Abstract
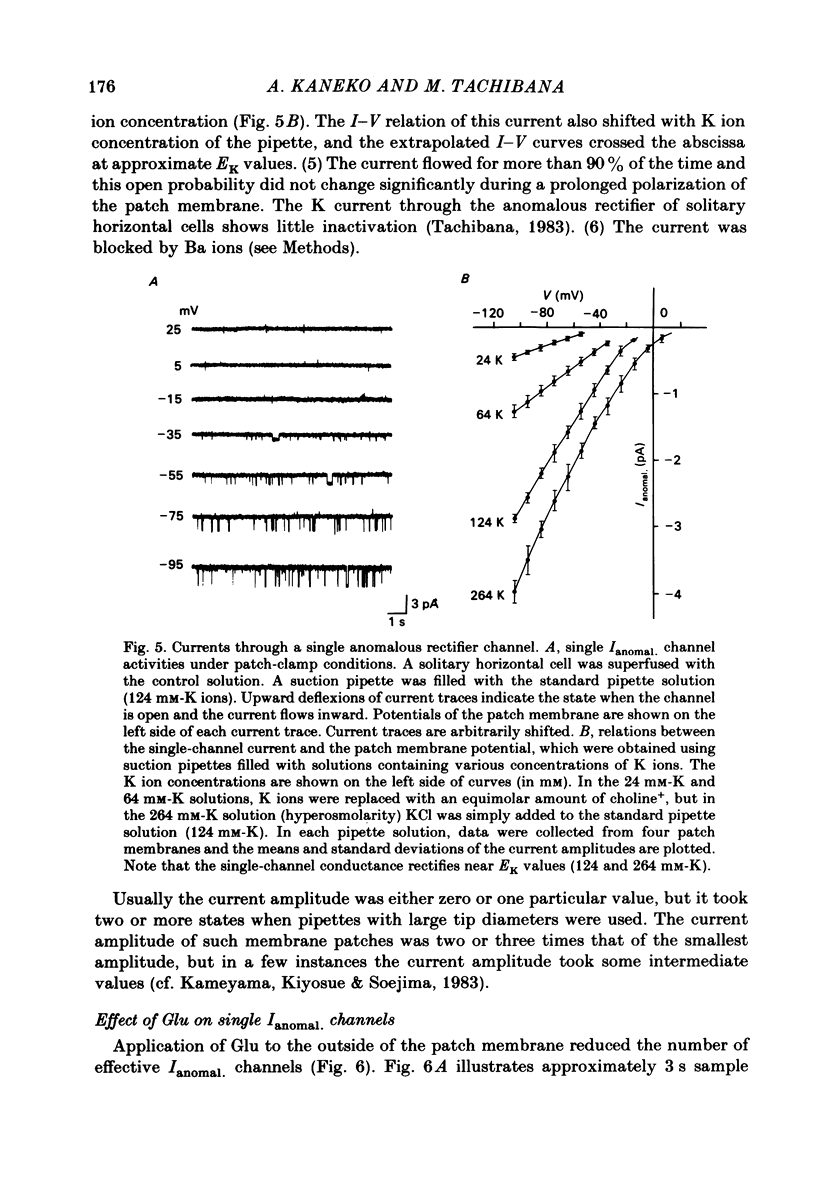
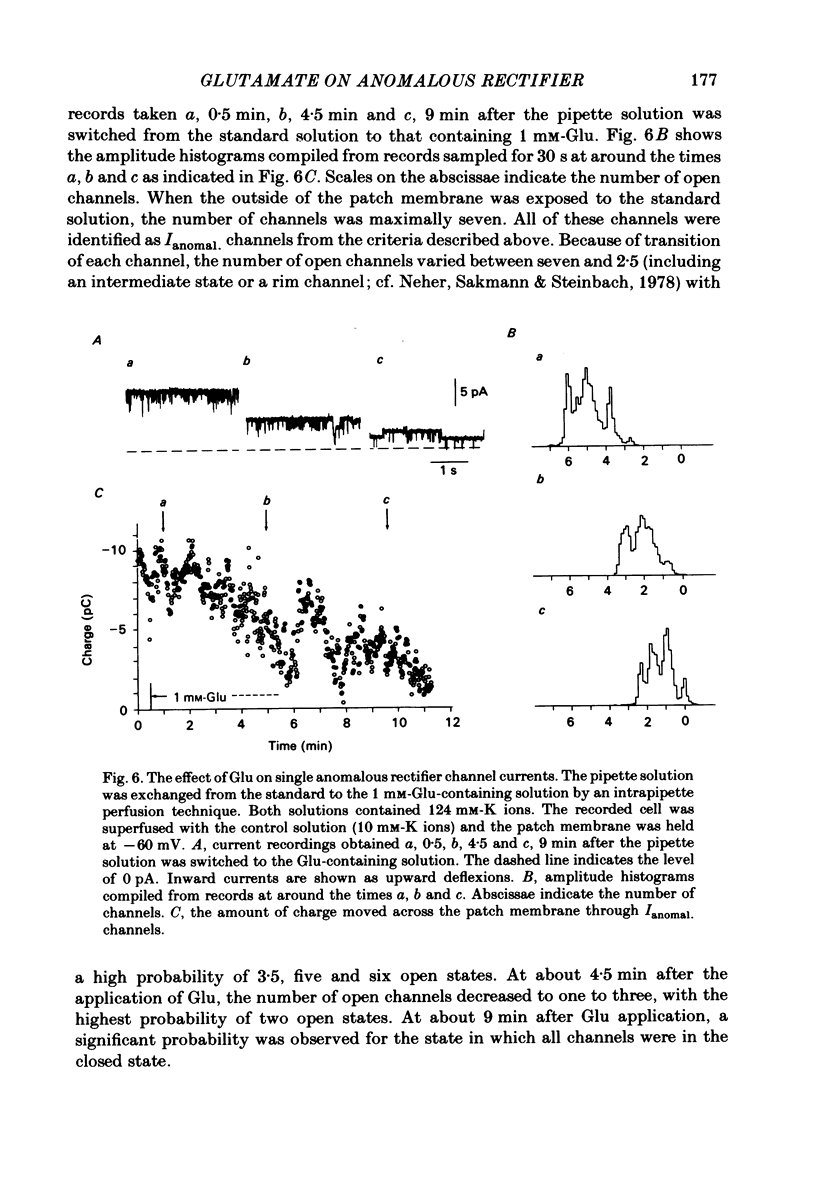
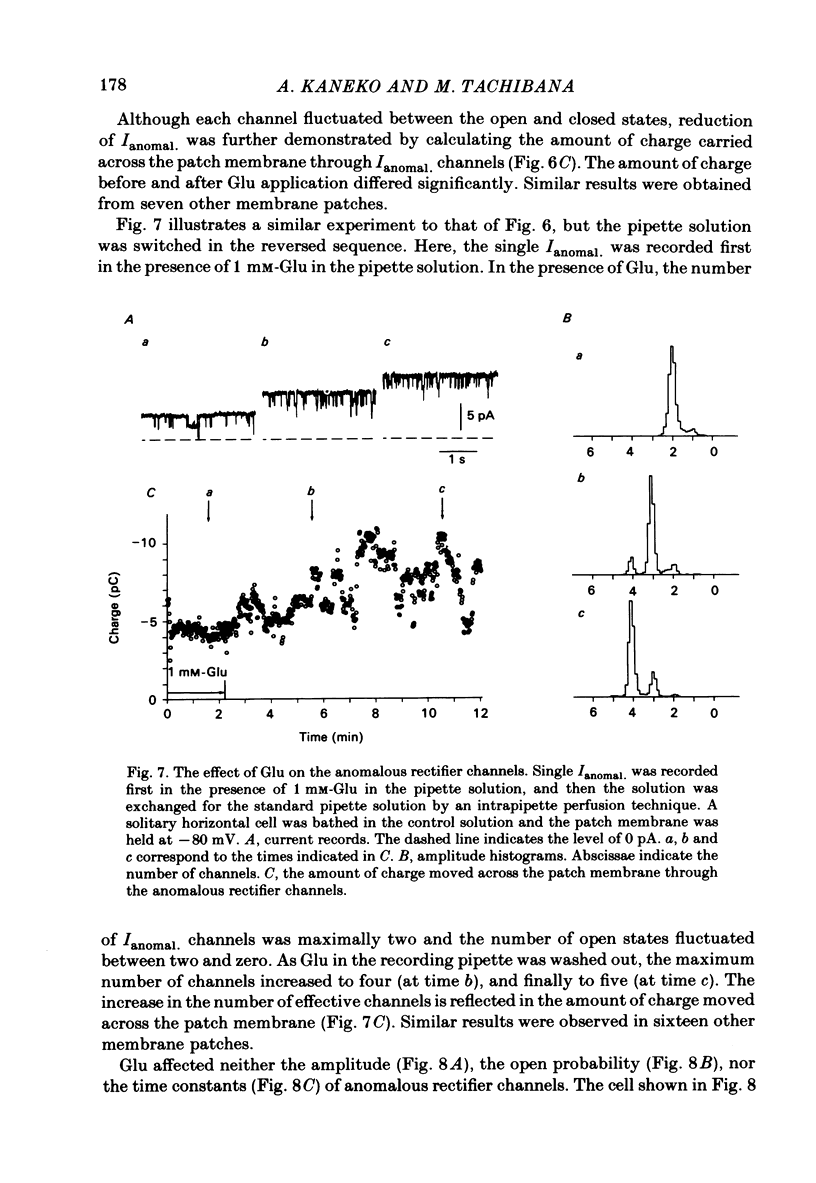
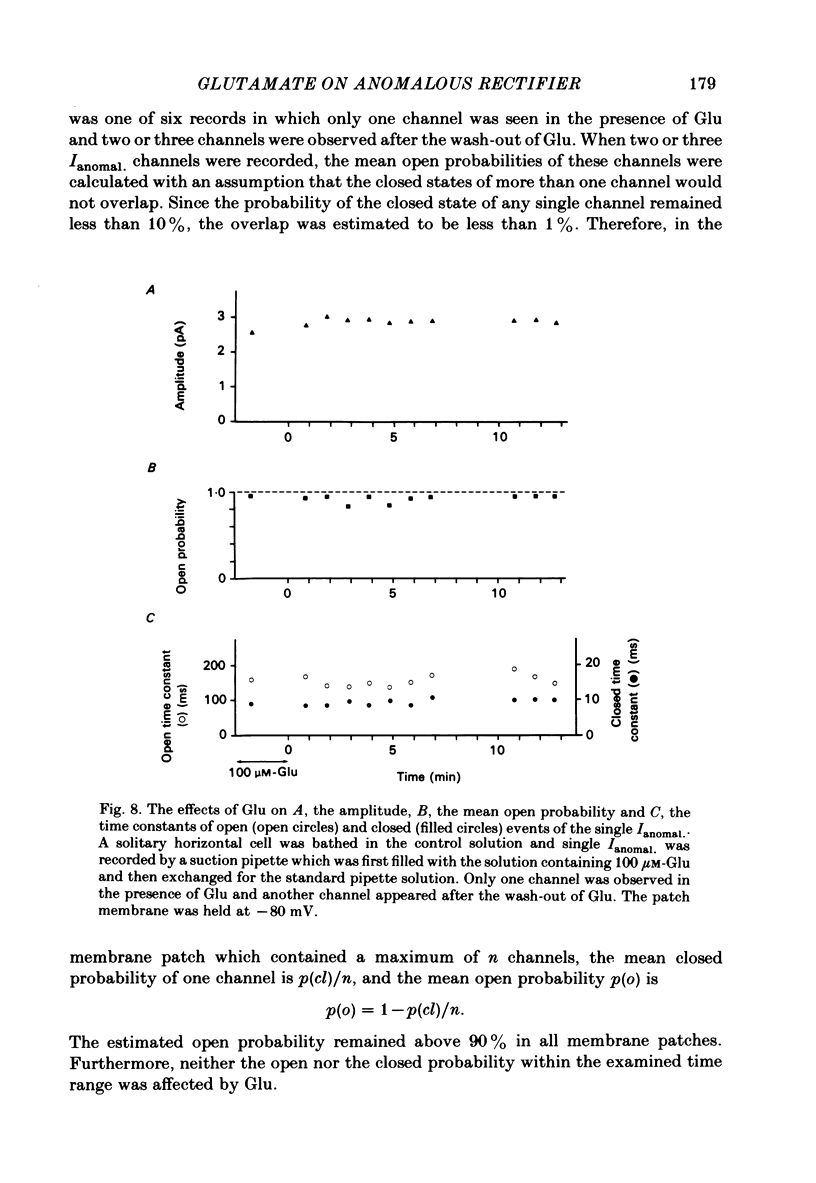
The effects of externally applied L-glutamate (Glu) on K currents through the anomalous rectifier were studied in solitary horizontal cells dissociated from goldfish retinae under whole-cell voltage-clamp or cell-attached patch-clamp conditions using 'giga-seal' suction pipettes. In the whole-cell clamp experiments, hyperpolarization of the membrane below the resting potential (ca. -57 mV) induced a large voltage-dependent inward current which has been identified as the K current through the anomalous rectifier (Ianomal.). Application of Glu to the external medium reduced Ianomal.. Reduction of the inward current was not seen in preparations in which Ianomal. has been blocked by an application of Cs or Ba ions to the external medium. Single-channel currents through the anomalous rectifier were recorded under cell-attached patch-clamp conditions. The current showed an inward rectification; its amplitude increased with hyperpolarization of the patch membrane, and became below the noise level near the equilibrium potential of K ions (EK). No polarity reversal was observed even by a strong membrane depolarization. The patch membrane potential at which the current amplitude became undetectable shifted in parallel to the shift of EK. The open probability changed little with polarization of the patch membrane. When Glu (greater than 100 microM) was applied to the outside of the patch membrane, the number of available Ianomal. channels was decreased, but neither the single-channel conductance, open or closed time constants, nor the open probability changed significantly. Removal of Glu produced the opposite sequence; i.e. the number of available Ianomal. channels increased with time. It was concluded that the reduction of the Glu-induced current at hyperpolarized potentials in the whole-cell recording configuration is due to the blocking action of Glu on Ianomal..
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Engberg I., Flatman J. A., Lambert J. D. The actions of excitatory amino acids on motoneurones in the feline spinal cord. J Physiol. 1979 Mar;288:227–261. [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y. Blocking kinetics of the anomalous potassium rectifier of tunicate egg studied by single channel recording. J Physiol. 1982 Oct;331:311–331. doi: 10.1113/jphysiol.1982.sp014374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Kaneko A., Tachibana M. Responses of solitary retinal horizontal cells from Carassius auratus to L-glutamate and related amino acids. J Physiol. 1984 Mar;348:255–270. doi: 10.1113/jphysiol.1984.sp015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Kiyosue T., Soejima M. Single channel analysis of the inward rectifier K current in the rabbit ventricular cells. Jpn J Physiol. 1983;33(6):1039–1056. doi: 10.2170/jjphysiol.33.1039. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Yoshida S., Hagiwara S. Single K+ channel currents of anomalous rectification in cultured rat myotubes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4960–4964. doi: 10.1073/pnas.78.8.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Velumian A. A. Mechanisms of post-synaptic excitation in amphibian motoneurones. J Physiol. 1978 Jun;279:437–455. doi: 10.1113/jphysiol.1978.sp012355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Ionic currents of solitary horizontal cells isolated from goldfish retina. J Physiol. 1983 Dec;345:329–351. doi: 10.1113/jphysiol.1983.sp014981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M. Permeability changes induced by L-glutamate in solitary retinal horizontal cells isolated from Carassius auratus. J Physiol. 1985 Jan;358:153–167. doi: 10.1113/jphysiol.1985.sp015545. [DOI] [PMC free article] [PubMed] [Google Scholar]


