Abstract
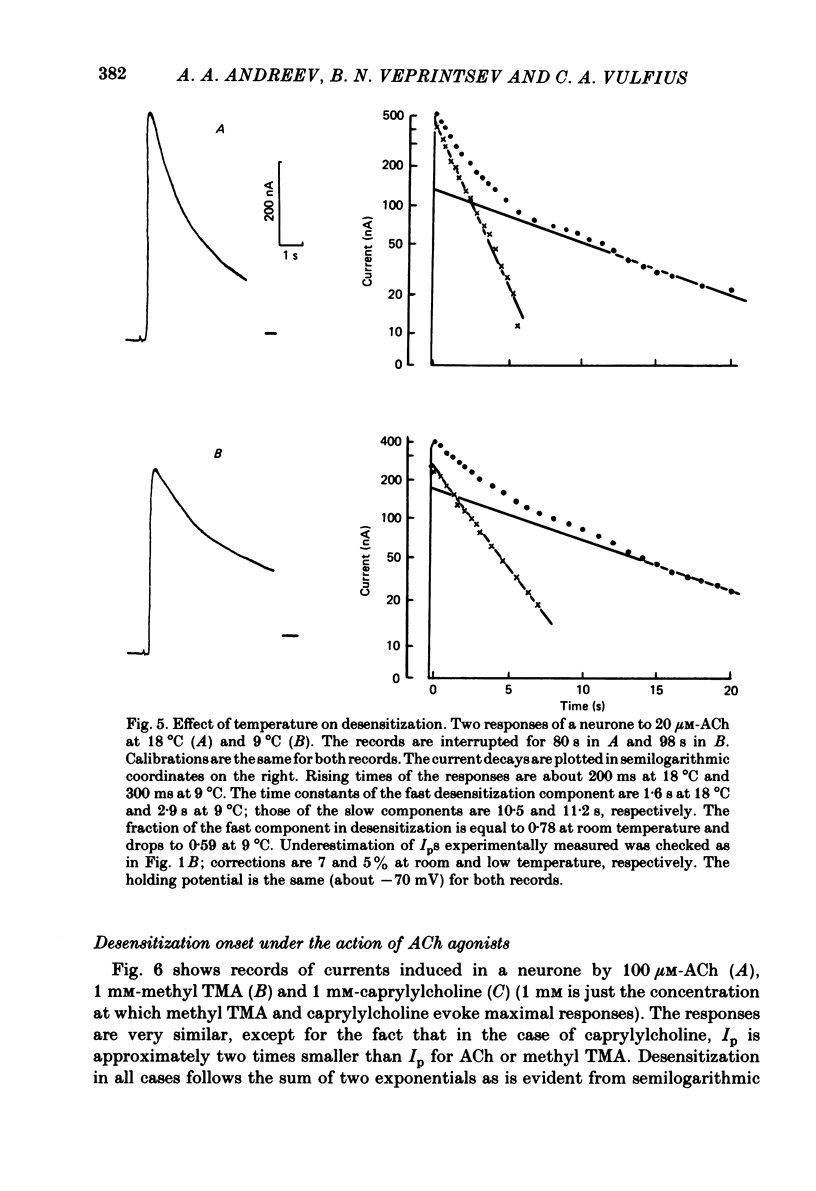
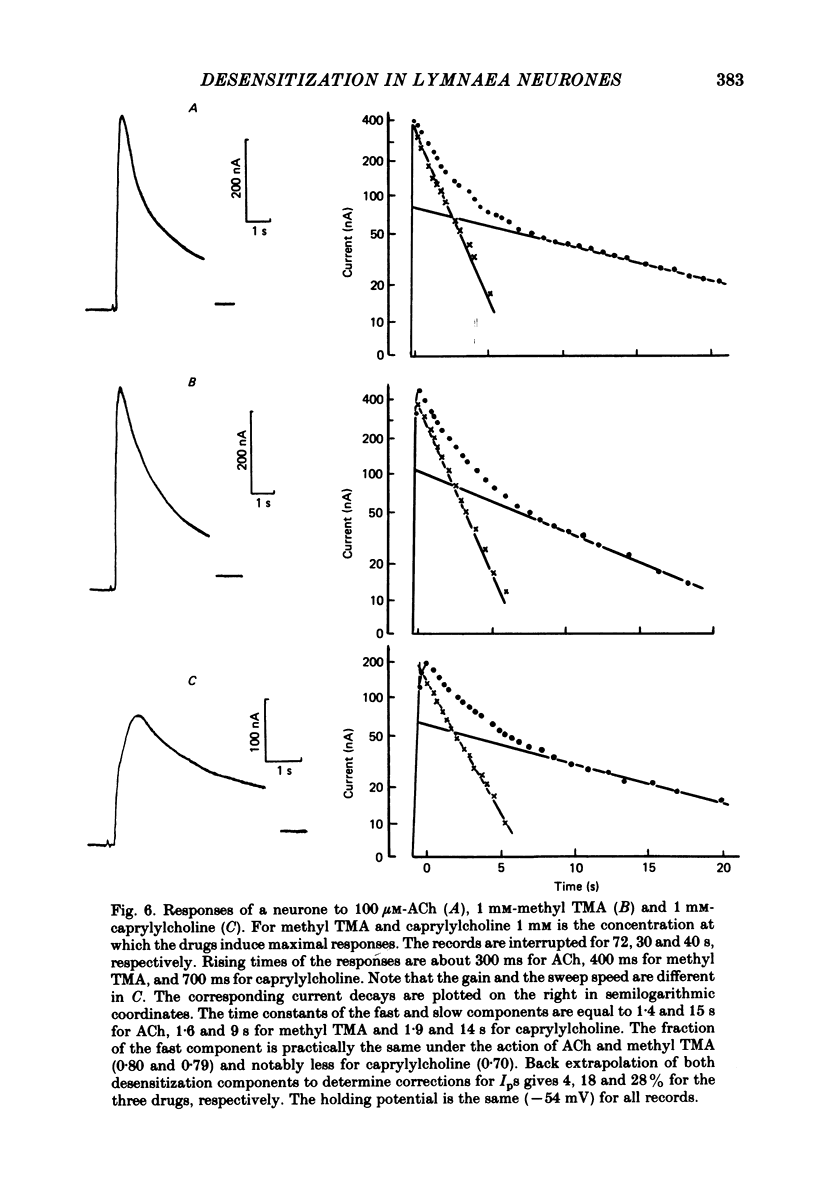
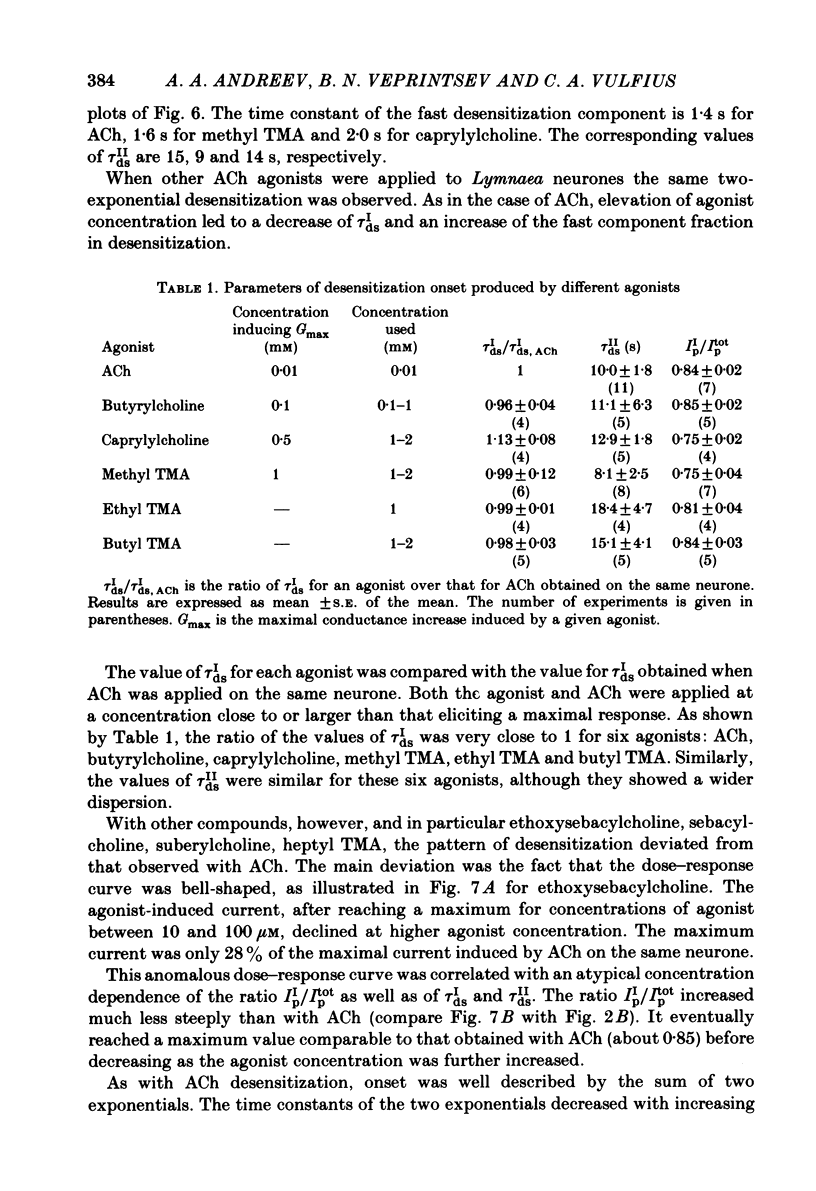
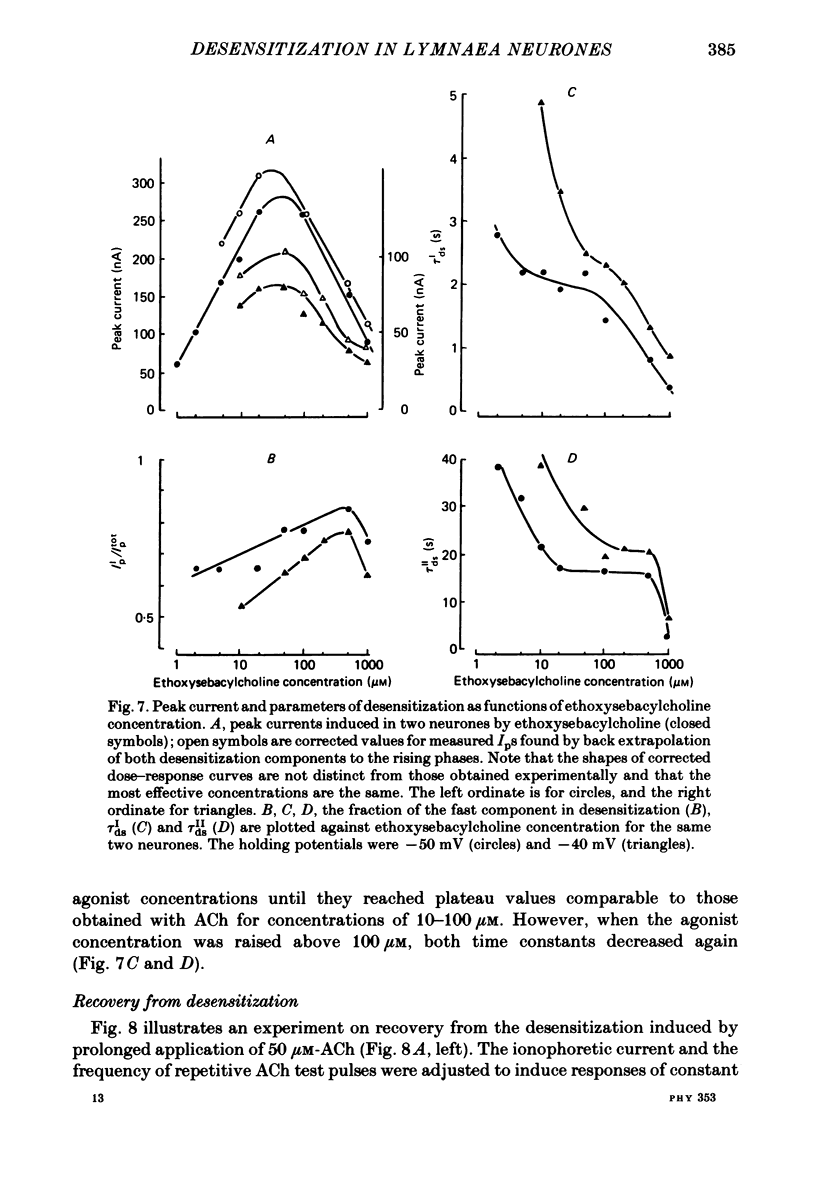
The kinetics of desensitization induced by different agonists of acetylcholine (ACh) as well as the kinetics of recovery from densensitization, have been studied using the voltage-clamp technique in isolated, identified Lymnaea stagnalis neurones. Desensitization follows the sum of two exponentials: one fast and one slow. The time constant of the fast desensitization component (tau Ids) under ACh application is in the range of seconds at room temperature (18-23 degrees C). It increases upon cooling (Q10 = 2.8 +/- 0.9), decreases with increasing ACh concentration and is independent of membrane voltage. The time constant of the slow component of densensitization (tau Ids) is in the range of tens of seconds. It decreases with increasing drug concentration and is weakly dependent upon temperature (Q10 = 1.3 +/- 0.4). The relative amplitude of the fast component, estimated by back extrapolation to the position of the peak current, increases with agonist concentration and decreases upon cooling. Recovery from desensitization follows the sum of two exponentials with time constants (tau Ir and tau IIr) of the order of seconds and minutes, respectively. Cooling prolongs the slow component (Q10 of tau IIr is approx. 3) and reduces its contribution during recovery. A comparison of the desensitization induced by various agonists indicates that for the small monoquaternary agonists the onset and recovery of desensitization resemble the onset and recovery observed with ACh. For more bulky agonists, like ethoxysebacylcholine, sebacylcholine and suberylcholine, the decay of the response during prolonged application of the agonist may involve an additional blocking process.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F. J. Agonist-mediated changes of the acetylcholine receptor in its membrane environment. J Mol Biol. 1978 Sep 5;124(1):1–26. doi: 10.1016/0022-2836(78)90144-4. [DOI] [PubMed] [Google Scholar]
- Bregestovksi P. D., Bukharaeva E. A., Iljin V. I. Voltage clamp analysis of acetylcholine receptor desensitization in isolated mollusc neurones. J Physiol. 1979 Dec;297(0):581–595. doi: 10.1113/jphysiol.1979.sp013058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregestovski P. D., Iljin V. I. Effect of "calcium antagonist" D-600 on the postsynaptic membrane. J Physiol (Paris) 1980 Sep;76(5):515–522. [PubMed] [Google Scholar]
- Chemeris N. K., Kazachenko V. N., Kislov A. N., Kurchikov A. L. Inhibition of acetylcholine responses by intracellular calcium in Lymnaea stagnalis neurones. J Physiol. 1982 Feb;323:1–19. doi: 10.1113/jphysiol.1982.sp014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Interaction between nerve-related acetylcholine and bath applied agonists at the frog end-plate. J Physiol. 1980 Feb;299:533–552. doi: 10.1113/jphysiol.1980.sp013141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiekers J. F., Spannbauer P. M., Scubon-Mulieri B., Parsons R. L. Voltage dependence of desensitization. Influence of calcium and activation kinetics. J Gen Physiol. 1980 May;75(5):511–529. doi: 10.1085/jgp.75.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Changeux J. P. Interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata in the millisecond time range: resolution of an "intermediate" conformational transition and evidence for positive cooperative effects. Biochem Biophys Res Commun. 1980 Dec 16;97(3):889–896. doi: 10.1016/0006-291x(80)91460-6. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenko M. A., Geletyuk V. I., Veprintsev B. N. Completely isolated neurons in the mollusc, Lymnaea stagnalis. A new objective for nerve cell biology investigation. Comp Biochem Physiol A Comp Physiol. 1974 Sep 1;49(1A):89–100. doi: 10.1016/0300-9629(74)90544-1. [DOI] [PubMed] [Google Scholar]
- Lester H. A., Changeux J. P., Sheridan R. E. Conductance increases produced by bath application of cholinergic agonists to Electrophorus electroplaques. J Gen Physiol. 1975 Jun;65(6):797–816. doi: 10.1085/jgp.65.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G. O mekhanizme desensitizatsii postsinapticheskoi membrany myshechnogo volokna. Biofizika. 1968 Jan-Feb;13(1):199–203. [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocit F. The effect of temperature on desensitization kinetics at the post-synaptic membrane of the frog muscle fibre. J Physiol. 1975 Jul;249(2):285–300. doi: 10.1113/jphysiol.1975.sp011016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey A. A. Further studies of the effect of calcium on the time course of action of carbamylcholine at the neuromuscular junction. J Gen Physiol. 1970 Sep;56(3):407–419. doi: 10.1085/jgp.56.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubig R. R., Cohen J. B. Permeability control by cholinergic receptors in Torpedo postsynaptic membranes: agonist dose-response relations measured at second and millisecond times. Biochemistry. 1980 Jun 10;19(12):2770–2779. doi: 10.1021/bi00553a036. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Webb G. D. The effects of external Ca++ and Mg++ on the voltage sensitivity of desensitization in Electrophorus electroplaques. J Gen Physiol. 1980 Jun;75(6):693–708. doi: 10.1085/jgp.75.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P. Acetylcholine receptors. Q Rev Biophys. 1974 Jul;7(3):283–399. doi: 10.1017/s0033583500001463. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. The relationship between desensitization and the metaphilic effect at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):383–390. [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization and recovery at the frog neuromuscular junction. J Gen Physiol. 1977 Apr;69(4):431–447. doi: 10.1085/jgp.69.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scubon-Mulieri B., Parsons R. L. Desensitization onset and recovery at the potassium-depolarized frog neuromuscular junction are voltage sensitive. J Gen Physiol. 1978 Mar;71(3):285–299. doi: 10.1085/jgp.71.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Isla B. A., Hucho F. Acetylcholine receptor: SH group reactivity as indicator of conformational changes and functional states. FEBS Lett. 1977 Mar 15;75(1):65–69. doi: 10.1016/0014-5793(77)80054-9. [DOI] [PubMed] [Google Scholar]
- TAUC L., BRUNER J. "Desensitization" of cholinergic receptors by acetylcholine in molluscan central neurones. Nature. 1963 Apr 6;198:33–34. doi: 10.1038/198033a0. [DOI] [PubMed] [Google Scholar]
- Weber M., David-Pfeuty T., Changeux J. P. Regulation of binding properties of the nicotinic receptor protein by cholinergic ligands in membrane fragments from Torpedo marmorata. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3443–3447. doi: 10.1073/pnas.72.9.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]


