Abstract
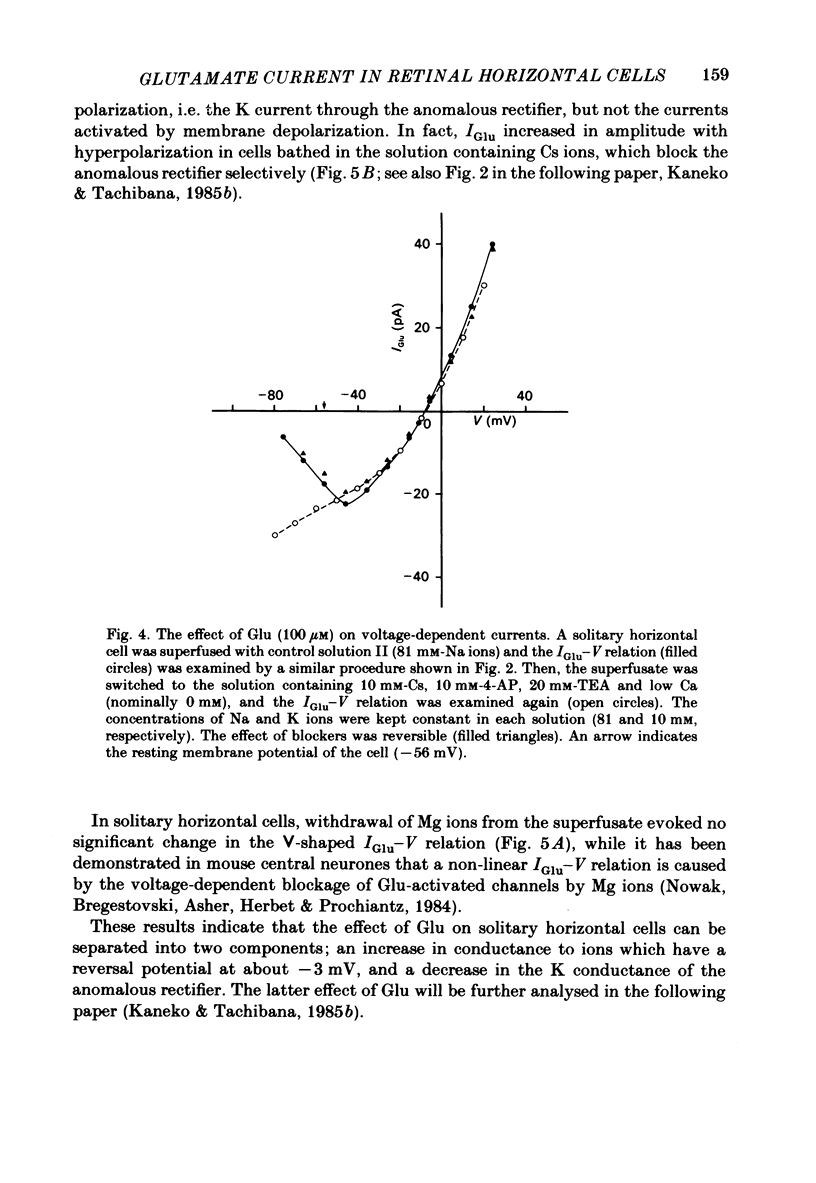
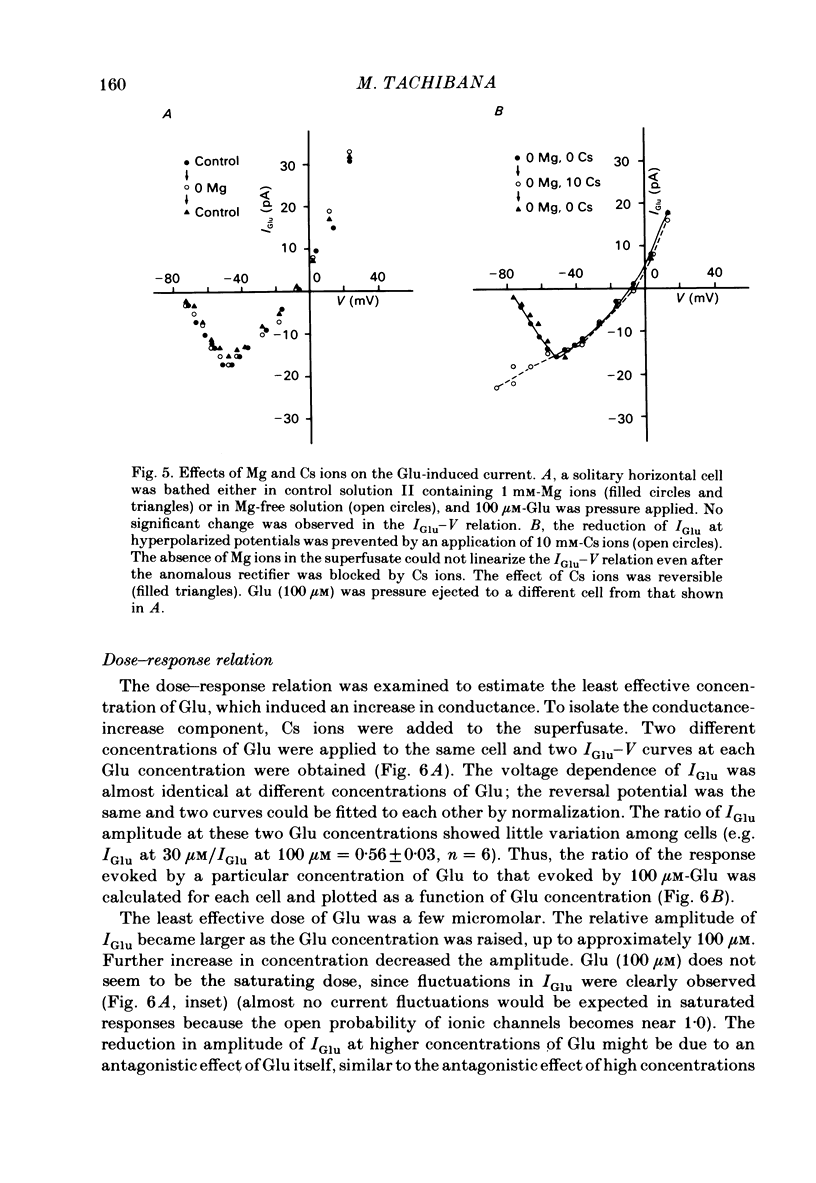
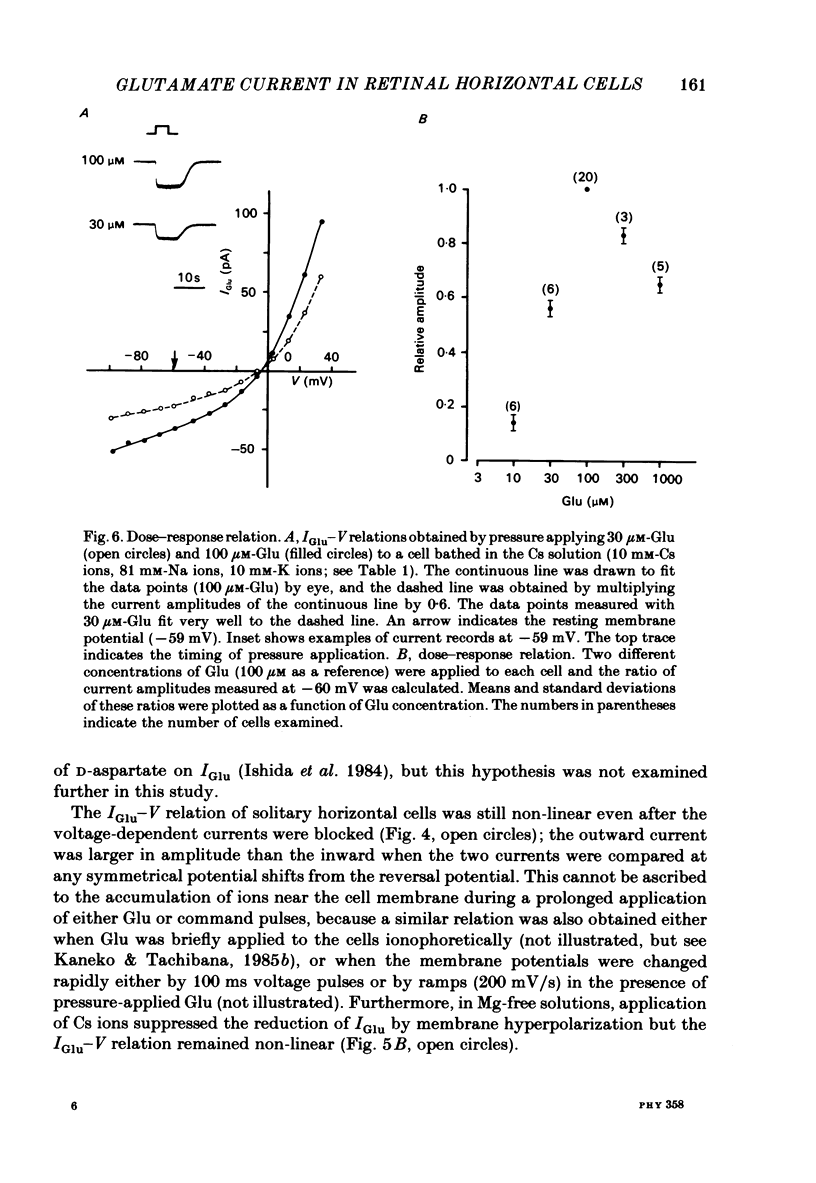
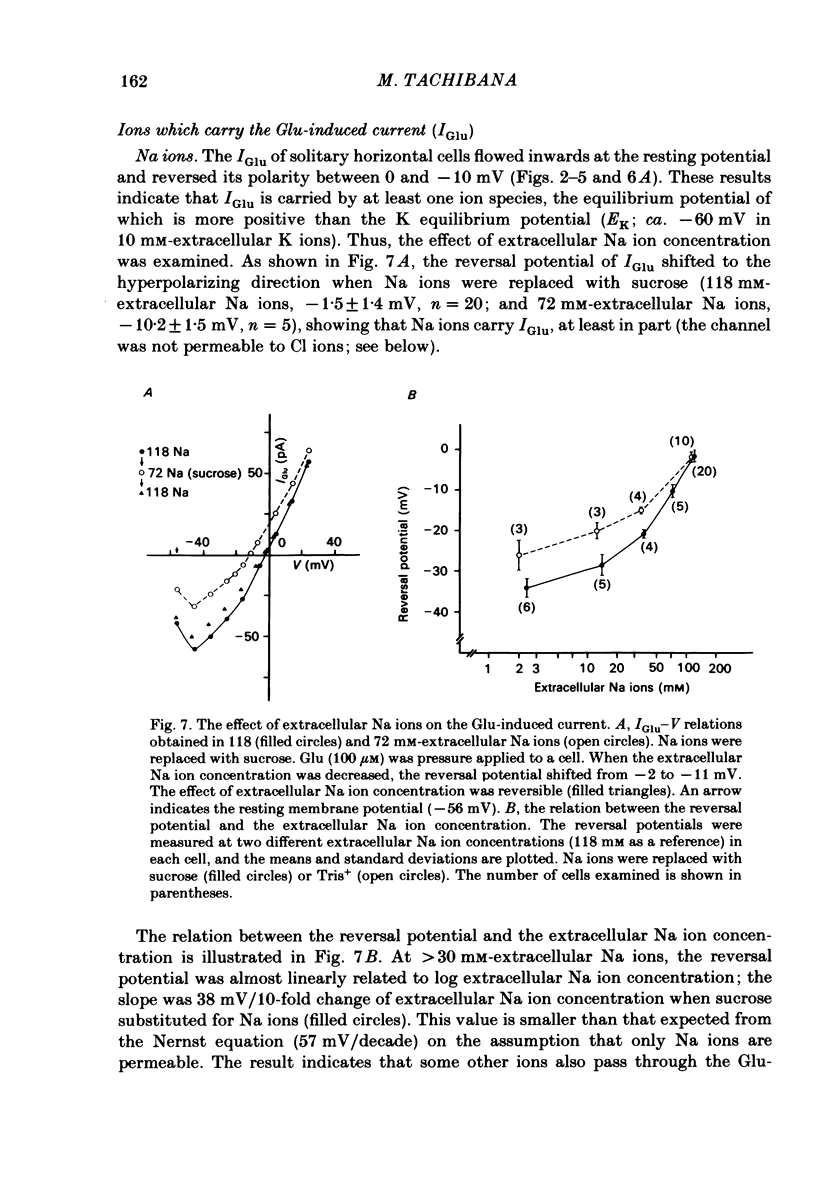
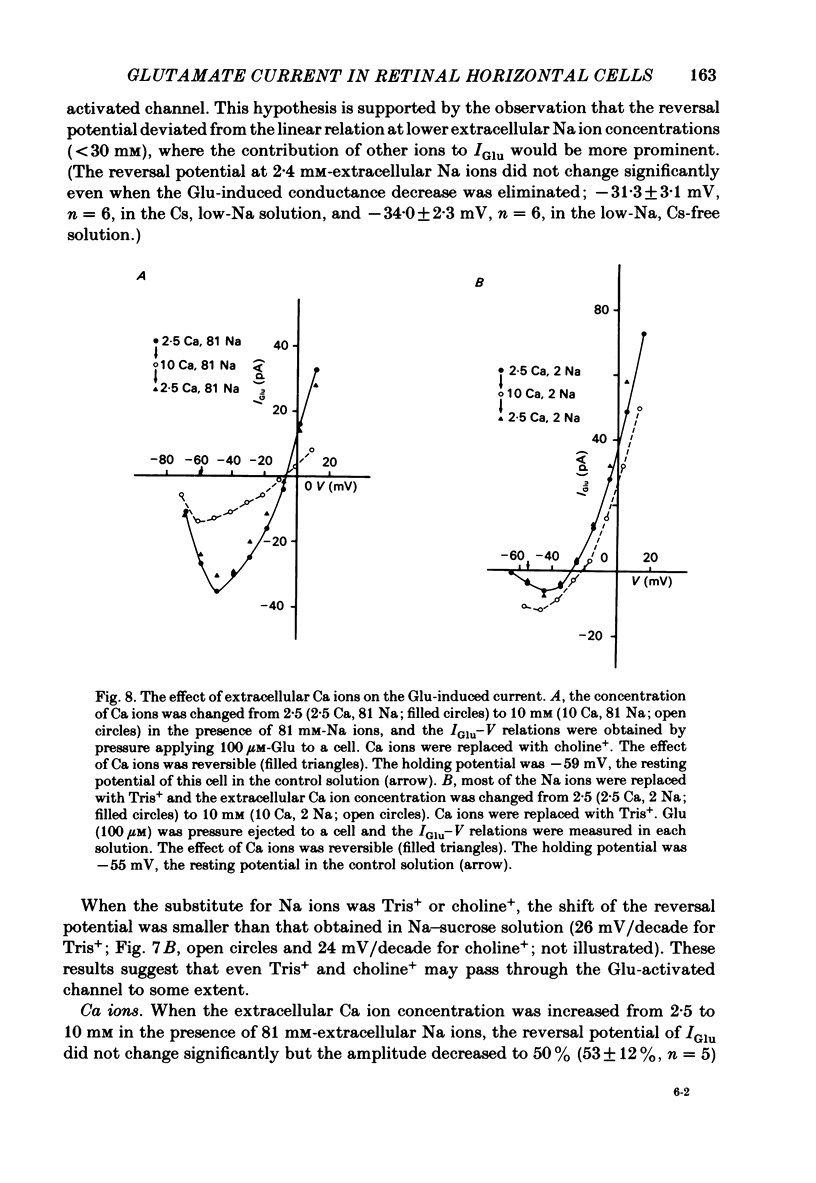
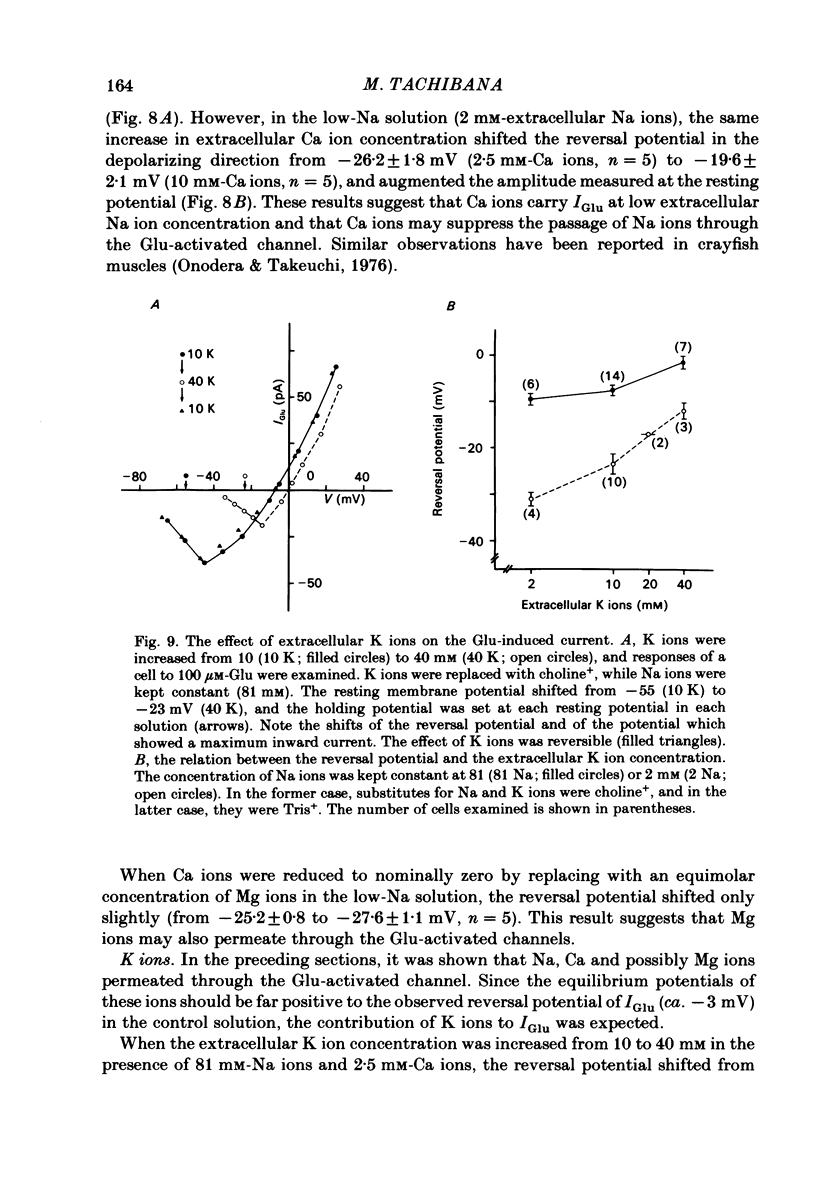
Solitary horizontal cells isolated from goldfish retinae are depolarized by L-glutamate (Glu) (Ishida, Kaneko & Tachibana, 1984), a possible candidate for the transmitter of photoreceptors. The underlying mechanisms were analysed under voltage-clamp conditions using 'giga-seal' suction pipettes in the whole-cell recording configuration. Glu induced an inward current at the resting membrane potential (ca. -57 mV). Membrane depolarization decreased the amplitude of Glu-induced current and reversed its polarity to outward beyond approximately -3 mV. Membrane hyperpolarization below the resting potential decreased the amplitude of the Glu-induced inward current. When a K current through the anomalous rectifier, which is activated by membrane hyperpolarization (Tachibana, 1983), was blocked by Cs ions, this phenomenon disappeared and the Glu-induced current increased in amplitude with hyperpolarization. Mg ions had no effect on the reduction of the Glu-induced current at hyperpolarized potentials. It was strongly suggested that Glu produced two types of conductance change; a conductance increase due to an activation of Glu channels and a conductance decrease due to a blockage of the K current through the anomalous rectifier. The latter effect is analysed in detail in the following paper (Kaneko & Tachibana, 1985b). The Glu-activated channel was permeable to cations (Na, K, Ca, Mg, Tris and choline ions) with low selectivity, but not to anions. The least effective dose of Glu was less than 10 microM. The relation between the Glu-induced current and the membrane potential curved upwards near the reversal potential, and this relation was not affected by Mg ions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate current noise: post-synaptic channel kinetics investigated under voltage clamp. J Physiol. 1978 Sep;282:219–242. doi: 10.1113/jphysiol.1978.sp012459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. The effect of foreign cations, pH and pharmacological agents on the ionic permeability of an excitatory glutamate synapse. J Physiol. 1977 Dec;273(2):389–404. doi: 10.1113/jphysiol.1977.sp012100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon C., Lam D. M. L-glutamic acid: a neurotransmitter candidate for cone photoreceptors in human and rat retinas. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5117–5121. doi: 10.1073/pnas.80.16.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzov A. L., Trifonov Y. A., Chailahian L. M., Golubtzov K. W. Amplification of graded potentials in horizontal cells of the retina. Vision Res. 1977 Feb;17(2):265–273. doi: 10.1016/0042-6989(77)90090-6. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Parker I. Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J Physiol. 1981 Dec;321:195–210. doi: 10.1113/jphysiol.1981.sp013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer T. M., Adams D. J., Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980 May;75(5):469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. The selectivity of ion channels in nerve and muscle. Neuroscience. 1982 Jun;7(6):1335–1366. doi: 10.1016/0306-4522(82)90249-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Kaneko A., Tachibana M. Responses of solitary retinal horizontal cells from Carassius auratus to L-glutamate and related amino acids. J Physiol. 1984 Mar;348:255–270. doi: 10.1113/jphysiol.1984.sp015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D., Lam D. M. Regenerative and passive membrane properties of isolated horizontal cells from a teleost retina. Nature. 1981 Jul 30;292(5822):451–454. doi: 10.1038/292451a0. [DOI] [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Effects of external ions on the synaptic transmission from photorecptors to horizontal cells in the carp retina. J Physiol. 1975 Nov;252(2):509–522. doi: 10.1113/jphysiol.1975.sp011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. A voltage-clamp analysis of membrane currents in solitary bipolar cells dissociated from Carassius auratus. J Physiol. 1985 Jan;358:131–152. doi: 10.1113/jphysiol.1985.sp015544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Tachibana M. Effects of L-glutamate on the anomalous rectifier potassium current in horizontal cells of Carassius auratus retina. J Physiol. 1985 Jan;358:169–182. doi: 10.1113/jphysiol.1985.sp015546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E. Carp horizontal cells in culture respond selectively to L-glutamate and its agonists. Proc Natl Acad Sci U S A. 1982 Feb;79(3):936–940. doi: 10.1073/pnas.79.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R. E., Lam D. M. Uptake of aspartic and glutamic acid by photoreceptors in goldfish retina. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7185–7189. doi: 10.1073/pnas.78.11.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. M., Schwartz E. A. Evidence for the identification of synaptic transmitters released by photoreceptors of the toad retina. J Physiol. 1983 Jan;334:325–349. doi: 10.1113/jphysiol.1983.sp014497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Otsu K., Otsuka T. Effects of chemicals on receptors and horizontal cells in the retina. J Physiol. 1972 Dec;227(3):899–913. doi: 10.1113/jphysiol.1972.sp010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Permeability changes produced by L-glutamate at the excitatory post-synaptic membrane of the crayfish muscle. J Physiol. 1976 Mar;255(3):669–685. doi: 10.1113/jphysiol.1976.sp011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Gration K. A., Usherwood P. N. Single glutamate-activated channels in locust muscle. Nature. 1979 Apr 12;278(5705):643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Shingai R., Christensen B. N. Sodium and calcium currents measured in isolated catfish horizontal cells under voltage clamp. Neuroscience. 1983 Nov;10(3):893–897. doi: 10.1016/0306-4522(83)90227-0. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Ionic currents of solitary horizontal cells isolated from goldfish retina. J Physiol. 1983 Dec;345:329–351. doi: 10.1113/jphysiol.1983.sp014981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Membrane properties of solitary horizontal cells isolated from goldfish retina. J Physiol. 1981 Dec;321:141–161. doi: 10.1113/jphysiol.1981.sp013976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waloga G., Pak W. L. Ionic mechanism for the generation of horizontal cell potentials in isolated axolotl retina. J Gen Physiol. 1978 Jan;71(1):69–92. doi: 10.1085/jgp.71.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]


