Abstract
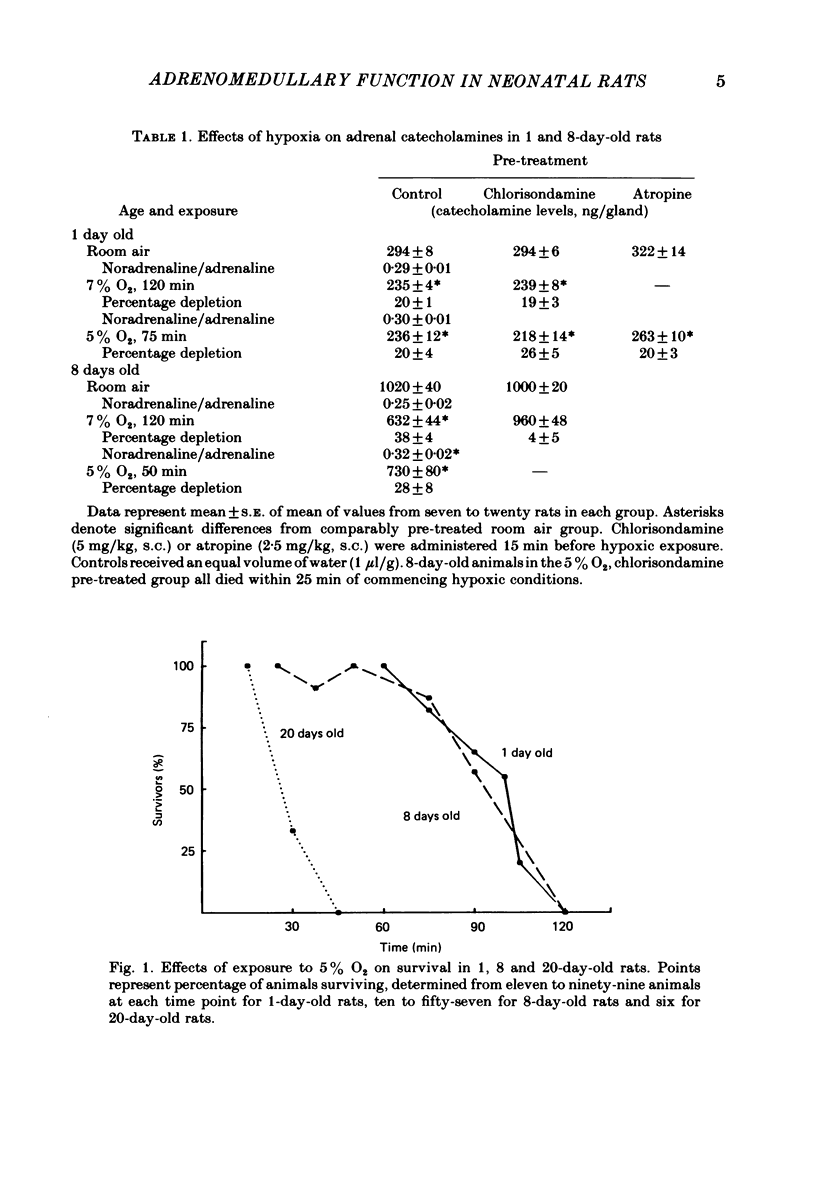
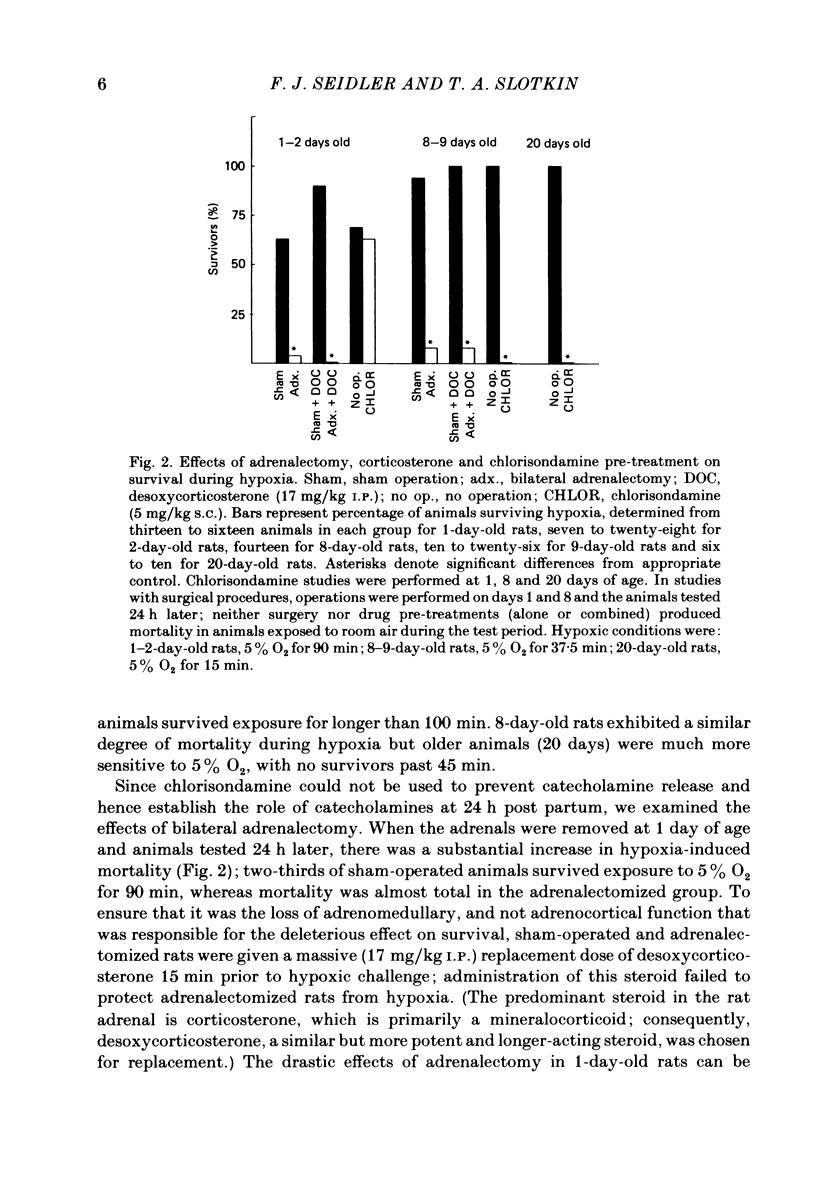
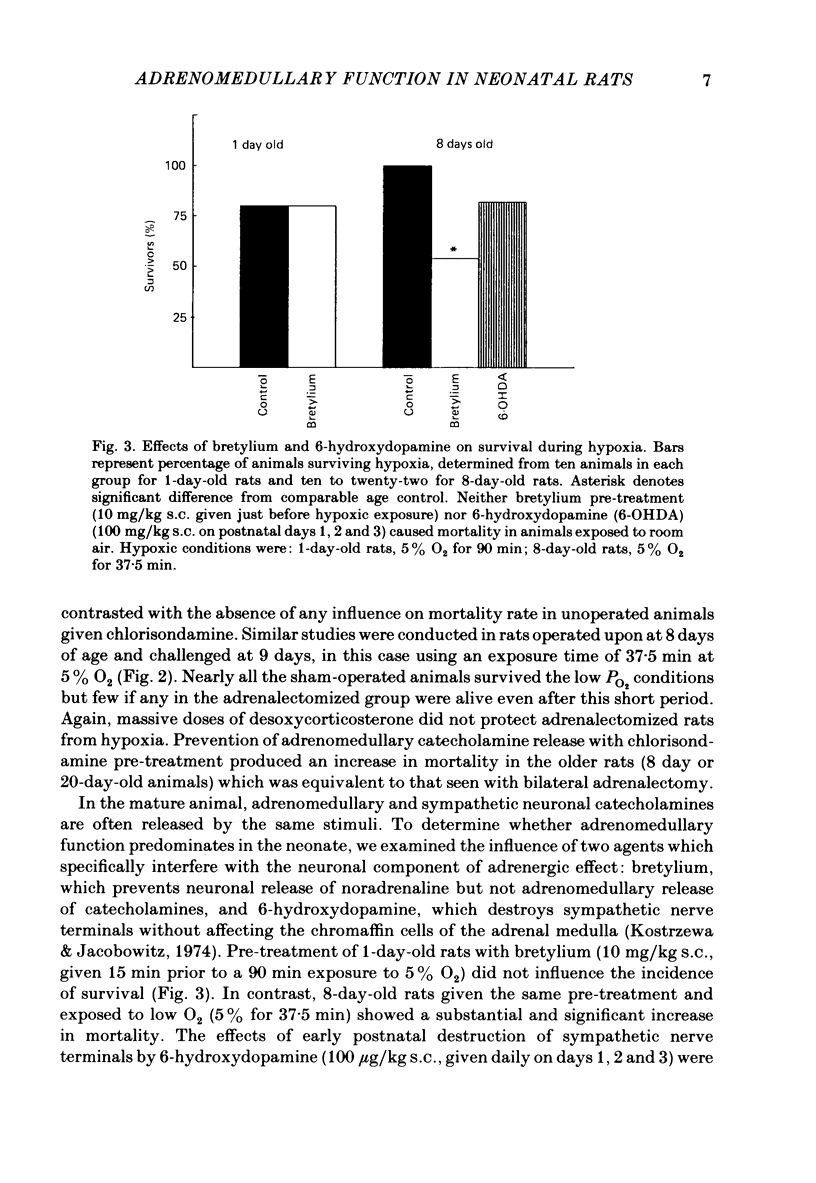
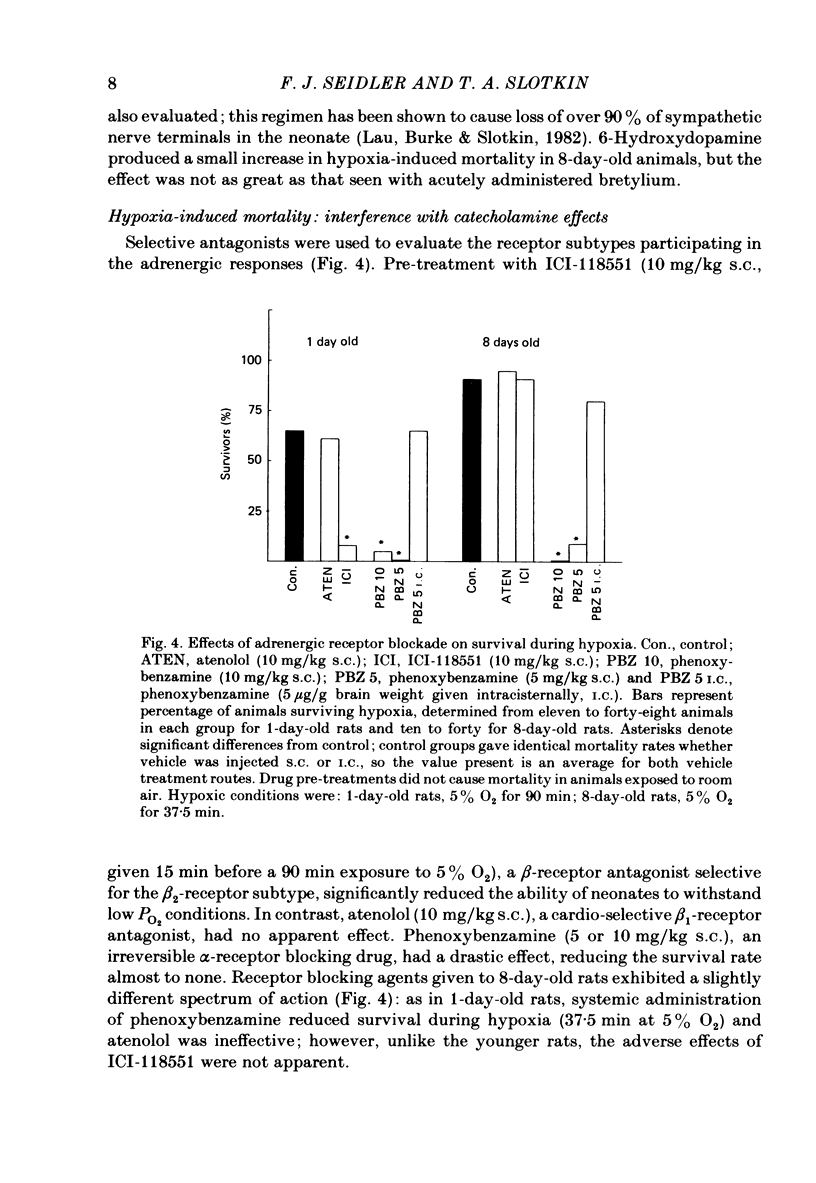
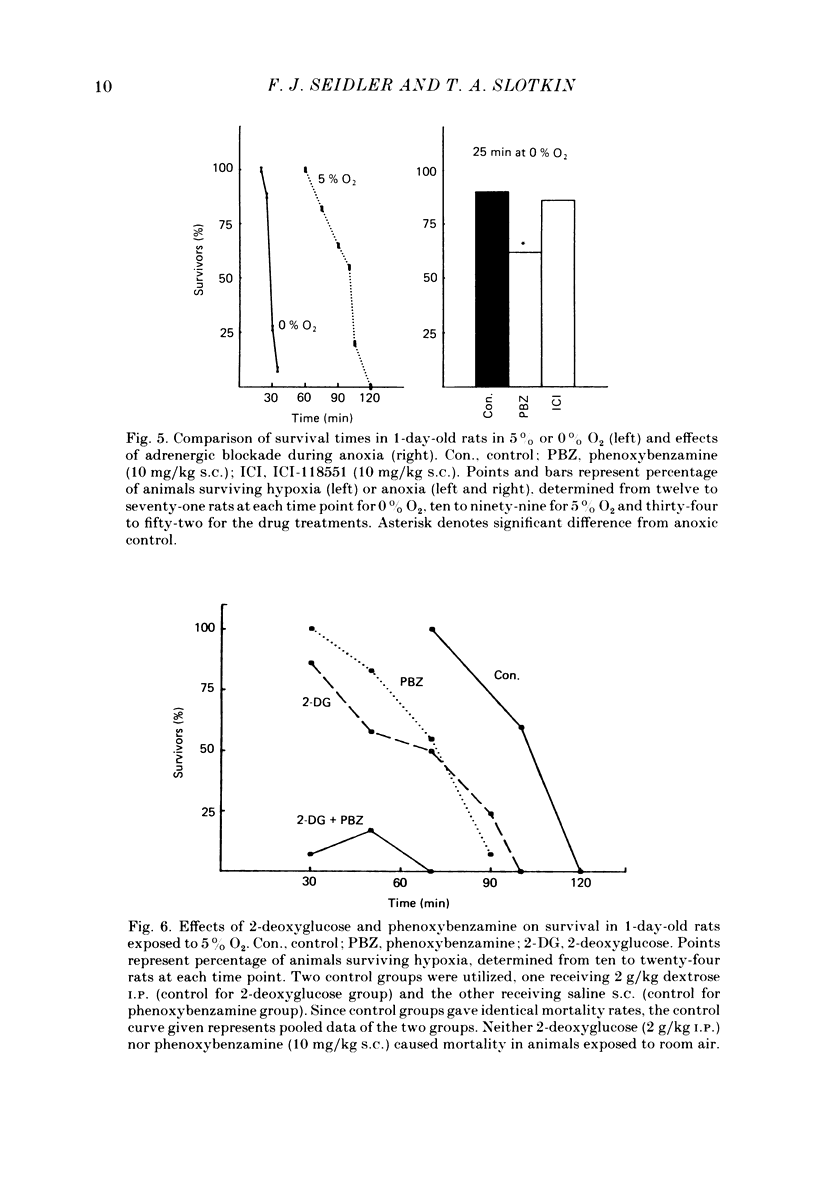
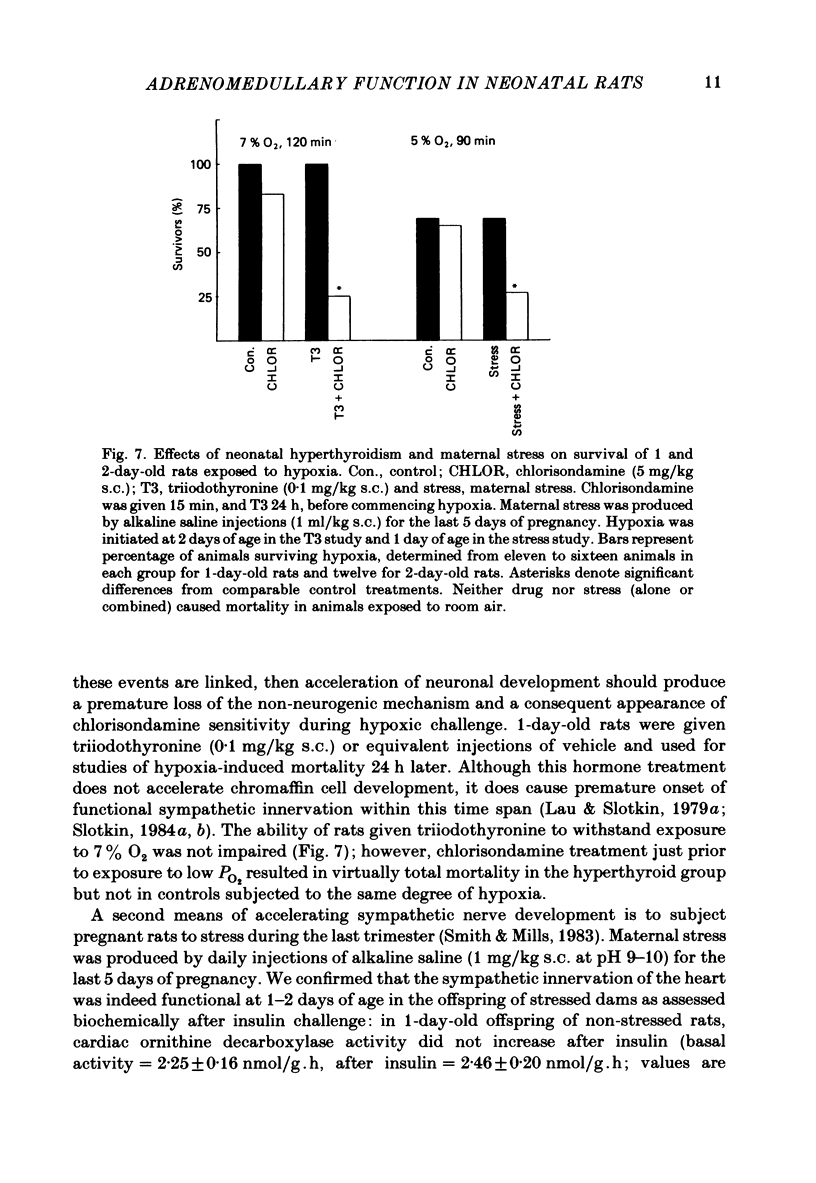
The mechanism of release of catecholamines from the adrenal medulla of neonatal rats was examined, together with the role of these amines in the ability of the organism to withstand acute O2 deprivation. Splanchnic innervation of the rat adrenal is non-functional until the end of the first postnatal week. Nevertheless, hypoxia caused depletion of adrenal catecholamines in 1-day-old rats as well as in 8-day-old animals. Pre-treatment with cholinergic receptor blocking agents did not prevent the catecholamine response at 1 day but did in older animals; these results indicate that the depletion mechanism is not neurogenic in 1-day-old animals but is neurogenic in 8-day-old animals. The proportions of noradrenaline and adrenaline released by hypoxic stress also differed at the two ages, with preferential release of adrenaline by the neurogenic mechanism but not by the non-neurogenic one. The ontogenetic replacement of non-neurogenic adrenomedullary responses by the neurogenic mechanism was directly related to the onset of splanchnic nerve function. Treatments which accelerated the development of neuronal connexions (neonatal hyperthyroidism, maternal stress) caused premature loss of the non-neurogenic response. Prior to the development of sympathetic nerve function, adrenal catecholamines plays a predominant role in enabling the neonate to survive hypoxia. Interference with the release of adrenal amines invariably increased mortality during hypoxia. In contrast, interference with sympathetic neural release of catecholamines did not affect the ability of 1-day-old rats to withstand hypoxia, indicating that survival during low PO2 conditions is not dependent on the sympathetic innervation at that stage of development. After functional development of the sympathetic nerves and disappearance of non-neurogenic adrenomedullary responses, the neonatal rats became partially dependent upon catecholamines derived from sympathetic terminals; administration of bretylium at 8 days significantly compromised survival during hypoxia. Interference with adrenergic receptor function also interfered with the ability of neonatal rats to withstand low PO2. At 1 day of age, either phenoxybenzamine or ICI-118551, but not atenolol, shortened the survival time during hypoxia. At 8 days, only phenoxybenzamine did so.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. R., Slotkin T. A. Maturation of the adrenal medulla--IV. Effects of morphine. Biochem Pharmacol. 1975 Aug 15;24(16):1469–1474. doi: 10.1016/0006-2952(75)90020-9. [DOI] [PubMed] [Google Scholar]
- Bareis D. L., Slotkin T. A. Maturation of sympathetic neurotransmission in the rat heart. I. Ontogeny of the synaptic vesicle uptake mechanism and correlation with development of synaptic function. Effects of neonatal methadone administration on development of synaptic vesicles. J Pharmacol Exp Ther. 1980 Jan;212(1):120–125. [PubMed] [Google Scholar]
- Bareis D. L., Slotkin T. A. Responses of heart ornithine decarboxylase and adrenal catecholamines to methadone and sympathetic stimulants in developing and adults rats. J Pharmacol Exp Ther. 1978 Apr;205(1):164–174. [PubMed] [Google Scholar]
- Bartolomé J., Bartolomé M., Seidler J., Anderson T. R., Slotkin T. A. Effects of early postnatal guanethidine administration on adrenal medulla and brain of developing rats. Biochem Pharmacol. 1976 Nov 1;25(21):2387–2390. doi: 10.1016/0006-2952(76)90033-2. [DOI] [PubMed] [Google Scholar]
- Bartolomé J., Lau C., Slotkin T. A. Ornithine decarboxylase in developing rat heart and brain: role of sympathetic development for responses to autonomic stimulants and the effects of reserpine on maturation. J Pharmacol Exp Ther. 1977 Sep;202(3):510–518. [PubMed] [Google Scholar]
- Bartolomé J., Slotkin T. A. Effects of postnatal reserpine administration on sympatho-adrenal development in the rat. Biochem Pharmacol. 1976 Jul 1;25(13):1513–1519. doi: 10.1016/0006-2952(76)90070-8. [DOI] [PubMed] [Google Scholar]
- Benfey B. G. Characterization of -adrenoceptors in the myocardium. Br J Pharmacol. 1973 May;48(1):132–138. doi: 10.1111/j.1476-5381.1973.tb08230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantry C. J., Seidler F. J., Slotkin T. A. Non-neurogenic mechanism for reserpine-induced release of catecholamines from the adrenal medulla of neonatal rats: possible modulation by opiate receptors. Neuroscience. 1982 Mar;7(3):673–678. doi: 10.1016/0306-4522(82)90073-2. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Patten G. S., Filsell O. H. Evidence for an alpha-adrenergic receptor-mediated control of energy production in heart. J Mol Cell Cardiol. 1982 Jun;14(6):313–321. doi: 10.1016/0022-2828(82)90246-2. [DOI] [PubMed] [Google Scholar]
- Comline R. S., Silver M. The development of the adrenal medulla of the foetal and new-born calf. J Physiol. 1966 Mar;183(2):305–340. doi: 10.1113/jphysiol.1966.sp007868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V., Cote J., Labrie F. Characteristics of the alpha-adrenergic stimulation of adrenocorticotropin secretion in rat anterior pituitary cells. Endocrinology. 1981 Sep;109(3):757–762. doi: 10.1210/endo-109-3-757. [DOI] [PubMed] [Google Scholar]
- Govier W. C. Myocardial alpha adrenergic receptors and their role in the production of a positive inotropic effect by sympathomimetic agents. J Pharmacol Exp Ther. 1968 Jan;159(1):82–90. [PubMed] [Google Scholar]
- Kostrzewa R. M., Jacobowitz D. M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974 Sep;26(3):199–288. [PubMed] [Google Scholar]
- Lagercrantz H., Bistoletti P. Catecholamine release in the newborn infant at birth. Pediatr Res. 1977 Aug;11(8):889–893. doi: 10.1203/00006450-197708000-00007. [DOI] [PubMed] [Google Scholar]
- Lau C., Burke S. P., Slotkin T. A. Maturation of sympathetic neurotransmission in the rat heart. IX. Development of transsynaptic regulation of cardiac adrenergic sensitivity. J Pharmacol Exp Ther. 1982 Dec;223(3):675–680. [PubMed] [Google Scholar]
- Lau C., Slotkin T. A. Accelerated development of rat sympathetic neurotransmission caused by neonatal triiodothyronine administration. J Pharmacol Exp Ther. 1979 Mar;208(3):485–490. [PubMed] [Google Scholar]
- Lau C., Slotkin T. A. Maturation of sympathetic neurotransmission in the rat heart. II. Enhanced development of presynaptic and postsynaptic components of noradrenergic synapses as a result of neonatal hyperthyroidism. J Pharmacol Exp Ther. 1980 Jan;212(1):126–130. [PubMed] [Google Scholar]
- Lau C., Slotkin T. A. Regulation of rat heart ornithine decarboxylase: change in affinity for ornithine evoked by neuronal, hormonal, and ontogenetic stimuli. Mol Pharmacol. 1979 Sep;16(2):504–512. [PubMed] [Google Scholar]
- Lawson E. E., Brown E. R., Torday J. S., Madansky D. L., Taeusch H. W., Jr The effect of epinephrine on tracheal fluid flow and surfactant efflux in fetal sheep. Am Rev Respir Dis. 1978 Dec;118(6):1023–1026. doi: 10.1164/arrd.1978.118.6.1023. [DOI] [PubMed] [Google Scholar]
- MERRILLS R. J. A SEMIAUTOMATIC METHOD FOR DETERMINATION OF CATECHOLAMINES. Anal Biochem. 1963 Sep;6:272–282. doi: 10.1016/0003-2697(63)90135-0. [DOI] [PubMed] [Google Scholar]
- Noguchi A., Whitsett J. A., Dickman L. Ontogeny of myocardial alpha 1-adrenergic receptor in the rat. Dev Pharmacol Ther. 1981;3(3):179–188. doi: 10.1159/000457439. [DOI] [PubMed] [Google Scholar]
- Nylund L., Lagercrantz H., Lunell N. O. Catecholamines in fetal blood during birth in man. J Dev Physiol. 1979 Dec;1(6):427–430. [PubMed] [Google Scholar]
- Pappano A. J. Ontogenetic development of autonomic neuroeffector transmission and transmitter reactivity in embryonic and fetal hearts. Pharmacol Rev. 1977 Mar;29(1):3–33. [PubMed] [Google Scholar]
- Rabinowitz B., Chuck L., Kligerman M., Parmley W. W. Positive inotropic effects of methoxamine: evidence for alpha-adrenergic receptors in ventricular myocardium. Am J Physiol. 1975 Sep;229(3):582–585. doi: 10.1152/ajplegacy.1975.229.3.582. [DOI] [PubMed] [Google Scholar]
- Seidler F. J., Slotkin T. A. Development of central control of norepinephrine turnover and release in the rat heart: responses to tyramine, 2-deoxyglucose and hydralazine. Neuroscience. 1981;6(10):2081–2086. doi: 10.1016/0306-4522(81)90047-6. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A., Bartolome J. Ornithine decarboxylase: marker of neuroendocrine and neurotransmitter actions. Methods Enzymol. 1983;103:590–603. doi: 10.1016/s0076-6879(83)03042-6. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. Maturation of the adrenal medulla. I. Uptake and storage of amines in isolated storage vesicles of the rat. Biochem Pharmacol. 1973 Aug 15;22(16):2023–2032. doi: 10.1016/0006-2952(73)90083-x. [DOI] [PubMed] [Google Scholar]
- Slotkin T. A. Maturation of the adrenal medulla. II. Content and properties of catecholamine storage vesicles of the rat. Biochem Pharmacol. 1973 Aug 15;22(16):2033–2044. doi: 10.1016/0006-2952(73)90084-1. [DOI] [PubMed] [Google Scholar]
- Smith P. G., Mills E. Abnormal development of blood pressure and growth in rats exposed to perinatal injection stress. Life Sci. 1983 May 23;32(21):2497–2501. doi: 10.1016/0024-3205(83)90376-4. [DOI] [PubMed] [Google Scholar]
- Walters D. V., Olver R. E. The role of catecholamines in lung liquid absorption at birth. Pediatr Res. 1978 Mar;12(3):239–242. doi: 10.1203/00006450-197803000-00017. [DOI] [PubMed] [Google Scholar]
- Winkler H. The biogenesis of adrenal chromaffin granules. Neuroscience. 1977;2(5):657–683. doi: 10.1016/0306-4522(77)90022-7. [DOI] [PubMed] [Google Scholar]


