Abstract
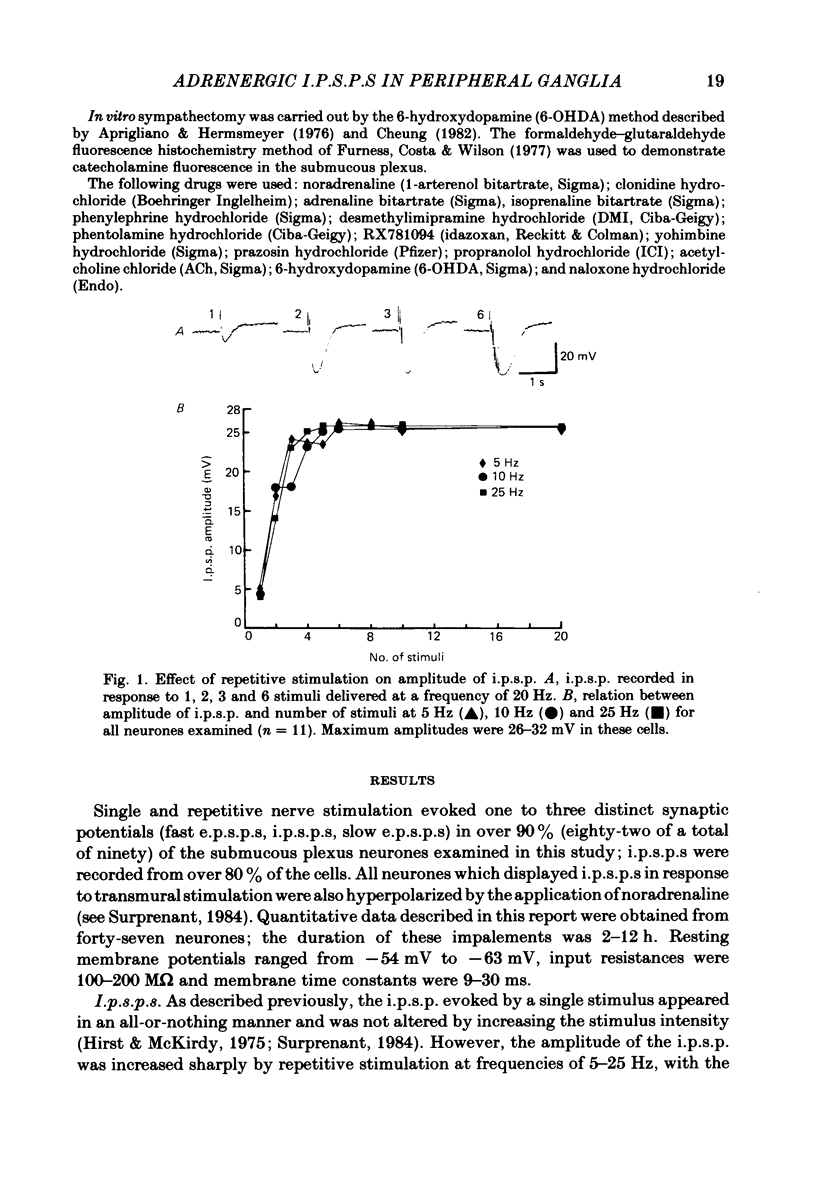
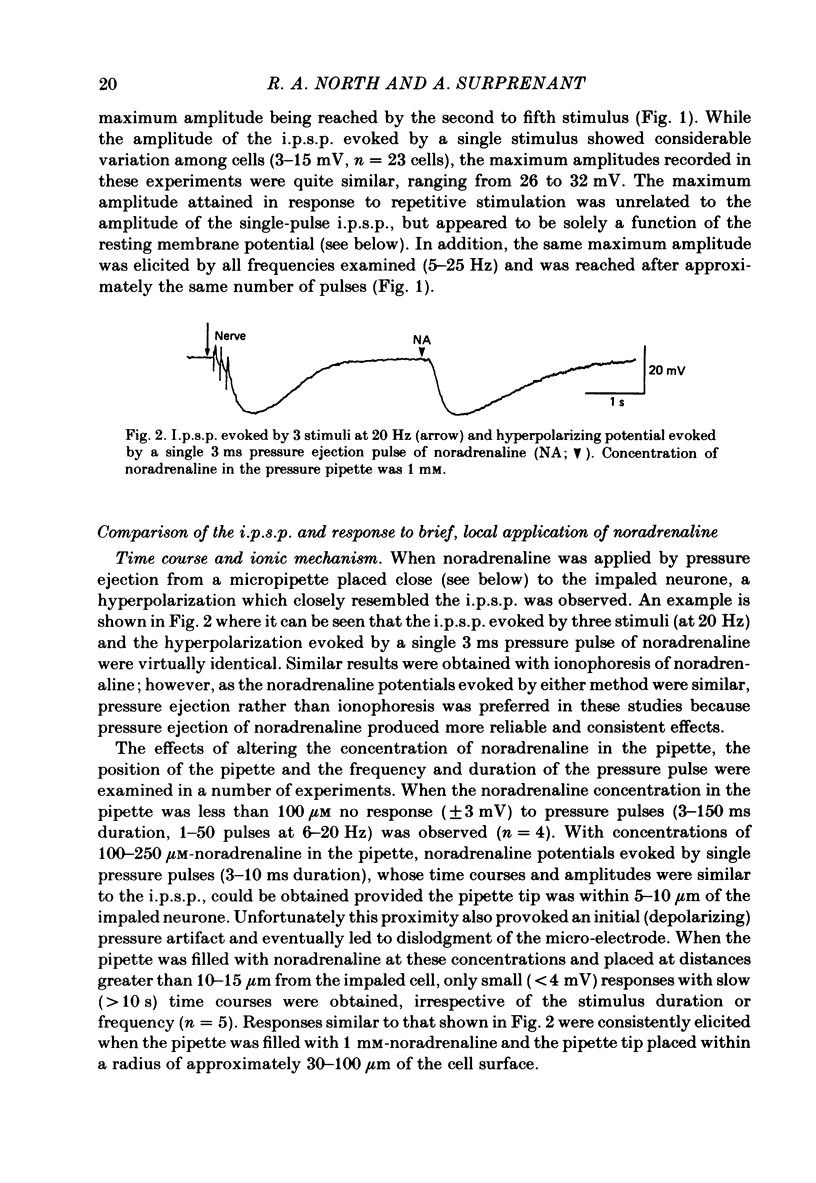
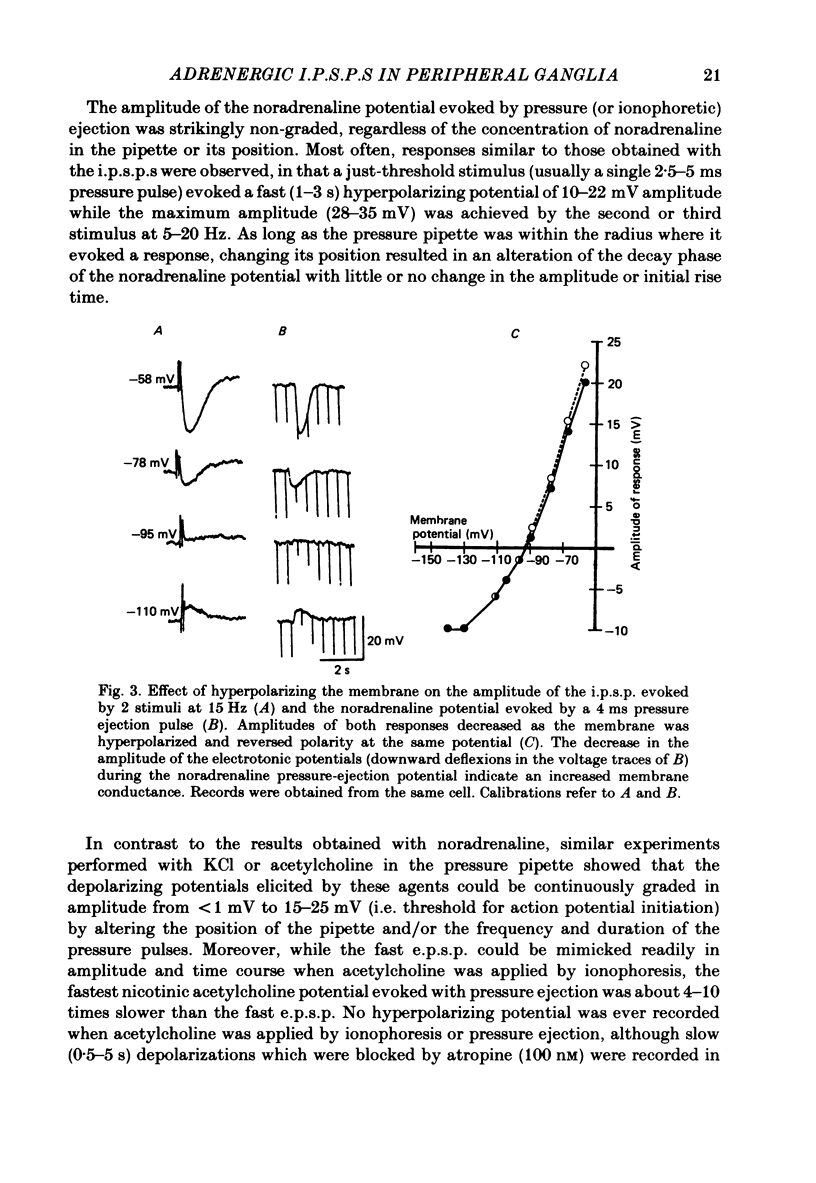
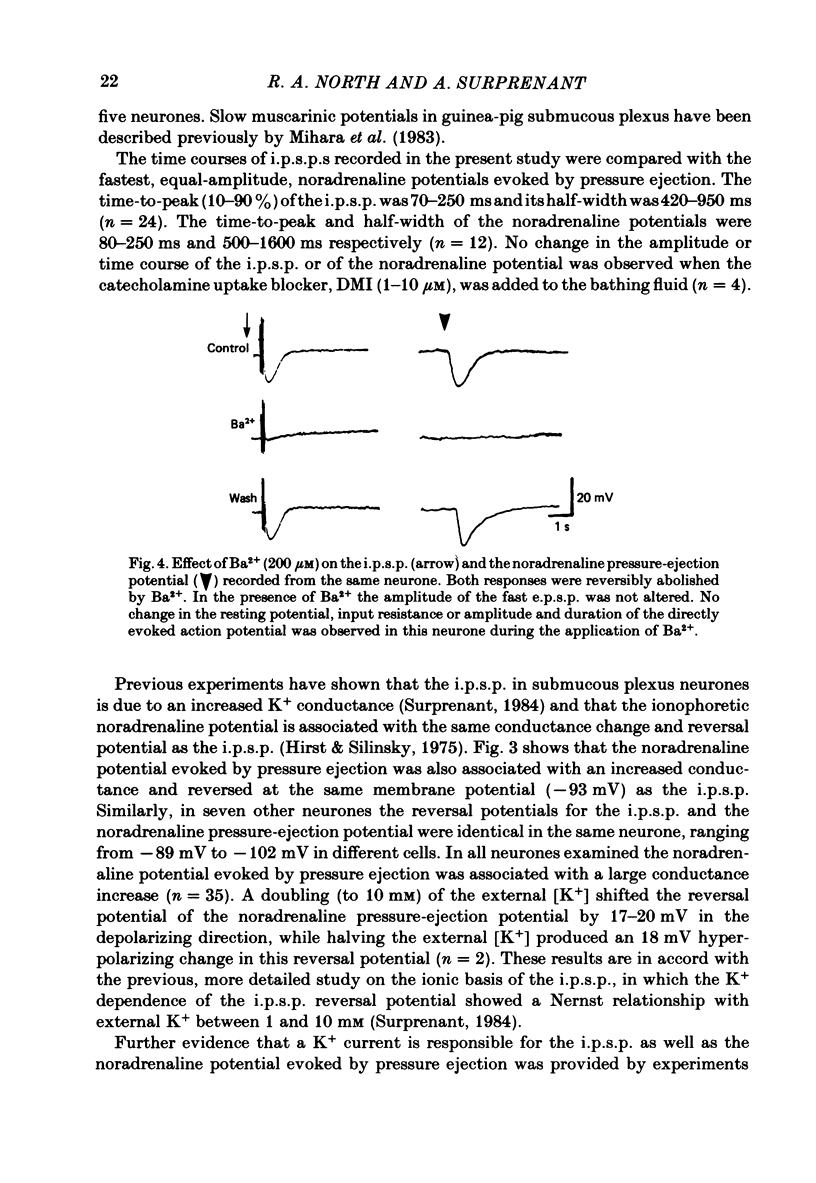
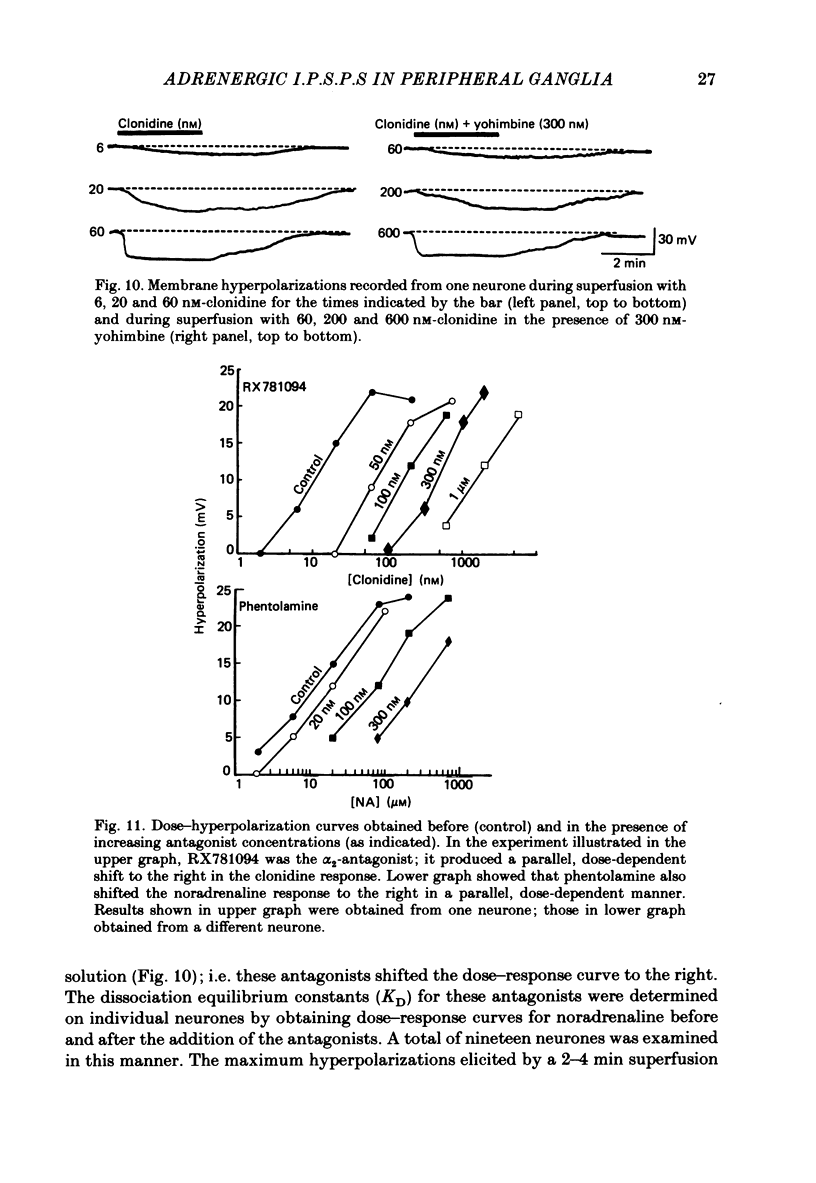
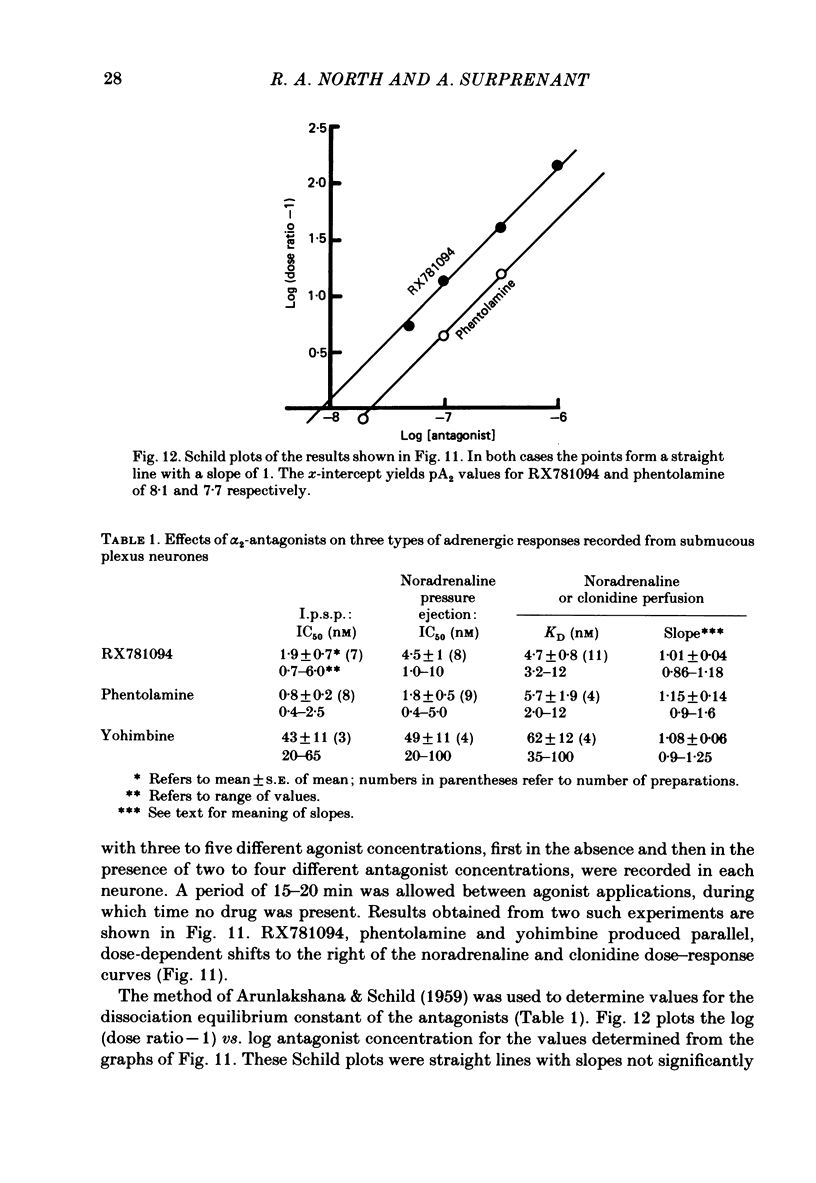
Intracellular recordings were obtained from neurones of the guinea-pig submucous plexus. Inhibitory synaptic potentials (i.p.s.p.s) were compared with hyperpolarizations evoked by brief, local applications of noradrenaline and by superfusion with adrenoceptor agonists. Hyperpolarizing potentials elicited by brief applications of noradrenaline were similar to the i.p.s.p. in latency of onset, amplitude, time course, conductance increase, reversal potential and ionic dependence. Both responses were blocked by low concentrations of Ba2+ and quinine. 6-hydroxydopamine selectively and irreversibly abolished the i.p.s.p. and resulted in a complete loss of catecholamine fluorescent nerve fibres in the submucous plexus. The alpha 2-adrenoceptor antagonists, phentolamine, yohimbine and RX781094, reversibly blocked the i.p.s.p. and the noradrenaline hyperpolarization. Prazosin, propranolol, atropine and naloxone had no effect on these responses. Superfusion with noradrenaline and clonidine produced dose-dependent membrane hyperpolarizations. Noradrenaline and clonidine dose-hyperpolarization curves were shifted to the right in a parallel fashion by alpha 2-adrenoceptor antagonists. Determination of the dissociation equilibrium constants for phentolamine, yohimbine and RX781094 showed that the hyperpolarization produced by noradrenaline perfusion is due to alpha 2-adrenoceptor activation. It is concluded that the release of noradrenaline from sympathetic nerves activates post-synaptic alpha 2-adrenoceptors, resulting in the K+ conductance increase which underlies the i.p.s.p. in submucous plexus neurones.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprigliano O., Hermsmeyer K. In vitro denervation of the portal vein and caudal artery of the rat. J Pharmacol Exp Ther. 1976 Sep;198(3):568–577. [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P. Hyperpolarizing 'alpha 2'-adrenoceptors in rat sympathetic ganglia. Br J Pharmacol. 1979 Mar;65(3):435–445. doi: 10.1111/j.1476-5381.1979.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W. Spontaneous and evoked excitatory junction potentials in rat tail arteries. J Physiol. 1982 Jul;328:449–459. doi: 10.1113/jphysiol.1982.sp014276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. E., Shinnick-Gallagher P. Comparison of the receptors mediating the catecholamine hyperpolarization and slow inhibitory postsynaptic potential in sympathetic ganglia. J Pharmacol Exp Ther. 1981 May;217(2):440–444. [PubMed] [Google Scholar]
- Diab I. M., Dinerstein R. J., Watanabe M., Roth L. J. (3H)morphine localization in myenteric plexus. Science. 1976 Aug 20;193(4254):689–691. doi: 10.1126/science.948744. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey J. C., Roach A. G., Smith C. F. Studies on RX 781094: a selective, potent and specific antagonist of alpha 2-adrenoceptors. Br J Pharmacol. 1983 Mar;78(3):489–505. doi: 10.1111/j.1476-5381.1983.tb08809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey J. C., Smith C. F., Walker J. M. Selectivity of blocking agents for pre-and postsynaptic alpha-adrenoceptors. Br J Pharmacol. 1977 May;60(1):91–96. doi: 10.1111/j.1476-5381.1977.tb16752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LIBET B. Origin and blockade of the synaptic responses of curarized sympathetic ganglia. J Physiol. 1961 Aug;157:484–503. doi: 10.1113/jphysiol.1961.sp006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert F. J., Roeske W. R., Yamamura H. I. Mathematical analysis of the kinetics of competitive inhibition in neurotransmitter receptor binding assays. Mol Pharmacol. 1981 May;19(3):367–371. [PubMed] [Google Scholar]
- Furness J. B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5(1):1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M., Wilson A. J. Water-stable fluorophores, produced by reaction with aldehyde solutions, for the histochemical localization of catechol- and indolethylamines. Histochemistry. 1977 May 20;52(2):159–170. doi: 10.1007/BF00492292. [DOI] [PubMed] [Google Scholar]
- Gallagher J. P., Griffith W. H., Shinnick-Gallagher P. Cholinergic transmission in cat parasympathetic ganglia. J Physiol. 1982 Nov;332:473–486. doi: 10.1113/jphysiol.1982.sp014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., McKirdy H. C. Synaptic potentials recorded from neurones of the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Jul;249(2):369–385. doi: 10.1113/jphysiol.1975.sp011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Silinsky E. M. Some effects of 5-hydroxytryptamine, dopamine and noradrenaline on neurones in the submucous plexus of guinea-pig small intestine. J Physiol. 1975 Oct;251(3):817–832. doi: 10.1113/jphysiol.1975.sp011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. Y., Skok V. I. Slow inhibitory postsynaptic potentials and hyperpolarization evoked by noradrenaline in the neurones of mammalian sympathetic ganglion. J Auton Nerv Syst. 1980 Mar;1(3):255–263. doi: 10.1016/0165-1838(80)90021-1. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., North R. A. Slow synaptic potentials in neurones of the myenteric plexus. J Physiol. 1980 Apr;301:505–516. doi: 10.1113/jphysiol.1980.sp013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K. Cholinergic synaptic potentials and the underlying ionic mechasims. Fed Proc. 1969 Jan-Feb;28(1):101–112. [PubMed] [Google Scholar]
- Koketsu K., Nishi S. Characteristics of the slow inhibitory postsynaptic potential of bullfrog sympathetic ganglion cells. Life Sci. 1967 Sep 1;6(17):1827–1836. doi: 10.1016/0024-3205(67)90211-1. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Libet B., Chichibu S., Tosaka T. Slow synaptic responses and excitability in sympathetic ganglia of the bullfrog. J Neurophysiol. 1968 May;31(3):383–395. doi: 10.1152/jn.1968.31.3.383. [DOI] [PubMed] [Google Scholar]
- Motulsky H. J., Mahan L. C. The kinetics of competitive radioligand binding predicted by the law of mass action. Mol Pharmacol. 1984 Jan;25(1):1–9. [PubMed] [Google Scholar]
- Neild T. O. The action of 5-hydroxytryptamine and possible 5-hydroxytryptamine antagonists on neurones of the guinea-pig submucous plexus. Gen Pharmacol. 1981;12(4):281–284. doi: 10.1016/0306-3623(81)90059-8. [DOI] [PubMed] [Google Scholar]
- Surprenant A. A comparative study of neuromuscular transmission in several mammalian muscular arteries. Pflugers Arch. 1980 Jul;386(1):85–91. doi: 10.1007/BF00584192. [DOI] [PubMed] [Google Scholar]
- Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. J Physiol. 1984 Jun;351:343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U'Prichard D. C., Mitrius J. C., Kahn D. J., Perry B. D. The alpha 2-adrenergic receptor: multiple affinity states and regulation of a receptor inversely coupled to adenylate cyclase. Adv Biochem Psychopharmacol. 1983;36:53–72. [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Intracellular study of tonic-type enteric neurons in guinea pig small intestine. J Neurophysiol. 1979 Mar;42(2):569–581. doi: 10.1152/jn.1979.42.2.569. [DOI] [PubMed] [Google Scholar]


