Abstract
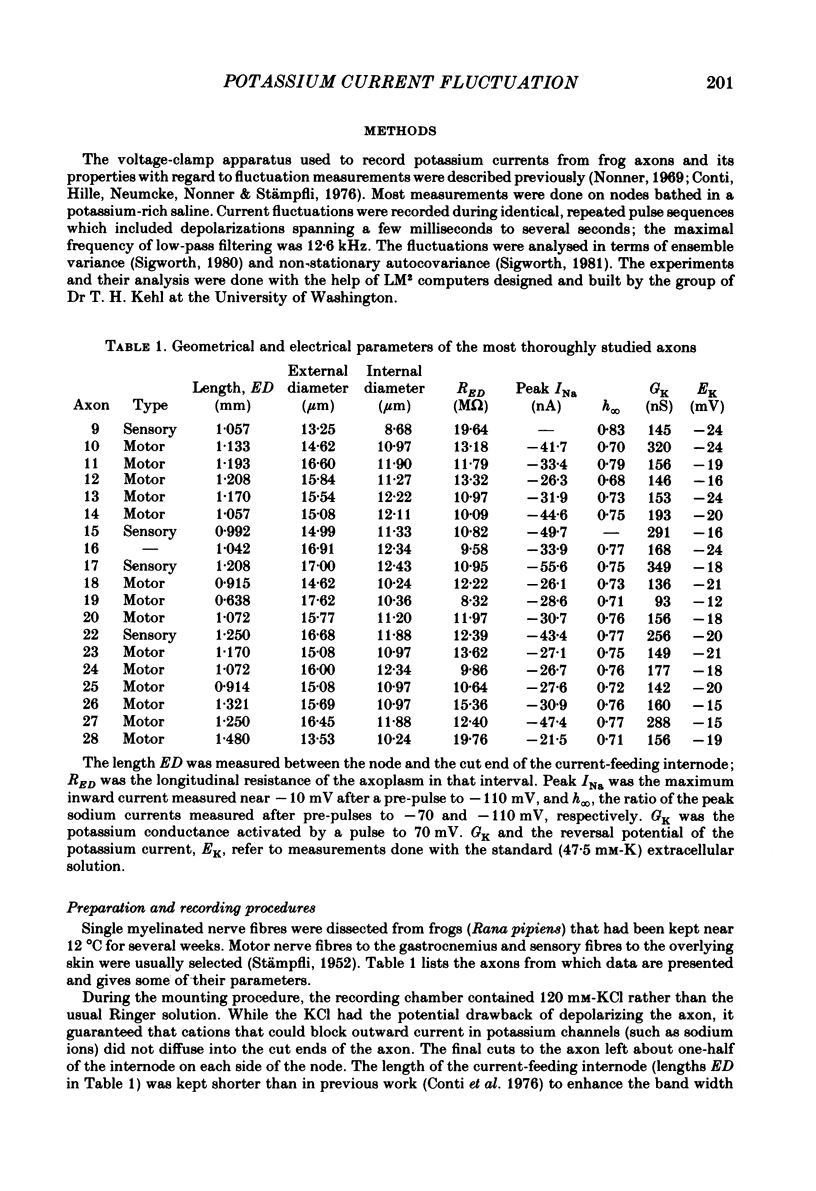
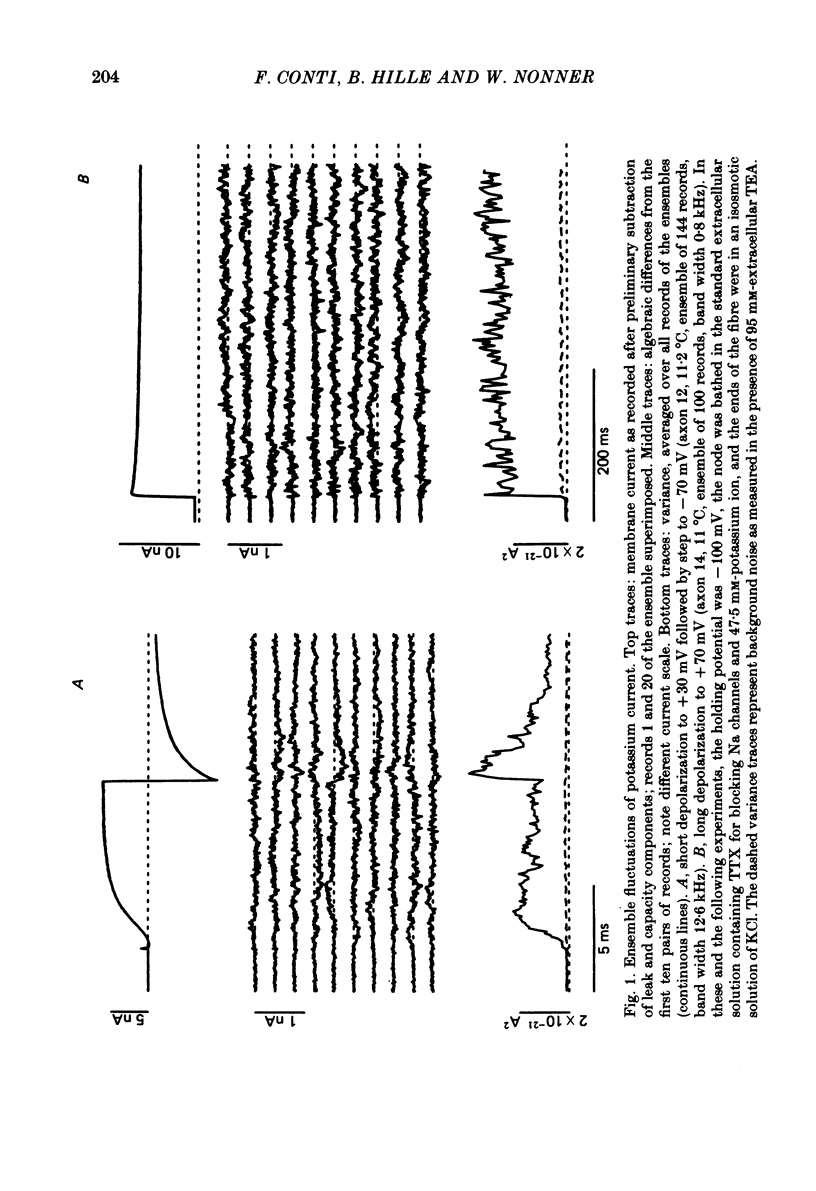
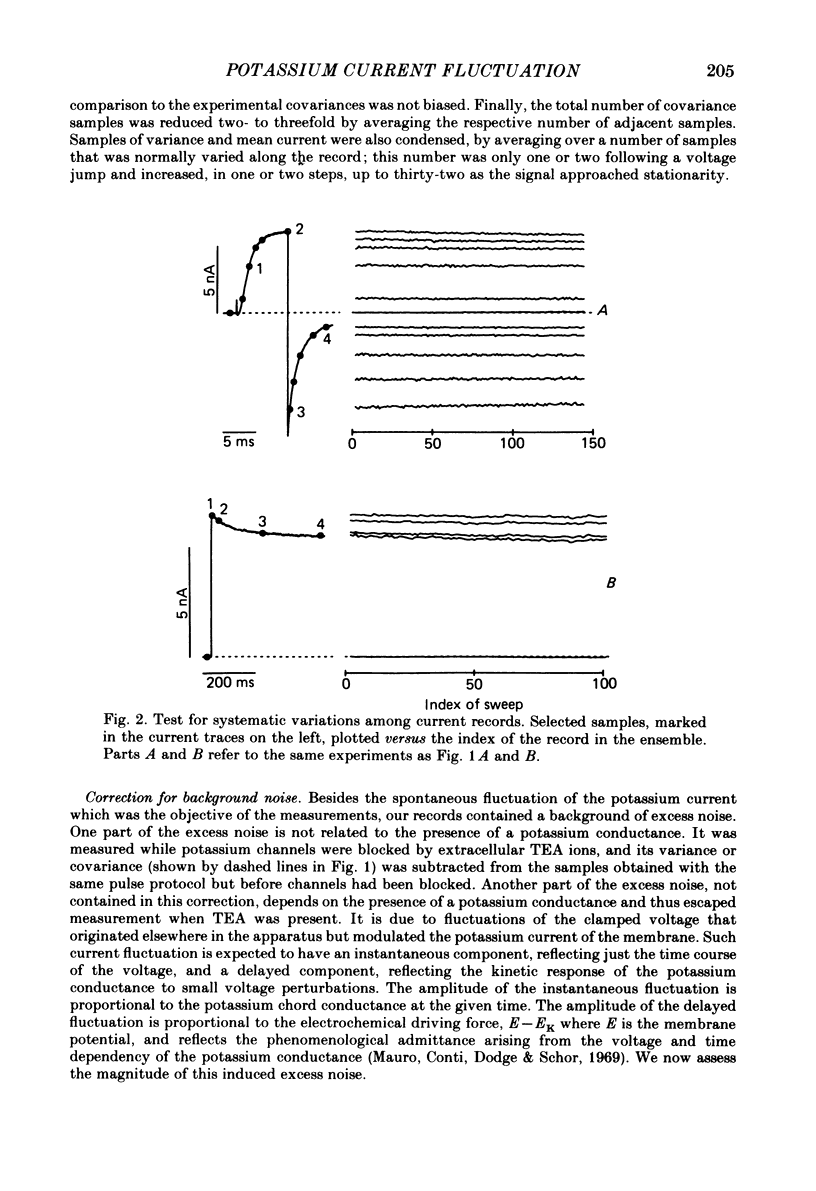
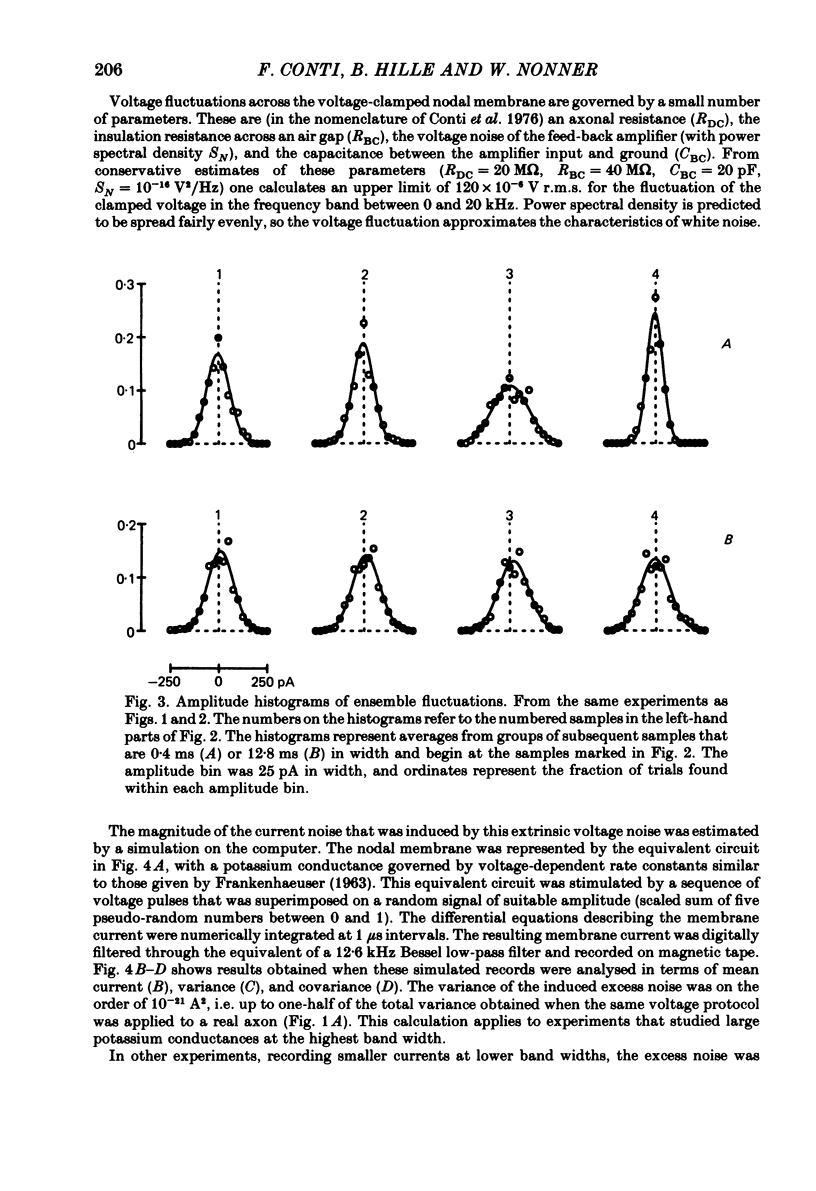
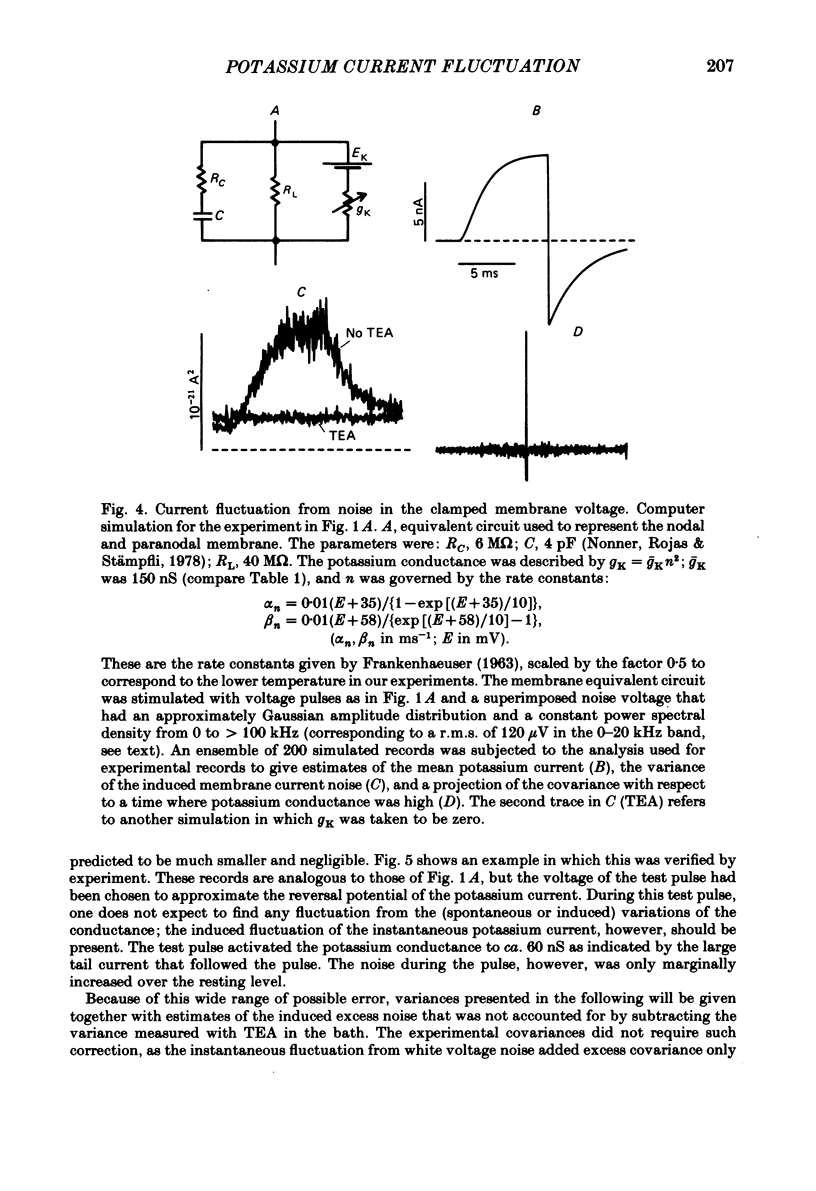
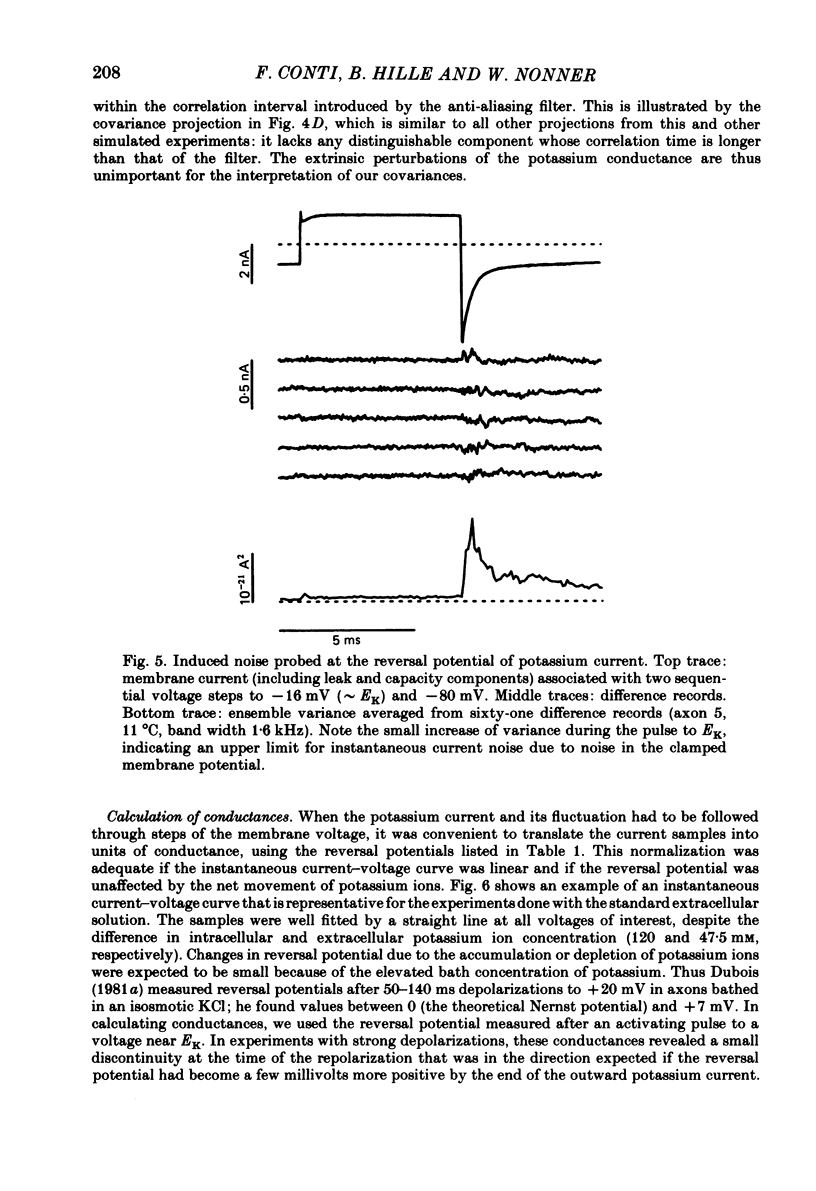
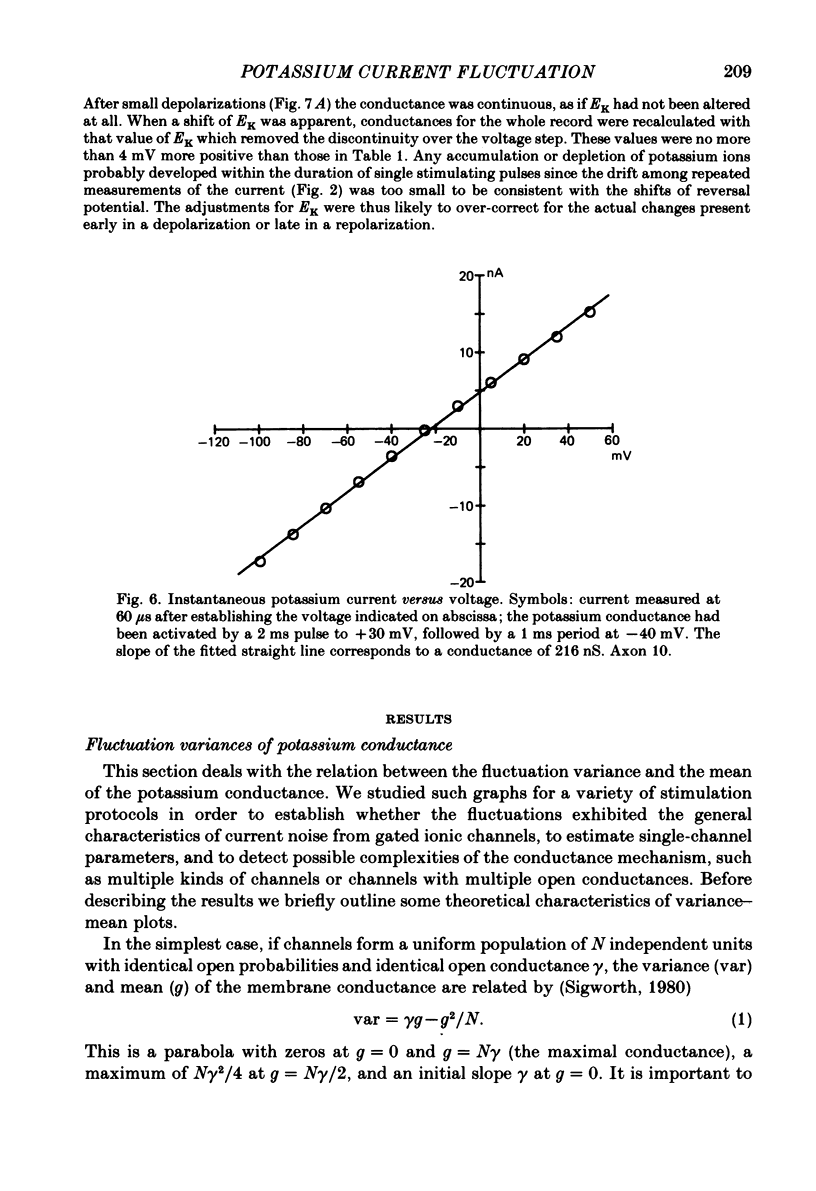
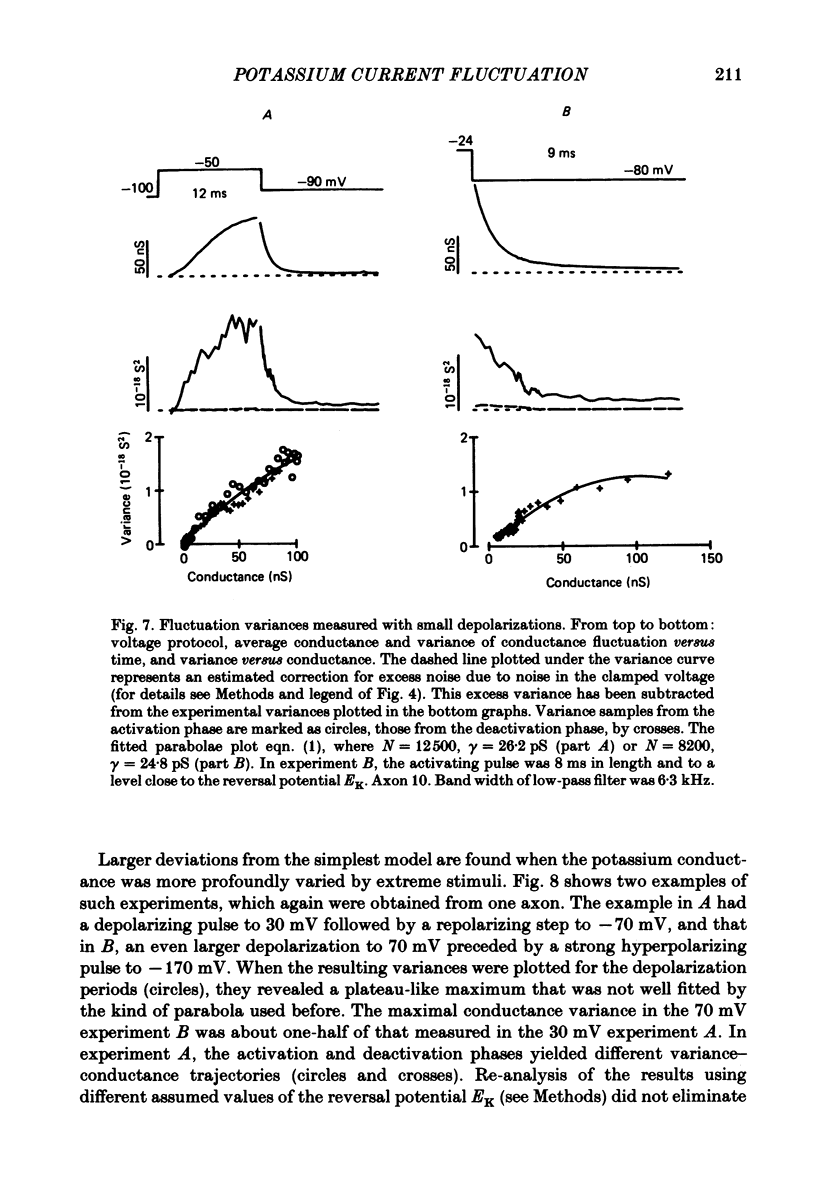
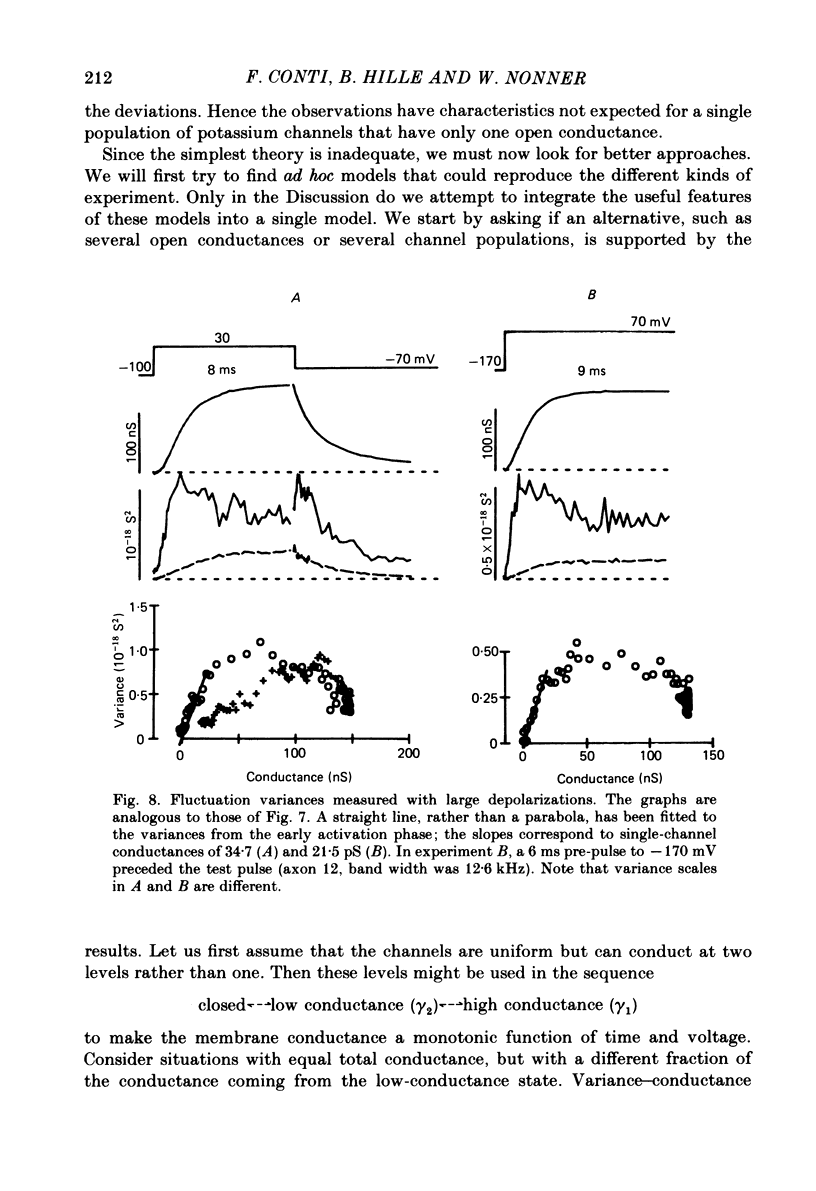
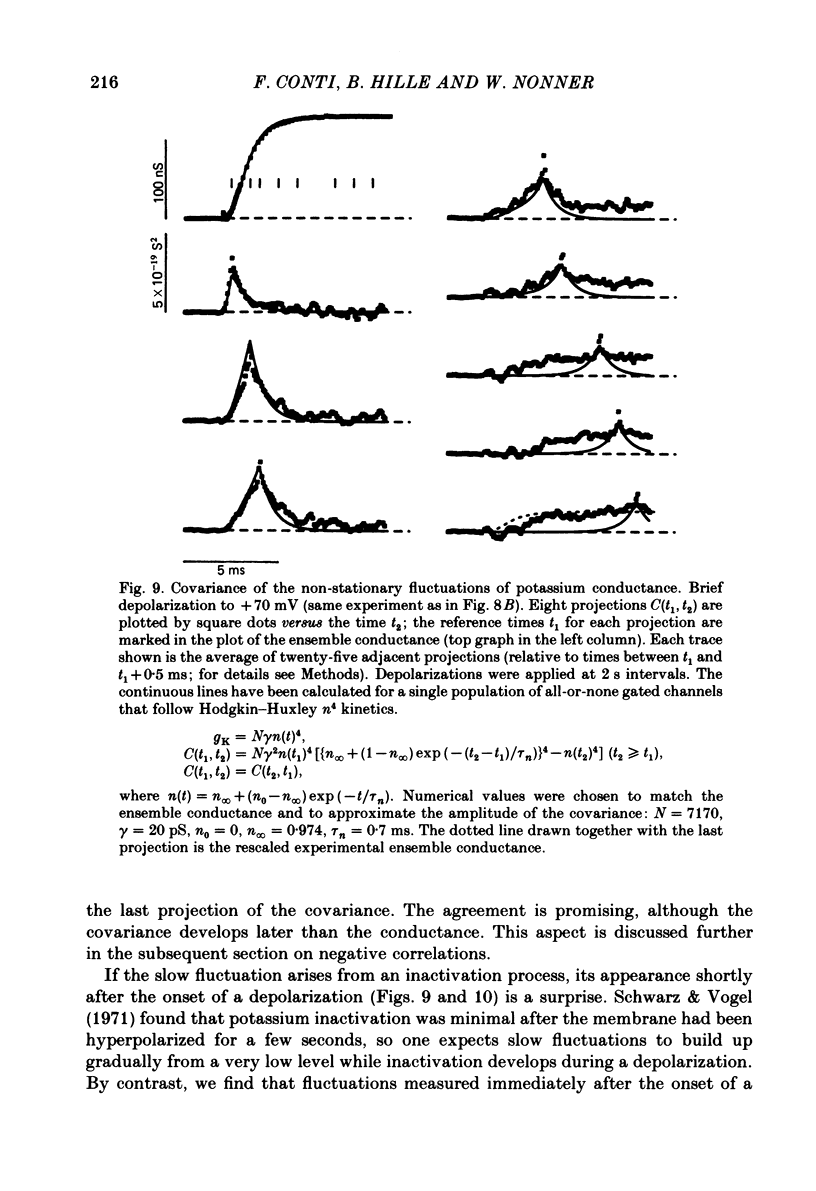
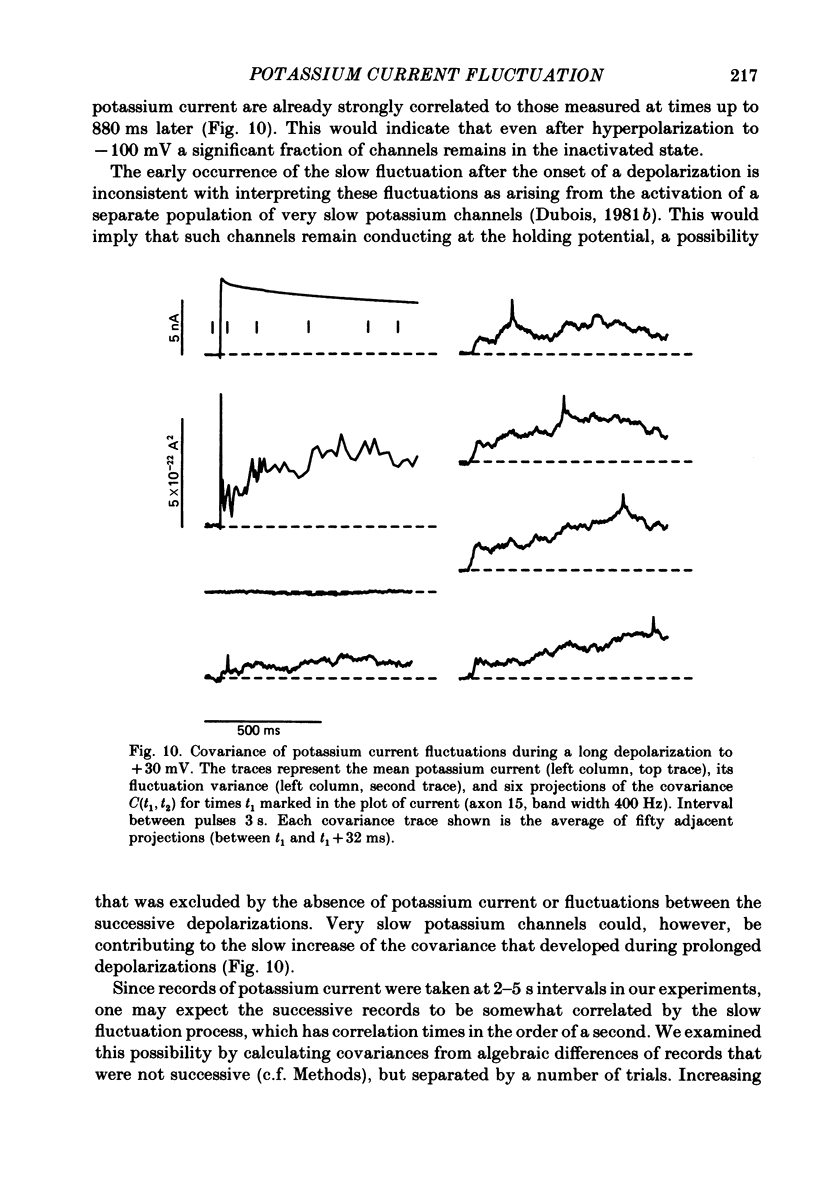
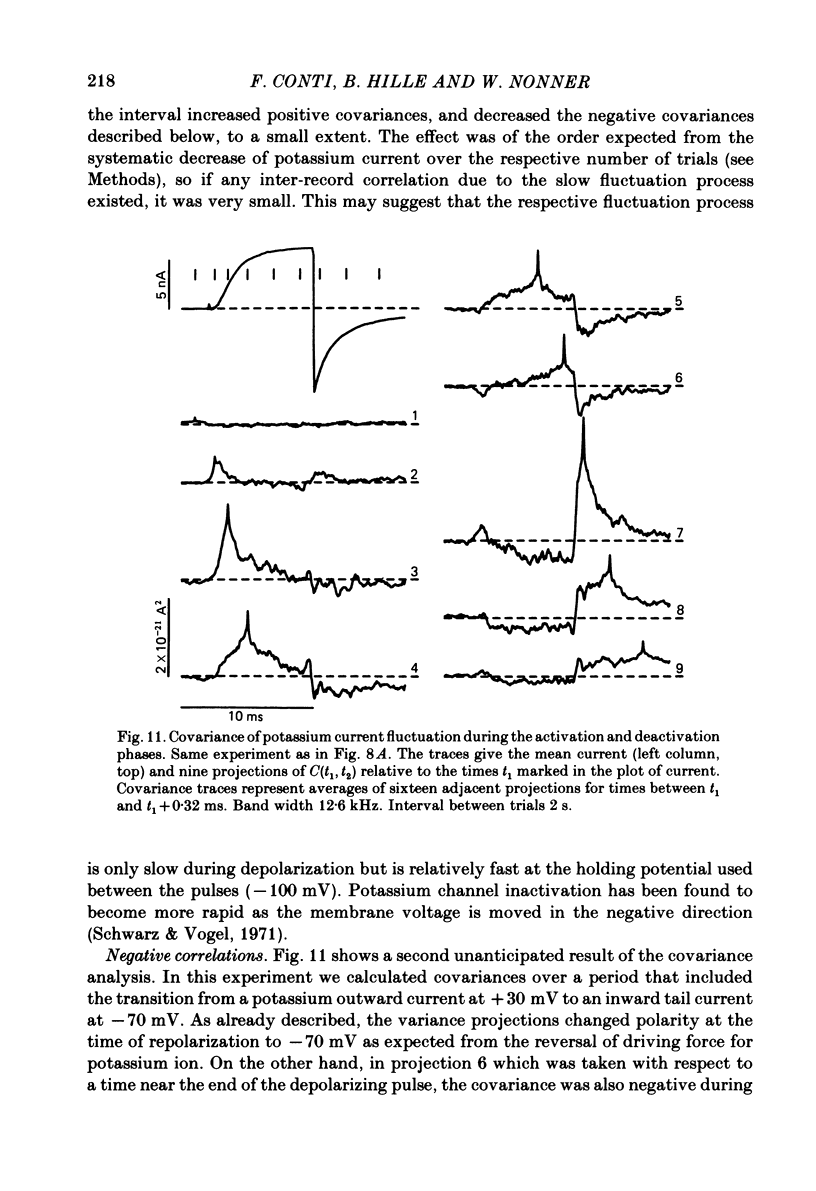
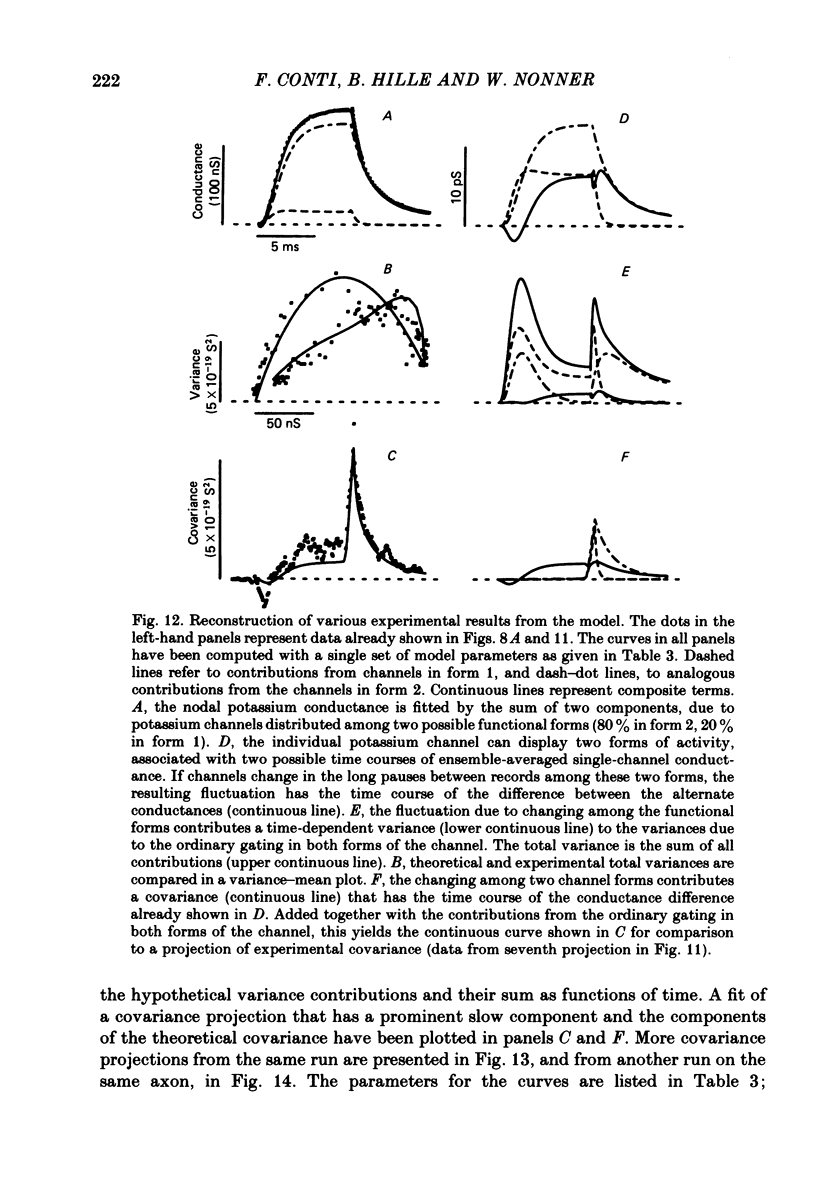
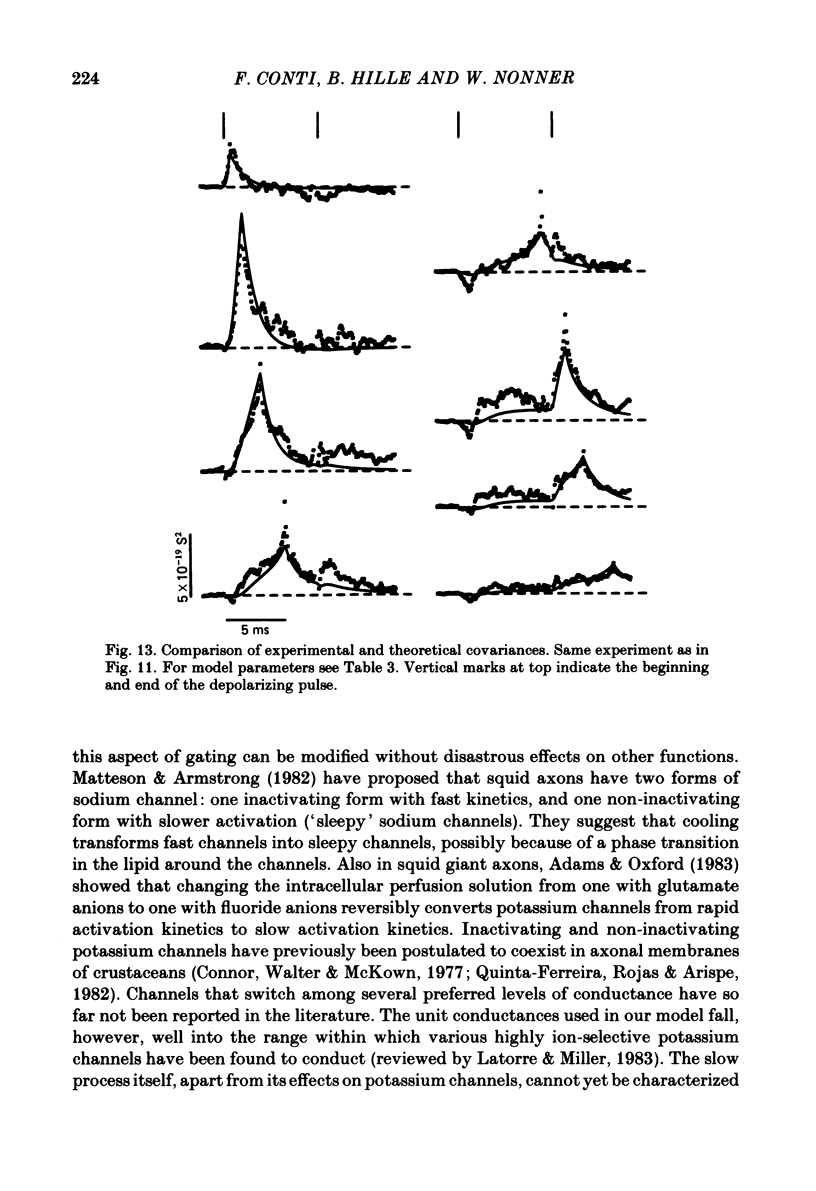
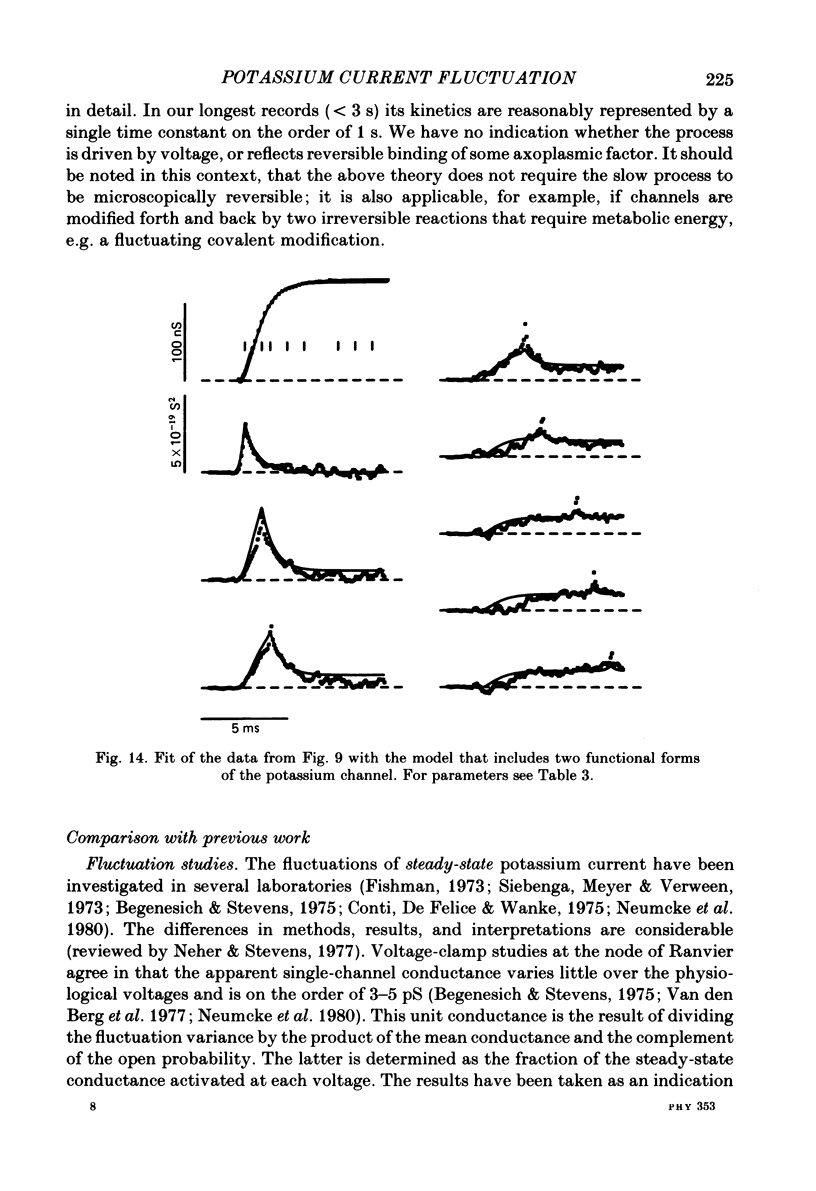
Potassium currents were recorded from voltage-clamped nodes of isolated, myelinated axons of Rana pipiens. Nodes were maintained in a modified Ringer solution containing tetrodotoxin to block sodium current and 47.5 mM-potassium to minimize effects of extracellular potassium accumulation. Voltage protocols included depolarizing pulses lasting a few milliseconds to several seconds. Fluctuations about the ensemble average of the current were characterized in terms of non-stationary variance and autocovariance. The fluctuations had a Gaussian amplitude distribution and were virtually free of contaminations from systematic variations of the membrane current. Corrections for background noise were based on measurements done while potassium current was blocked with tetraethylammonium, and on simulations of extrinsic current fluctuations expected to arise from noise in the actual membrane voltage. The fluctuations were attributed to variations of nodal potassium conductance, since they were absent at the reversal potential of the potassium current and at membrane voltages that do not activate potassium current. Covariances indicated that voltage steps that reversed a macroscopic potassium current also reversed the sign of the fluctuation. Plots of the conductance variance versus the mean potassium conductance were generated from both the activation and deactivation (tail) phases of the potassium currents at various voltages between -80 and +70 mV. When the current was activated by a small depolarization (-50 mV) the trajectories from both phases were indistinguishable and were fitted by the parabola expected for a single population of channels with only one open-channel conductance. Apparent single-channel conductance from the early activation phase averaged 24 pS and was not significantly voltage dependent. In contrast, experiments with large depolarizations (+10 to +70 mV) gave significantly different variance--mean trajectories during activation and deactivation and these trajectories were poorly fitted by parabola. This result indicates that the fluctuations reflect several populations of channels and/or a population of channels that can have several levels of non-zero conductance. Projections of the fluctuation covariance showed long correlations, as well as the rapidly decaying component expected from the activation gating of channels. A slow fluctuation arose at a time slightly later than the rise of potassium current, spanned the entire length of brief depolarizations, and extended up to 880 ms during long depolarizations.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Oxford G. S. Interaction of internal anions with potassium channels of the squid giant axon. J Gen Physiol. 1983 Oct;82(4):429–448. doi: 10.1085/jgp.82.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., Stevens C. F. How many conductance states do potassium channels have? Biophys J. 1975 Aug;15(8):843–846. doi: 10.1016/S0006-3495(75)85858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Walter D., McKown R. Neural repetitive firing: modifications of the Hodgkin-Huxley axon suggested by experimental results from crustacean axons. Biophys J. 1977 Apr;18(1):81–102. doi: 10.1016/S0006-3495(77)85598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., De Felice L. J., Wanke E. Potassium and sodium ion current noise in the membrane of the squid giant axon. J Physiol. 1975 Jun;248(1):45–82. doi: 10.1113/jphysiol.1975.sp010962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Measurement of the conductance of the sodium channel from current fluctuations at the node of Ranvier. J Physiol. 1976 Nov;262(3):699–727. doi: 10.1113/jphysiol.1976.sp011616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980 May 15;285(5761):140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Capsaicin blocks one class of K+ channels in the frog node of Ranvier. Brain Res. 1982 Aug 12;245(2):372–375. doi: 10.1016/0006-8993(82)90820-4. [DOI] [PubMed] [Google Scholar]
- Dubois J. M. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981 Sep;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M. Potassium currents in the frog node of Ranvier. Prog Biophys Mol Biol. 1983;42(1):1–20. doi: 10.1016/0079-6107(83)90002-0. [DOI] [PubMed] [Google Scholar]
- Dubois J. M. Simultaneous changes in the equilibrium potential and potassium conductance in voltage clamped Ranvier node in the frog. J Physiol. 1981 Sep;318:279–295. doi: 10.1113/jphysiol.1981.sp013864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B. A QUANTITATIVE DESCRIPTION OF POTASSIUM CURRENTS IN MYELINATED NERVE FIBRES OF XENOPUS LAEVIS. J Physiol. 1963 Nov;169:424–430. doi: 10.1113/jphysiol.1963.sp007268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M. Relaxation spectra of potassium channel noise from squid axon membranes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):876–879. doi: 10.1073/pnas.70.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyin V. I., Katina I. E., Lonskii A. V., Makovsky V. S., Polishchuk E. V. The Cole-Moore effect in nodal membrane of the frog Rana ridibunda: evidence for fast and slow potassium channels. J Membr Biol. 1980 Dec 30;57(3):179–193. doi: 10.1007/BF01869586. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Evidence for a population of sleepy sodium channels in squid axon at low temperature. J Gen Physiol. 1982 May;79(5):739–758. doi: 10.1085/jgp.79.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A., Conti F., Dodge F., Schor R. Subthreshold behavior and phenomenological impedance of the squid giant axon. J Gen Physiol. 1970 Apr;55(4):497–523. doi: 10.1085/jgp.55.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Inactivation of the sodium permeability in squid giant nerve fibres. Prog Biophys Mol Biol. 1978;33(2):207–230. doi: 10.1016/0079-6107(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Neumcke B. 1/f noise in membranes. Biophys Struct Mech. 1978 Jul 12;4(3):179–199. doi: 10.1007/BF02426084. [DOI] [PubMed] [Google Scholar]
- Neumcke B., Schwarz W., Stämpfli R. Differences between K channels in motor and sensory nerve fibres of the frog as revealed by fluctuation analysis. Pflugers Arch. 1980 Aug;387(1):9–16. doi: 10.1007/BF00580838. [DOI] [PubMed] [Google Scholar]
- Nonner W. A new voltage clamp method for Ranvier nodes. Pflugers Arch. 1969;309(2):176–192. doi: 10.1007/BF00586967. [DOI] [PubMed] [Google Scholar]
- Nonner W., Rojas E., Stämpfli R. Asymmetrical displacement currents in the membrane of frog myelinated nerve: early time course and effects of membrane potential. Pflugers Arch. 1978 Jun 21;375(1):75–85. doi: 10.1007/BF00584151. [DOI] [PubMed] [Google Scholar]
- Palti Y., Ganot G., Stämpfli R. Effect of conditioning potential on potassium current kinetics in the frog node. Biophys J. 1976 Mar;16(3):261–273. doi: 10.1016/S0006-3495(76)85686-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinta-Ferreira M. E., Rojas E., Arispe N. Potassium currents in the giant axon of the crab Carcinus maenas. J Membr Biol. 1982;66(3):171–181. doi: 10.1007/BF01868492. [DOI] [PubMed] [Google Scholar]
- STAMPFLI R. Bau und Funktion isolierter markhaltiger Nervenfasern. Ergeb Physiol. 1952;47:70–165. [PubMed] [Google Scholar]
- Schwarz J. R., Vogel W. Potassium inactivation in single myelinated nerve fibres of Xenopus laevis. Pflugers Arch. 1971;330(1):61–73. doi: 10.1007/BF00588735. [DOI] [PubMed] [Google Scholar]
- Siebenga E., Meyer A. W., Verveen A. A. Membrane shot-noise in electrically depolarized nodes of Ranvier. Pflugers Arch. 1973 Jun 26;341(2):87–96. doi: 10.1007/BF00587315. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. Covariance of nonstationary sodium current fluctuations at the node of Ranvier. Biophys J. 1981 Apr;34(1):111–133. doi: 10.1016/S0006-3495(81)84840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. The variance of sodium current fluctuations at the node of Ranvier. J Physiol. 1980 Oct;307:97–129. doi: 10.1113/jphysiol.1980.sp013426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg R. J., Siebenga E., de Bruin G. Potassium ion noise currents and inactivation in voltage-clamped node of Ranvier. Nature. 1977 Jan 13;265(5590):177–179. doi: 10.1038/265177a0. [DOI] [PubMed] [Google Scholar]


