Abstract
Biochemical and electrophysiological measurements were made on photoreceptor cells from Limulus ventral eyes to investigate the possible role of cyclic AMP and adenylate cyclase in the visual transduction mechanism. Cyclic AMP content in a photoreceptor-enriched fraction (the end organs) of Limulus ventral eyes was approximately 15 pmol/mg protein. The cyclic AMP content was increased by bathing eyes in 1-methyl-3-isobutyl xanthine or forskolin and was increased almost 100-fold when bathed in both. Illumination did not change cyclic AMP content significantly in any of these conditions. Discrete events that can be recorded electrophysiologically occur spontaneously in darkness. An increase in the frequency of discrete events is evoked by dim illumination. The discrete events are a sign of excitation of Limulus photoreceptor cells. Drug-induced changes in the rate of occurrence of discrete events recorded electrophysiologically in darkness were not correlated with changes in cyclic AMP content. Adenylate cyclase activity measured from a small number of pooled photoreceptor clusters was stimulated by fluoride and vanadate ions, hydrolysis-resistant analogues of GTP, cholera toxin and forskolin. The Limulus enzyme is similar pharmacologically to mammalian and avian adenylate cyclases. Activation of adenylate cyclase by drugs was not correlated with changes in the rate of occurrence of discrete events recorded electrophysiologically in darkness. A heat-treated Lubrol extract of membranes from Limulus ventral eyes reconstituted the adenylate cyclase activity of membranes from S49 mouse lymphoma cyc- mutant cells which lack a functional regulatory protein. These findings suggest that Limulus ventral eye photoreceptors contain a regulatory protein that mediates the activation of adenylate cyclase by guanine nucleotides, fluoride or cholera toxin. This regulatory protein is homologous with that found in mammalian and avian adenylate cyclases. Our findings suggest that neither cyclic AMP nor adenylate cyclase activation is a necessary or obligatory component of the excitation mechanism in Limulus ventral photoreceptors.
Full text
PDF


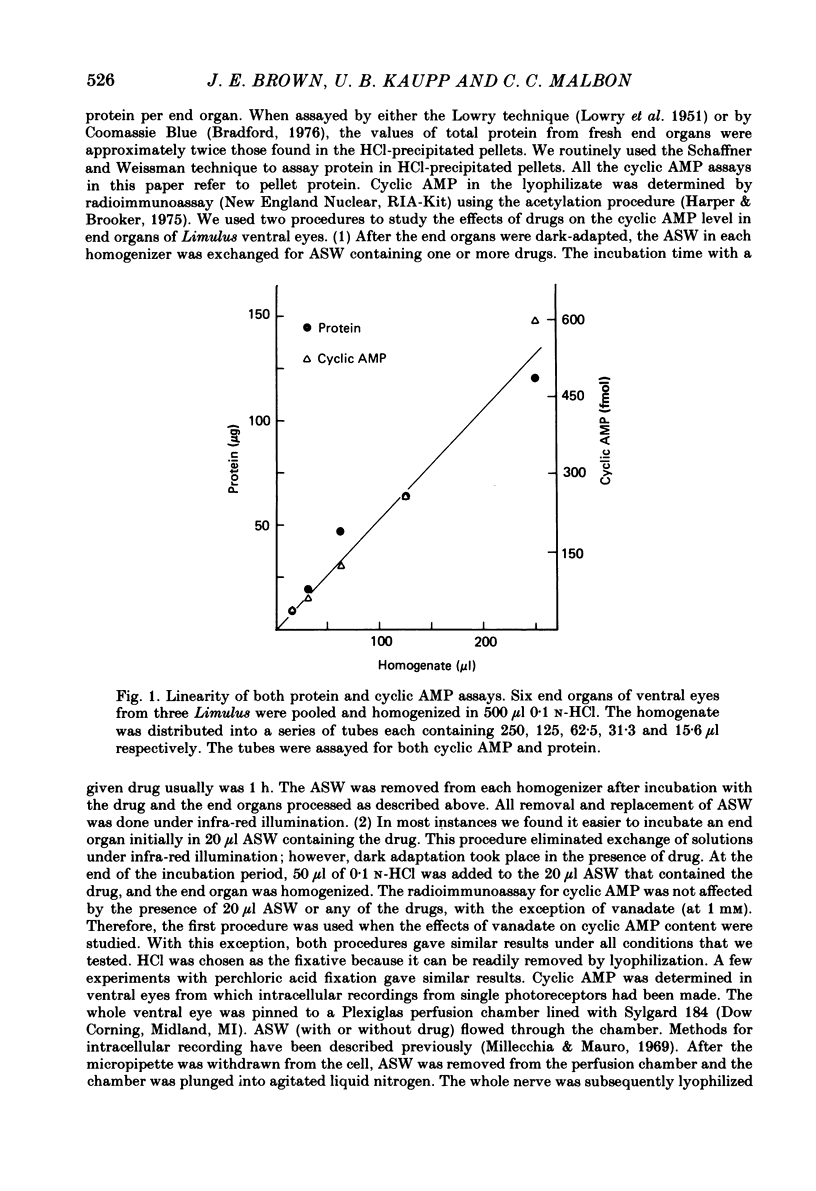



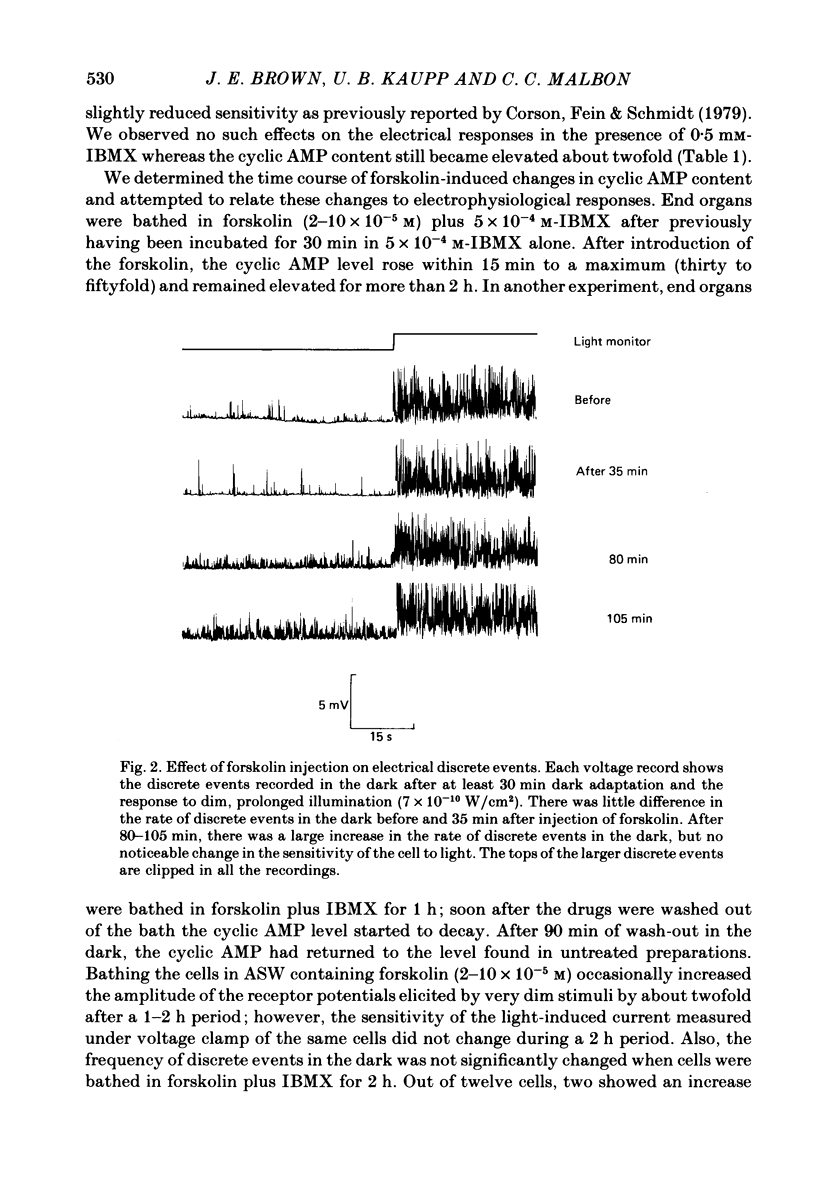



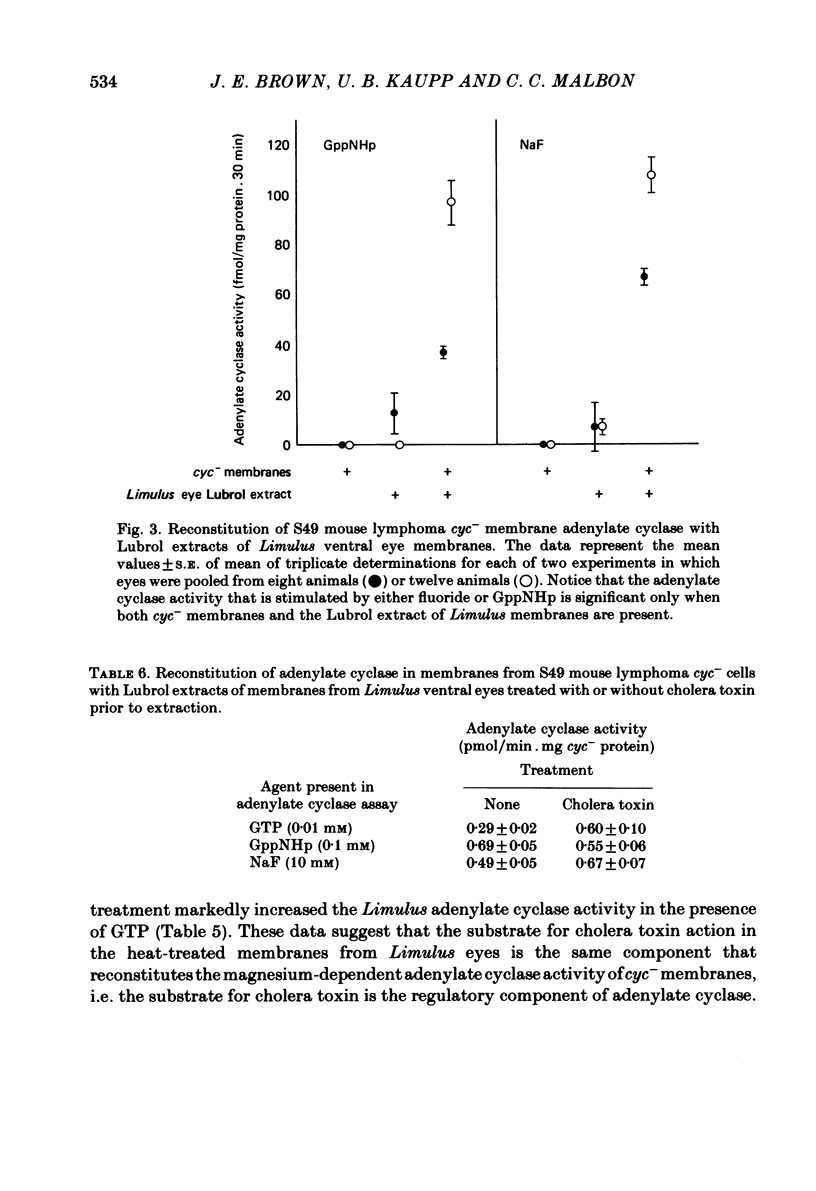





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow R. B., Jr, Chamberlain S. C., Levinson J. Z. Limulus brain modulates the structure and function of the lateral eyes. Science. 1980 Nov 28;210(4473):1037–1039. doi: 10.1126/science.7434015. [DOI] [PubMed] [Google Scholar]
- Batelle B. A., Evans J. A., Chamberlain S. C. Efferent fibers to Limulus eyes synthesize and release octopamine. Science. 1982 Jun 11;216(4551):1250–1252. doi: 10.1126/science.6123151. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Miller W. H. Digitonin effects on photoreceptor adenylate cyclase. Science. 1972 Mar 24;175(4028):1363–1364. doi: 10.1126/science.175.4028.1363. [DOI] [PubMed] [Google Scholar]
- Bolsover S. R., Brown J. E. Injection of guanosine and adenosine nucleotides into Limulus ventral photoreceptor cells. J Physiol. 1982 Nov;332:325–342. doi: 10.1113/jphysiol.1982.sp014416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Blinks J. R. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. Detection with aequorin. J Gen Physiol. 1974 Dec;64(6):643–665. doi: 10.1085/jgp.64.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E. Calcium ion, a putative intracellular messenger for light-adaptation in Limulus ventral photoreceptors. Biophys Struct Mech. 1977 Jun 29;3(2):141–143. doi: 10.1007/BF00535809. [DOI] [PubMed] [Google Scholar]
- Calman B. G., Chamberlain S. C. Distinct lobes of Limulus ventral photoreceptors. II. Structure and ultrastructure. J Gen Physiol. 1982 Dec;80(6):839–862. doi: 10.1085/jgp.80.6.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. I. The microanatomy. J Gen Physiol. 1969 Sep;54(3):289–309. doi: 10.1085/jgp.54.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Brown J. E. Effects of increased intracellular pH-buffering capacity on the light response of Limulus ventral photoreceptor. Biochim Biophys Acta. 1976 Jun 4;436(1):140–153. doi: 10.1016/0005-2736(76)90226-1. [DOI] [PubMed] [Google Scholar]
- Corson D. W., Fein A., Schmidt J. Two effects of phosphodiesterase inhibitors on Limulus ventral photoreceptors. Brain Res. 1979 Nov 2;176(2):365–368. doi: 10.1016/0006-8993(79)90990-9. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Knight B. W., Toyoda J. Voltage noise in Limulus visual cells. Science. 1968 Apr 5;160(3823):88–90. doi: 10.1126/science.160.3823.88. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Malbon C. C. Regulation of adenylate cyclase by adenosine. Mol Cell Biochem. 1979 Jun 15;25(3):143–169. doi: 10.1007/BF00235364. [DOI] [PubMed] [Google Scholar]
- Fein A., Corson D. W. Both photons and fluoride ions excite limulus ventral photoreceptors. Science. 1979 Apr 6;204(4388):77–79. doi: 10.1126/science.34877. [DOI] [PubMed] [Google Scholar]
- Fein A., Corson D. W. Excitation of Limulus photoreceptors by vanadate and by a hydrolysis-resistant analog of guanosine triphosphate. Science. 1981 May 1;212(4494):555–557. doi: 10.1126/science.6782676. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Reconstitution of cholera toxin-activated adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3113–3117. doi: 10.1073/pnas.75.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow H. R., Johnson G. L., Brothers V. M., Bourne H. R. A regulatory component of adenylate cyclase from human erythrocyte membranes. J Biol Chem. 1980 Apr 25;255(8):3736–3741. [PubMed] [Google Scholar]
- Kaupp U. B., Malbon C. C., Battelle B. A., Brown J. E. Octopamine stimulated rise of cAMP in Limulus ventral photoreceptors. Vision Res. 1982;22(12):1503–1506. doi: 10.1016/0042-6989(82)90216-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972 Jun;59(6):701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Strong J. A. The initiation of excitation and light adaptation in Limulus ventral photoreceptors. J Gen Physiol. 1979 Feb;73(2):219–243. doi: 10.1085/jgp.73.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litosch I., Saito Y., Fain J. N. Forskolin as an activator of cyclic AMP accumulation and secretion in blowfly salivary glands. Biochem J. 1982 Apr 15;204(1):147–151. doi: 10.1042/bj2040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon C. C., Gill D. M. ADP-ribosylation of membrane proteins and activation of adenylate cyclase by cholera toxin in fat cell ghosts from euthyroid and hypothyroid rats. Biochim Biophys Acta. 1979 Sep 3;586(3):518–527. doi: 10.1016/0304-4165(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Malbon C. C. Liver cell adenylate cyclase and beta-adrenergic receptors. Increased beta-adrenergic receptor number and responsiveness in the hypothyroid rat. J Biol Chem. 1980 Sep 25;255(18):8692–8699. [PubMed] [Google Scholar]
- Millecchia R., Mauro A. The ventral photoreceptor cells of Limulus. II. The basic photoresponse. J Gen Physiol. 1969 Sep;54(3):310–330. doi: 10.1085/jgp.54.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. H., Gorman R. E., Bitensky M. W. Cyclic adenosine monophosphate: function in photoreceptors. Science. 1971 Oct 15;174(4006):295–297. doi: 10.1126/science.174.4006.295. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase activity: interactions of solubilized components with receptor-replete membranes. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3715–3719. doi: 10.1073/pnas.74.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Farber D. B. Light-induced changes in cAMP levels in Limulus photoreceptors. Biochem Biophys Res Commun. 1980 May 30;94(2):438–442. doi: 10.1016/0006-291x(80)91250-4. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Puchstein C., Hannemann H., Söchtig E. Activation of adenylate cyclase by vanadate. Nature. 1979 Jan 11;277(5692):143–145. doi: 10.1038/277143a0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F., Knight B. W., Dodge F. A. Adapting bump model for ventral photoreceptors of Limulus. J Gen Physiol. 1982 Jun;79(6):1089–1113. doi: 10.1085/jgp.79.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff V. J. The effect of cyclic AMP and aminophylline on Limulus lateral eye retinular cells. Vision Res. 1973 Dec;13(12):2335–2344. doi: 10.1016/0042-6989(73)90233-2. [DOI] [PubMed] [Google Scholar]
- Wulff V. J. The effect of cyclic amp on Limulus lateral eye retinular cells. Vision Res. 1971 Dec;11(12):1493–1495. doi: 10.1016/0042-6989(71)90071-x. [DOI] [PubMed] [Google Scholar]


