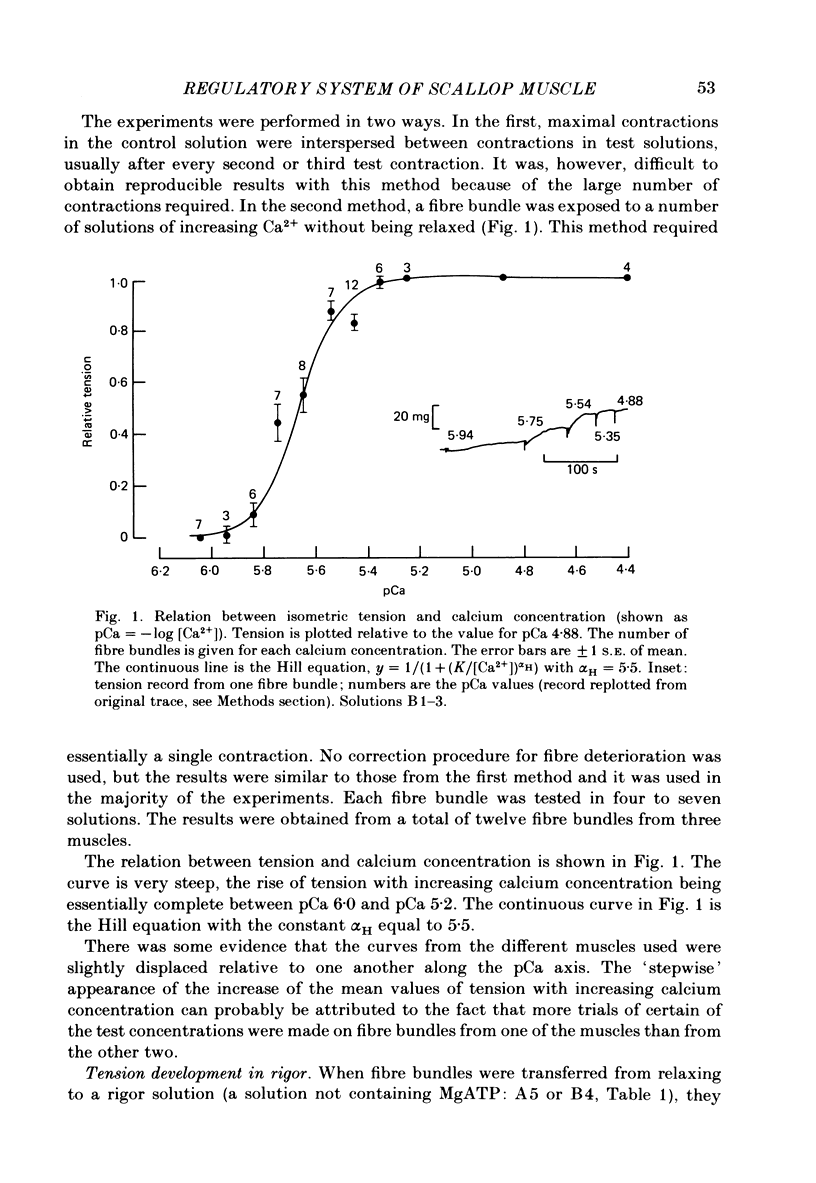
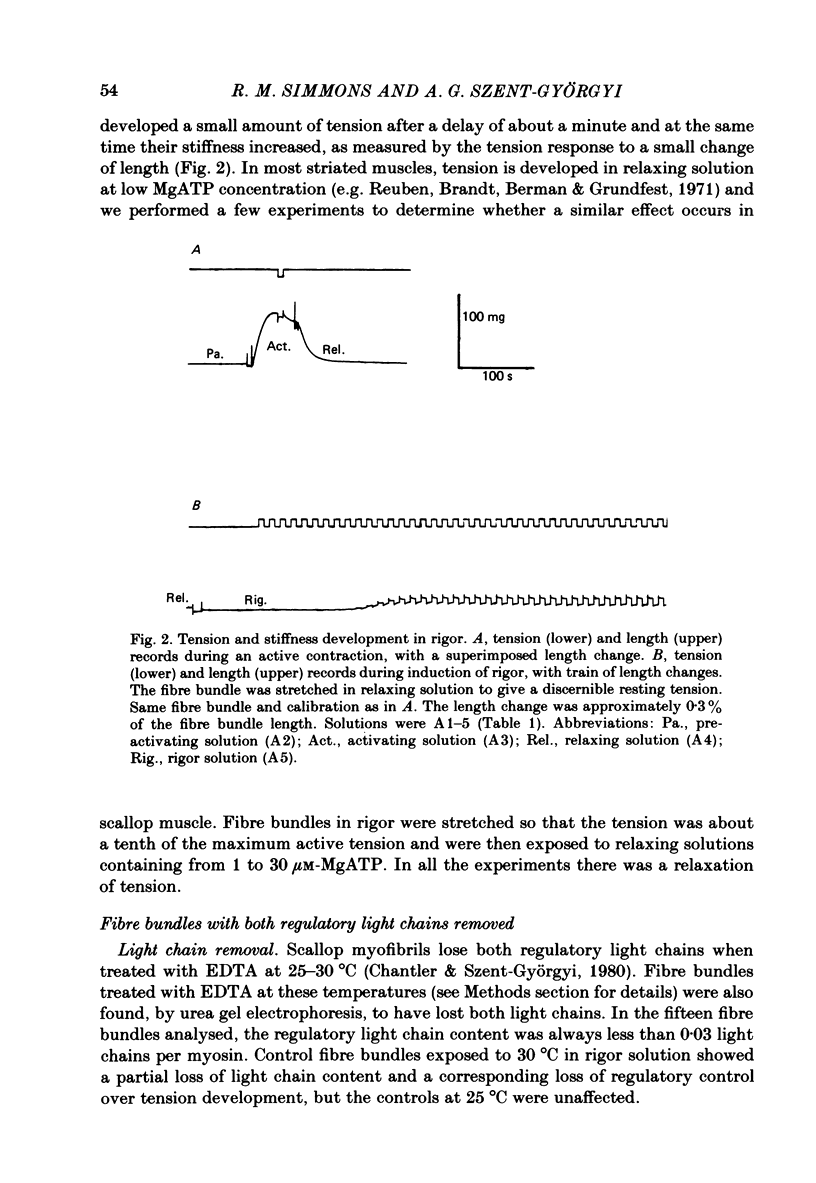
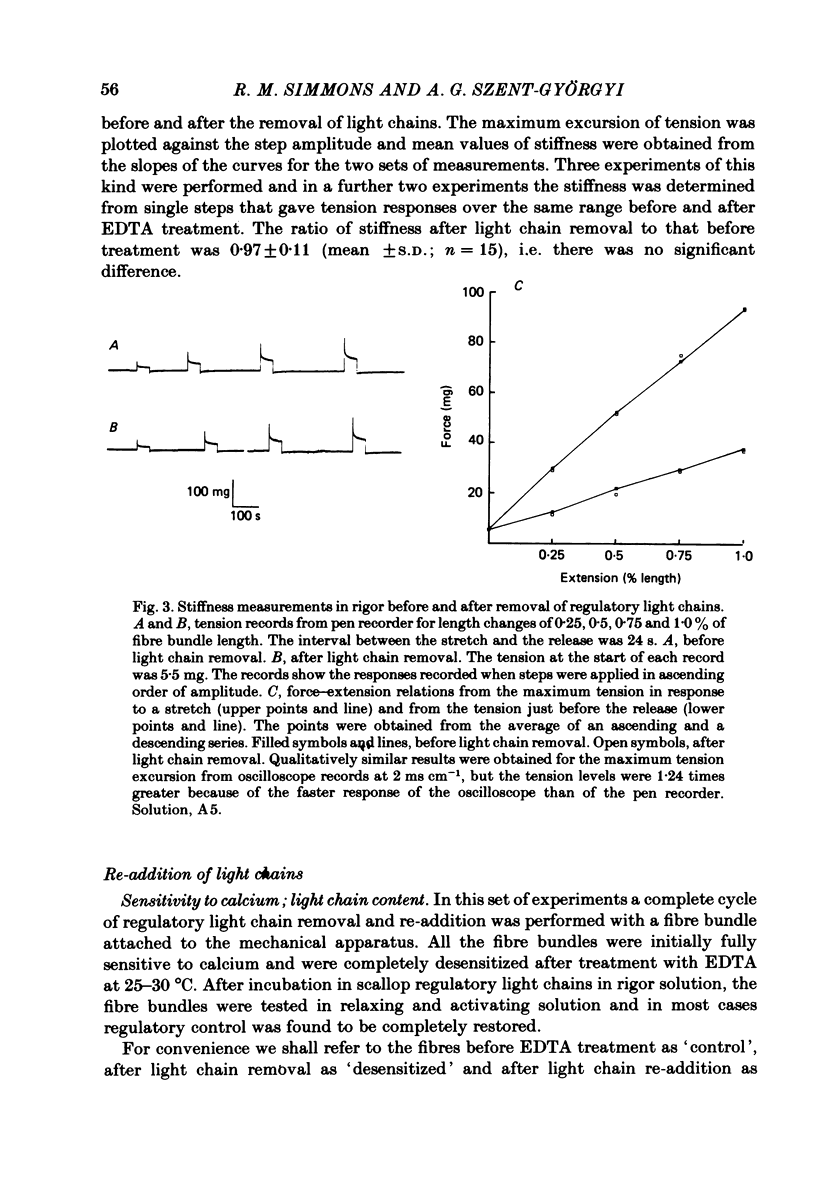
Abstract
Chemically skinned fibre bundles were prepared from the striated adductor muscle of the sea scallop, Placopecten magellanicus. The relation between tension and calcium concentration was determined in activating solutions containing 5 mM-MgATP, ionic strength 0.2, pH 7.1 at 20 degrees C. The isometric tension rose from zero to its maximum value between pCa 6.0 and 5.2. The steepness of the relation cannot be accounted for in terms of the binding of calcium to the two known sites on myosin and suggests that there must be an additional, co-operative mechanism. The regulatory light chain content of the fibre bundles was determined by urea gel electrophoresis and was found to be approximately 2 light chains per myosin molecule. The regulatory light chains were removed completely by treatment with EDTA at 25-30 degrees C. Fibre bundles then showed a total loss of control over contraction; a high tension was generated whether or not calcium was present in the bathing solution. Complete removal of the regulatory light chains did not greatly affect the tension generated or the stiffness in the rigor state. Control of contraction could be restored completely by the addition of regulatory light chains from scallop muscle. Treatment with EDTA at 0-12 degrees C resulted in the removal of 0.76-2.0 regulatory light chains per myosin molecule. Fibre bundles for which removal was less than complete were partially sensitive to calcium, i.e. tension was higher in the presence of calcium than in its absence. The results indicate that the normal mechanism of tension generation in scallop muscle is mediated primarily through myosin and not thin filament control. This finding is consistent with previous studies of the ATPase activity of myofibrils from scallop muscle.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Moisescu D. G. Tension changes in isolated bundles of frog and barnacle myofibrils in response to sudden changes in the external free calcium concentration. J Physiol. 1973 Aug;233(1):8P–9P. [PubMed] [Google Scholar]
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D. Myosin linked calcium regulation in vertebrate smooth muscle. Nature. 1974 Nov 29;252(5482):405–407. doi: 10.1038/252405a0. [DOI] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Chacko S., Conti M. A., Adelstein R. S. Effect of phosphorylation of smooth muscle myosin on actin activation and Ca2+ regulation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):129–133. doi: 10.1073/pnas.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler P. D., Sellers J. R., Szent-Györgyi A. G. Cooperativity in scallop myosin. Biochemistry. 1981 Jan 6;20(1):210–216. doi: 10.1021/bi00504a035. [DOI] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Regulatory light-chains and scallop myosin. Full dissociation, reversibility and co-operative effects. J Mol Biol. 1980 Apr 15;138(3):473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- EBASHI S., EBASHI F. A NEW PROTEIN COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF MYOSIN B. J Biochem. 1964 Jun;55:604–613. doi: 10.1093/oxfordjournals.jbchem.a127933. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol. 1981 Feb;311:219–249. doi: 10.1113/jphysiol.1981.sp013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORNALL A. G., BARDAWILL C. J., DAVID M. M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949 Feb;177(2):751–766. [PubMed] [Google Scholar]
- Goldberg A., Lehman W. Troponin-like proteins from muscles of the scallop, Aequipecten irradians. Biochem J. 1978 May 1;171(2):413–418. doi: 10.1042/bj1710413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Two rigor states in skinned crayfish single muscle fibers. J Gen Physiol. 1976 Sep;68(3):267–280. doi: 10.1085/jgp.68.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick-Jones J., Jakes R., Tooth P., Craig R., Scholey J. Role of the myosin light chains in the regulation of contractile activity. Soc Gen Physiol Ser. 1982;37:255–272. [PubMed] [Google Scholar]
- Kendrick-Jones J., Lehman W., Szent-Györgyi A. G. Regulation in molluscan muscles. J Mol Biol. 1970 Dec 14;54(2):313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory light chains in myosins. J Mol Biol. 1976 Jul 15;104(4):747–775. doi: 10.1016/0022-2836(76)90180-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman W., Szent-Györgyi A. G. Regulation of muscular contraction. Distribution of actin control and myosin control in the animal kingdom. J Gen Physiol. 1975 Jul;66(1):1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman W. Thin-filament-linked regulation in molluscan muscles. Biochim Biophys Acta. 1981 May 29;668(3):349–356. doi: 10.1016/0005-2795(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Matsubara I., Elliott G. F. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–669. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall J. A. Mechanics and energetics of contraction in striated muscle of the sea scallop, Placopecten magellanicus. J Physiol. 1981 Dec;321:287–295. doi: 10.1113/jphysiol.1981.sp013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben J. P., Brandt P. W., Berman M., Grundfest H. Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa is greater than 9). J Gen Physiol. 1971 Apr;57(4):385–407. doi: 10.1085/jgp.57.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway E. B., Gordon A. M., Martyn D. A. Hysteresis in the force-calcium relation in muscle. Science. 1983 Mar 4;219(4588):1075–1077. doi: 10.1126/science.6823567. [DOI] [PubMed] [Google Scholar]
- Sanger J. W. Sarcoplasmic reticulum in the cross-striated adductor muscle of the bay scallop, Aequipecten irridians. Z Zellforsch Mikrosk Anat. 1971;118(2):156–161. doi: 10.1007/BF00341560. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Taylor K. A., Kendrick-Jones J. The role of myosin light chains in regulating actin-myosin interaction. Biochimie. 1981 Apr;63(4):255–271. doi: 10.1016/s0300-9084(81)80115-0. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Chantler P. D., Szent-Györgyi A. G. Hybrid formation between scallop myofibrils and foreign regulatory light-chains. J Mol Biol. 1980 Dec 15;144(3):223–245. doi: 10.1016/0022-2836(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- Simmons R. M., Szent-Györgyi A. G. Control of tension development in scallop muscle fibres with foreign regulatory light chains. Nature. 1980 Aug 7;286(5773):626–628. doi: 10.1038/286626a0. [DOI] [PubMed] [Google Scholar]
- Simmons R. M., Szent-Györgyi A. G. Reversible loss of calcium control of tension in scallop striated muscle associated with the removal of regulatory light chains. Nature. 1978 May 4;273(5657):62–64. doi: 10.1038/273062a0. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Myosin-linked calcium regulation in vertebrate smooth muscle. J Mol Biol. 1976 Mar 25;102(1):75–92. doi: 10.1016/0022-2836(76)90074-7. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Konno K., Arai K., Watanabe S. ATP-induced tension development in glycerinated fibers of scallop adductor striated muscle. Role of regulatory light chain of myosin in calcium regulation of muscle contraction. J Biochem. 1980 Sep;88(3):909–911. doi: 10.1093/oxfordjournals.jbchem.a133047. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Toyo-Oka T. Effects of various concentrations of MgATP on the superprecipitation and ATPase activity of scallop striated muscle myosin B1. J Biochem. 1979 Mar;85(3):871–877. [PubMed] [Google Scholar]
- Vibert P., Szent-Györgyi A. G., Craig R., Wray J., Cohen C. Changes in crossbridge attachment in a myosin-regulated muscle. Nature. 1978 May 4;273(5657):64–66. doi: 10.1038/273064a0. [DOI] [PubMed] [Google Scholar]


