neuron: implications of a polyenkephalin precursor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):393–404. doi: 10.1101/sqb.1983.048.01.043. [DOI] [PubMed] [Google Scholar]
- Rossier J. Opioid peptides have found their roots. Nature. 1982 Jul 15;298(5871):221–222. doi: 10.1038/298221a0. [DOI] [PubMed] [Google Scholar]
- Stine S. M., Yang H. Y., Costa E. Release of enkephalin-like immunoreactive material from isolated bovine chromaffin cells. Neuropharmacology. 1980 Jul;19(7):683–685. doi: 10.1016/0028-3908(80)90045-3. [DOI] [PubMed] [Google Scholar]