Abstract
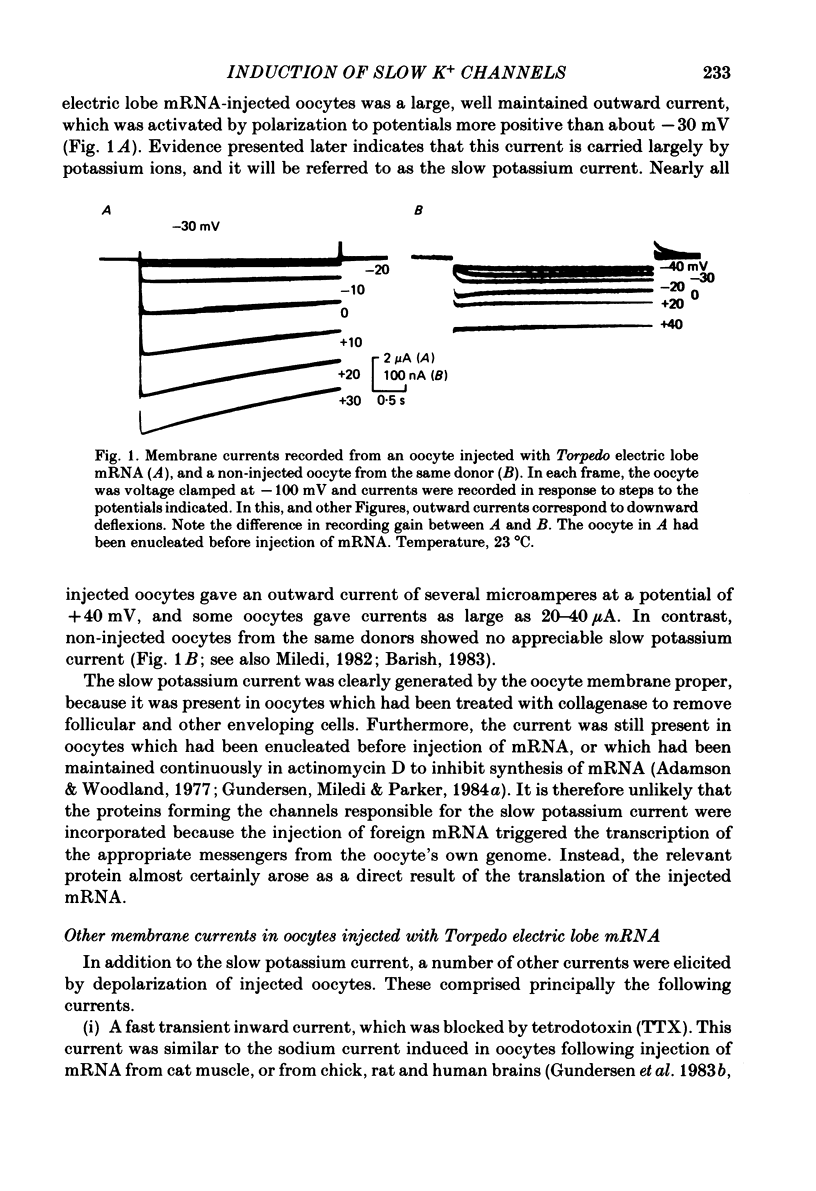
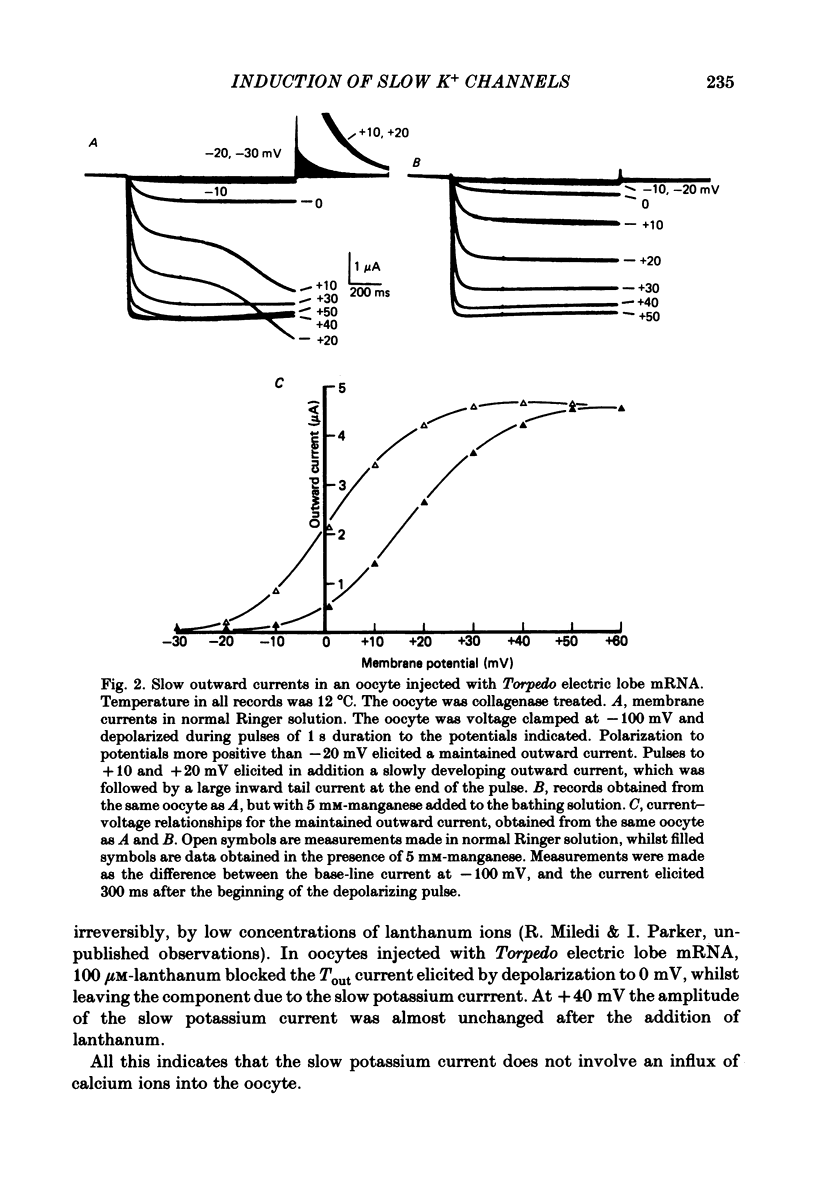
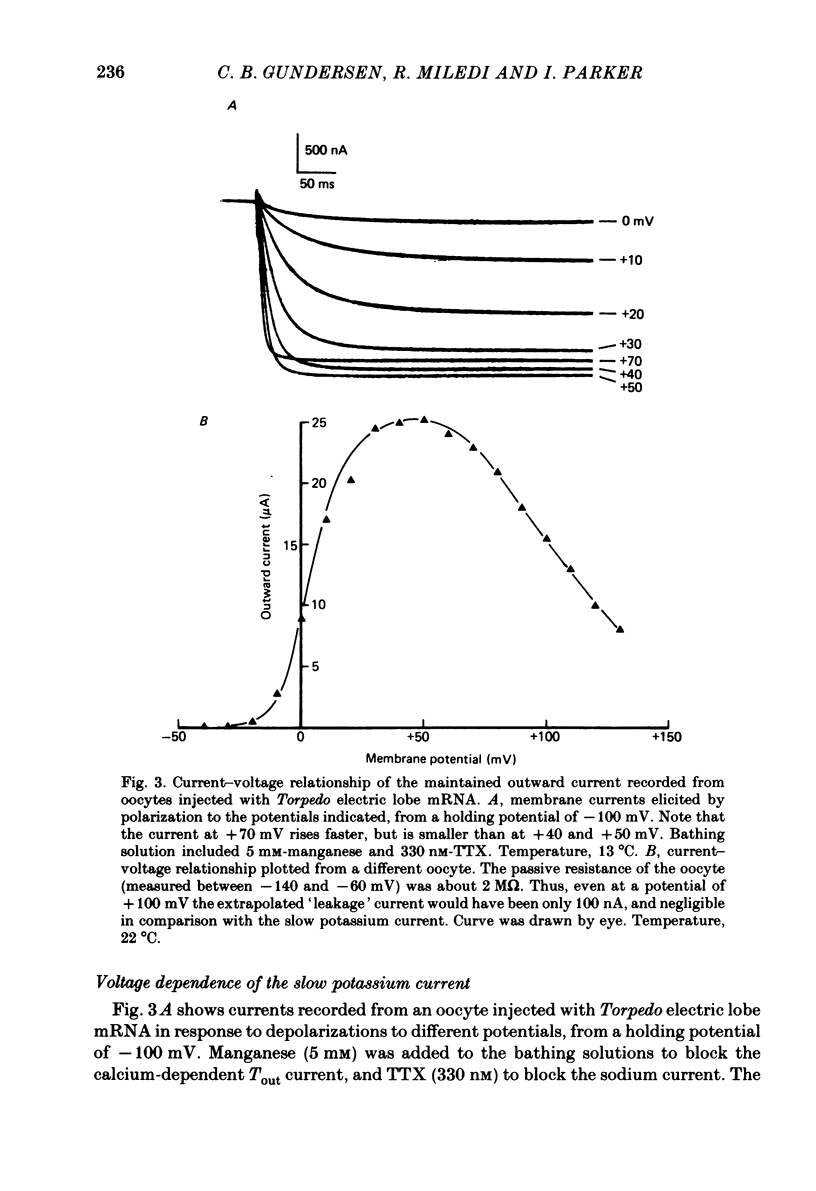
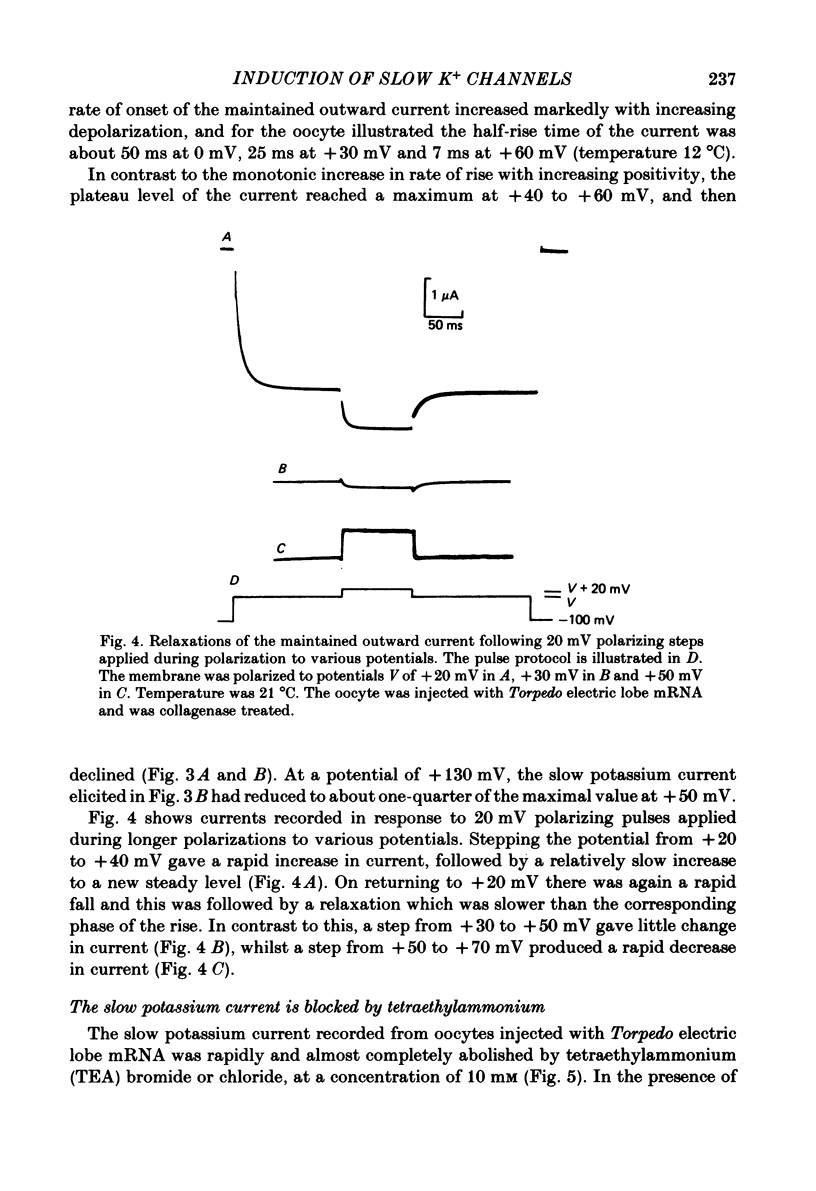
Poly(A+) messenger RNA was extracted from the electric lobe and medulla of Torpedo and injected into oocytes of Xenopus laevis. The synthesis and processing of proteins coded by the injected messenger RNA led to the incorporation of voltage-activated channels in the oocyte membrane. A large, well maintained outward current was recorded from injected oocytes in response to depolarization. Non-injected oocytes did not show this current. The reversal potential of the current varied according to the Nernst equation with external potassium concentration, indicating that it was largely carried by potassium ions. The maintained potassium current was not reduced by manganese (5 mM) or lanthanum ions (0.1 mM). Tetraethylammonium and aminopyridines blocked the potassium current. The block produced by 3,4-diaminopyridine was enhanced by previous activation, but diminished by strong depolarization. The amplitude of the potassium current increased over the approximate voltage range -30 to +50 mV, but reduced at more positive potentials. The decline of the current during maintained depolarization became slower as the membrane potential was made more positive, and the rate of onset of the current became faster. Estimates from noise analysis indicated that the slow potassium current passes through channels with a mean lifetime of about 14 ms and conductance of 14 pS (at -10 mV and room temperature). Injection of the messenger RNA also induced the formation of fast sodium and potassium channels activated by voltage, and channels activated by kainate.
Full text
PDF


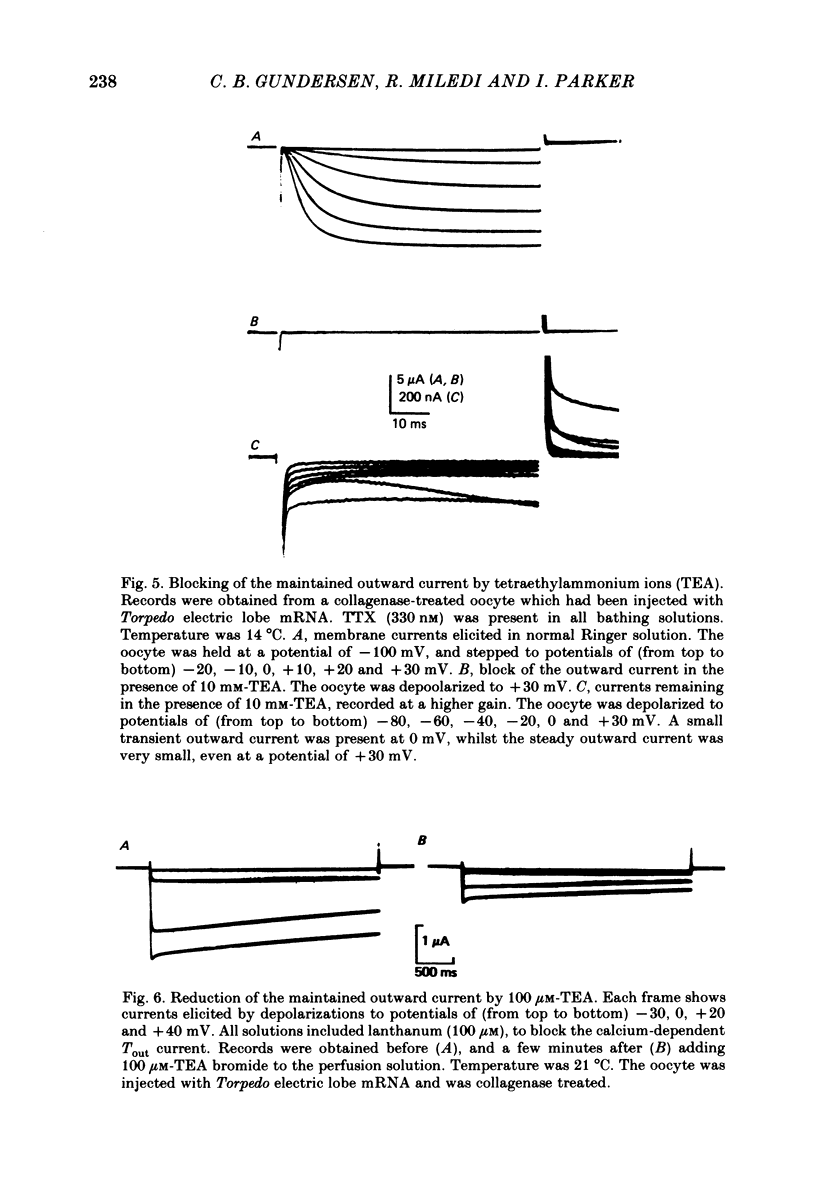
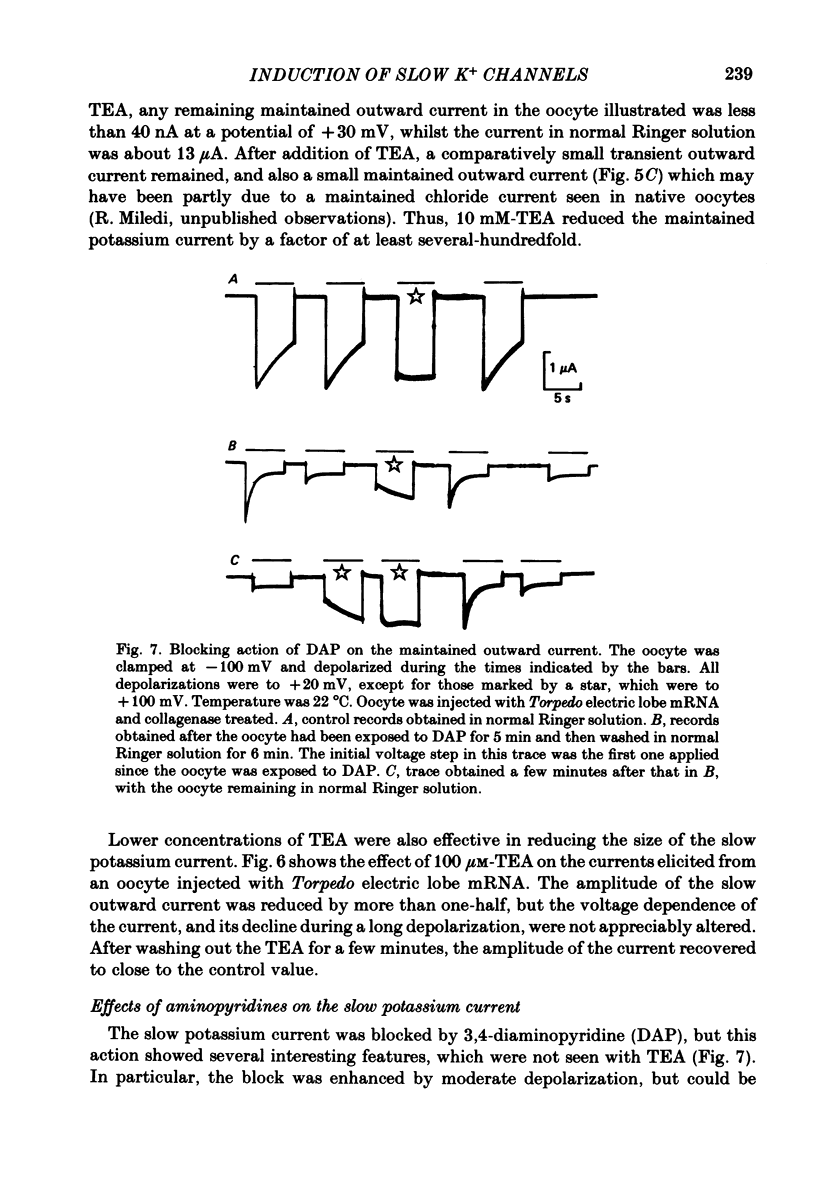

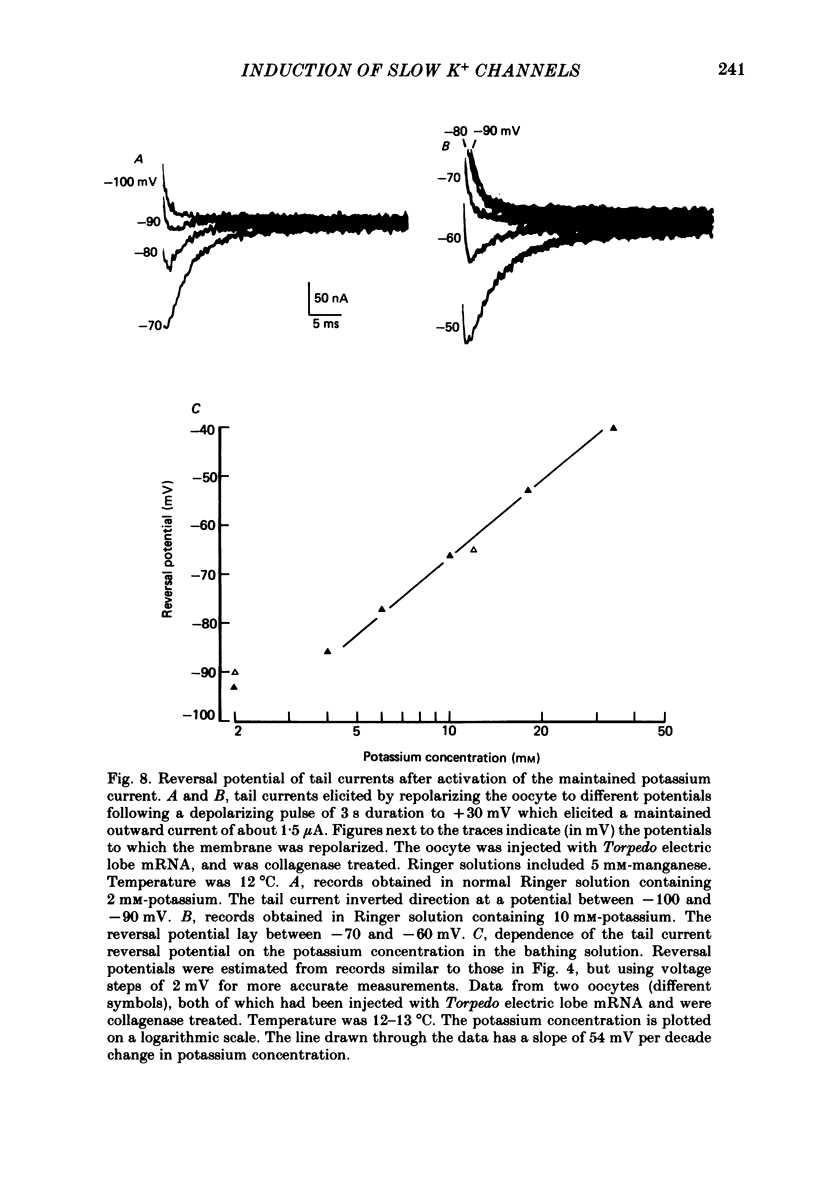
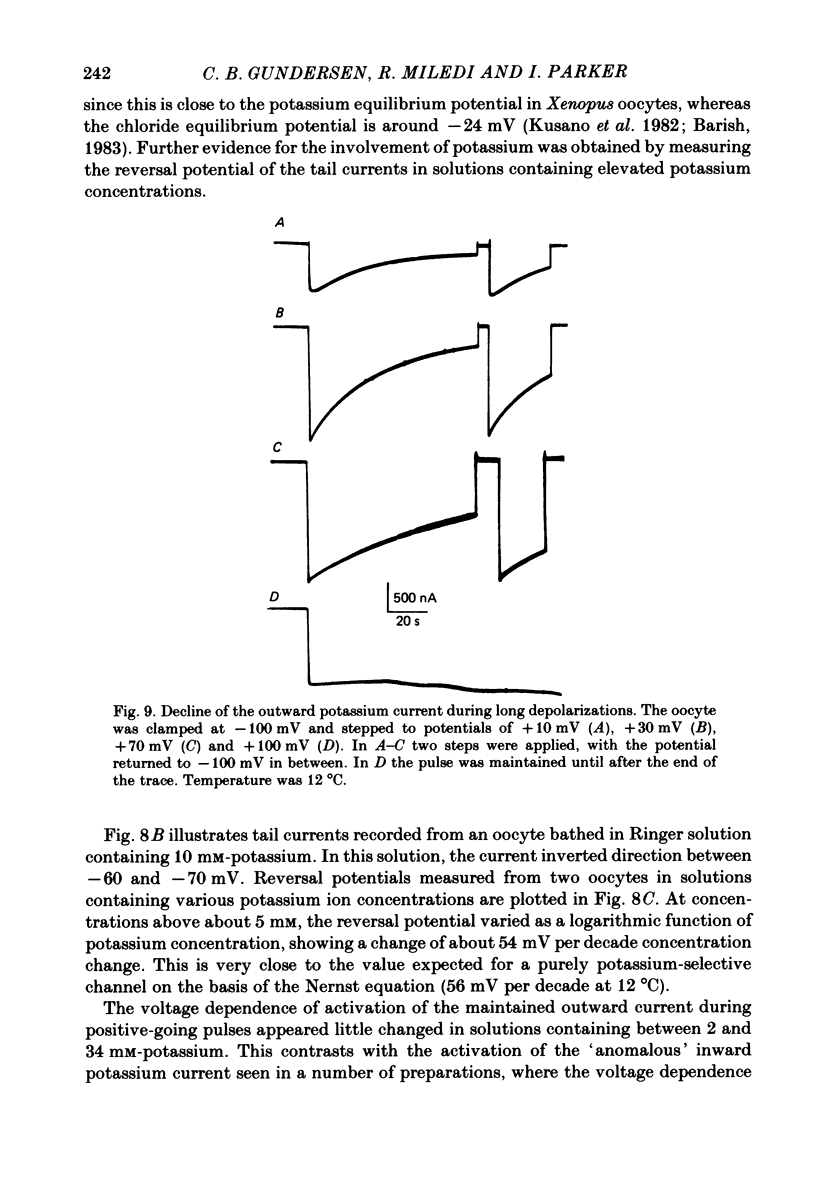





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBE-FESSARD D., BUSER P. Analyse microphysiologique de la transmission réflexe au niveau du lobe électrique de la torpille (torpedo marmorata). J Physiol (Paris) 1954;46(4):932–946. [PubMed] [Google Scholar]
- Adamson E. D., Woodland H. R. Changes in the rate of histone synthesis during oocyte maturation and very early development of Xenopus laevis. Dev Biol. 1977 May;57(1):136–149. doi: 10.1016/0012-1606(77)90360-8. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud C., Kado R. T., Marcher K. Sodium channels induced by depolarization of the Xenopus laevis oocyte. Proc Natl Acad Sci U S A. 1982 May;79(10):3188–3192. doi: 10.1073/pnas.79.10.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V. Comparative physiology: electric organs. Annu Rev Physiol. 1970;32:471–528. doi: 10.1146/annurev.ph.32.030170.002351. [DOI] [PubMed] [Google Scholar]
- Davies L. P., Dowe G. H. Amino acids in the CNS and electromotor system of Torpedo marmorata: association of beta-alanine with cholinergic cells of the electric lobe. Comp Biochem Physiol C. 1979;62C(1):107–113. doi: 10.1016/0306-4492(79)90108-4. [DOI] [PubMed] [Google Scholar]
- Ehrenstein G., Gilbert D. L. Slow changes of potassium permeability in the squid giant axon. Biophys J. 1966 Sep;6(5):553–566. doi: 10.1016/S0006-3495(66)86677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Messenger RNA from human brain induces drug- and voltage-operated channels in Xenopus oocytes. 1984 Mar 29-Apr 4Nature. 308(5958):421–424. doi: 10.1038/308421a0. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Serotonin receptors induced by exogenous messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Aug 22;219(1214):103–109. doi: 10.1098/rspb.1983.0062. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Voltage-operated channels induced by foreign messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Nov 22;220(1218):131–140. doi: 10.1098/rspb.1983.0092. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshida S., Yoshii M. Transient and delayed potassium currents in the egg cell membrane of the coelenterate, Renilla koellikeri. J Physiol. 1981 Sep;318:123–141. doi: 10.1113/jphysiol.1981.sp013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Suppression of transmitter release at the neuromuscular junction. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):465–469. doi: 10.1098/rspb.1977.0051. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech C. A., Stanfield P. R. Inward rectification in frog skeletal muscle fibres and its dependence on membrane potential and external potassium. J Physiol. 1981;319:295–309. doi: 10.1113/jphysiol.1981.sp013909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H., Nilsson O., Rosén I. 4-aminopyridine--a new drug tested in the treatment of Eaton-Lambert syndrome. J Neurol Neurosurg Psychiatry. 1977 Nov;40(11):1109–1112. doi: 10.1136/jnnp.40.11.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundh H., Nilsson O., Rosén I. Effects of 4-aminopyridine in myasthenia gravis. J Neurol Neurosurg Psychiatry. 1979 Feb;42(2):171–175. doi: 10.1136/jnnp.42.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Nakajima S., Parker I. Endplate currents in sucrose solution. Proc R Soc Lond B Biol Sci. 1980 Dec 31;211(1182):135–141. doi: 10.1098/rspb.1980.0161. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Sumikawa K. Synthesis of chick brain GABA receptors by frog oocytes. Proc R Soc Lond B Biol Sci. 1982 Nov 22;216(1205):509–515. doi: 10.1098/rspb.1982.0089. [DOI] [PubMed] [Google Scholar]
- Pappas G. D., Waxman S. G., Bennett M. V. Morphology of spinal electromotor neurons and presynaptic coupling in the gymnotid Sternarchus albifrons. J Neurocytol. 1975 Aug;4(4):469–478. doi: 10.1007/BF01261376. [DOI] [PubMed] [Google Scholar]
- Peres A., Bernardini G. A hyperpolarization-activated chloride current in Xenopus laevis oocytes under voltage-clamp. Pflugers Arch. 1983 Oct;399(2):157–159. doi: 10.1007/BF00663914. [DOI] [PubMed] [Google Scholar]
- SZABO T. Un relais dans le système des connexions du lobe électrique de lar torpille. Arch Anat Microsc Morphol Exp. 1954;43(3):187–201. [PubMed] [Google Scholar]
- Schmid D., Stadler H., Whittaker V. P. The isolation, from electromotor neurone perikarya, of messenger RNAs coding for synaptic proteins. Eur J Biochem. 1982 Mar 1;122(3):633–639. doi: 10.1111/j.1432-1033.1982.tb06486.x. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Passow H. Ca2+-activated K+ channels in erythrocytes and excitable cells. Annu Rev Physiol. 1983;45:359–374. doi: 10.1146/annurev.ph.45.030183.002043. [DOI] [PubMed] [Google Scholar]
- TASAKI I., HAGIWAR A. S. Demonstration of two stable potential states in the squid giant axon under tetraethylammonium chloride. J Gen Physiol. 1957 Jul 20;40(6):859–885. doi: 10.1085/jgp.40.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]


