Abstract
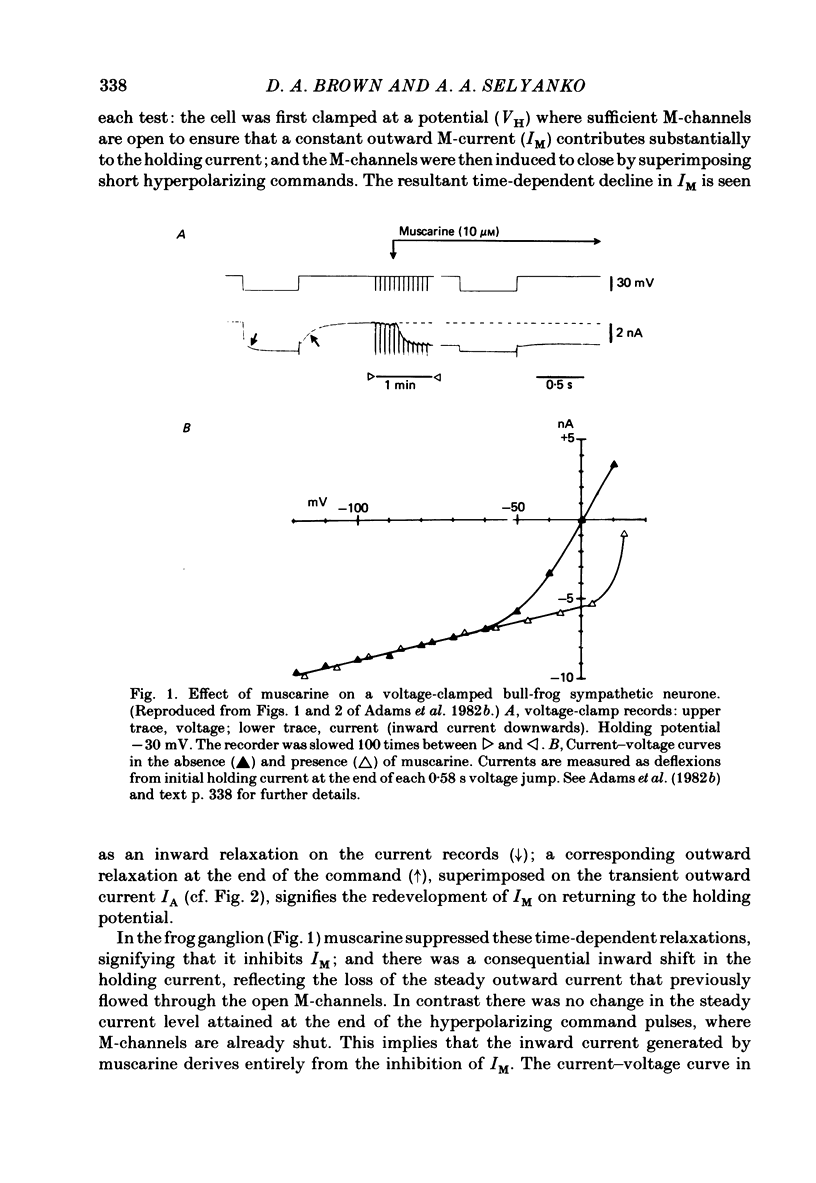
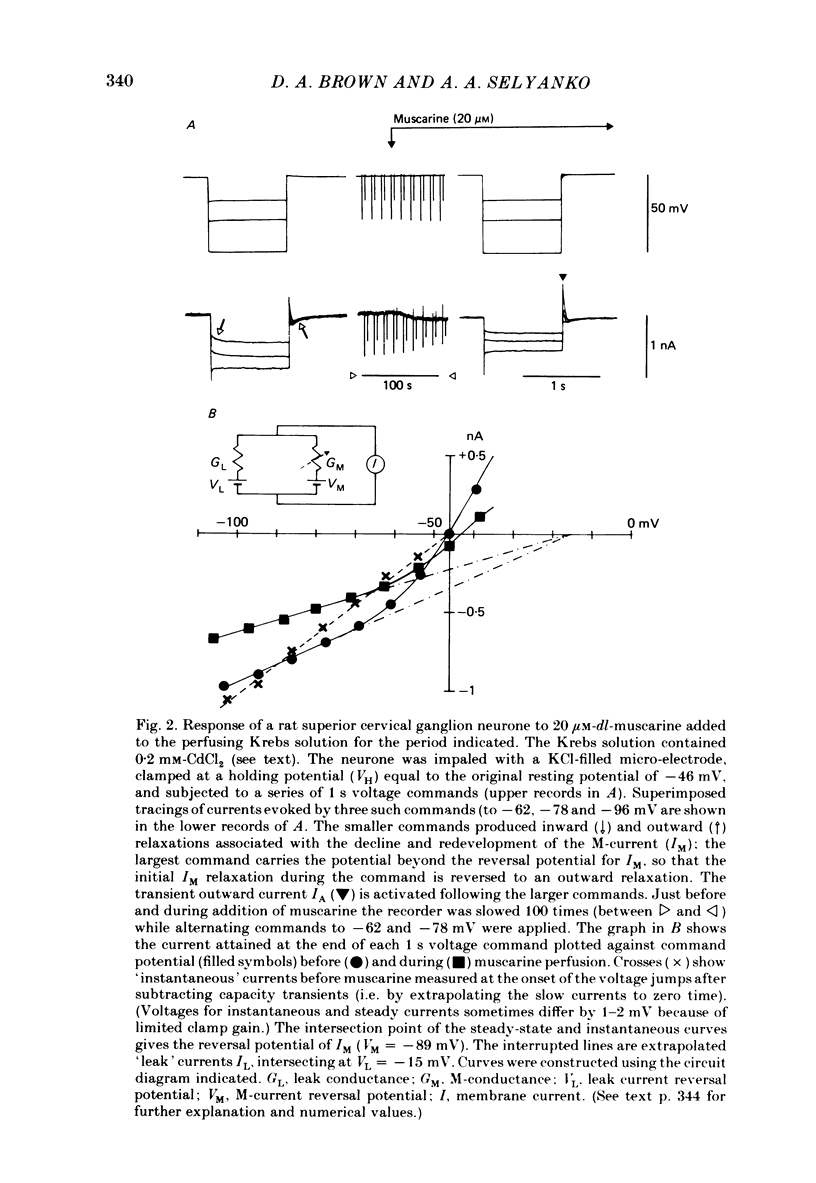
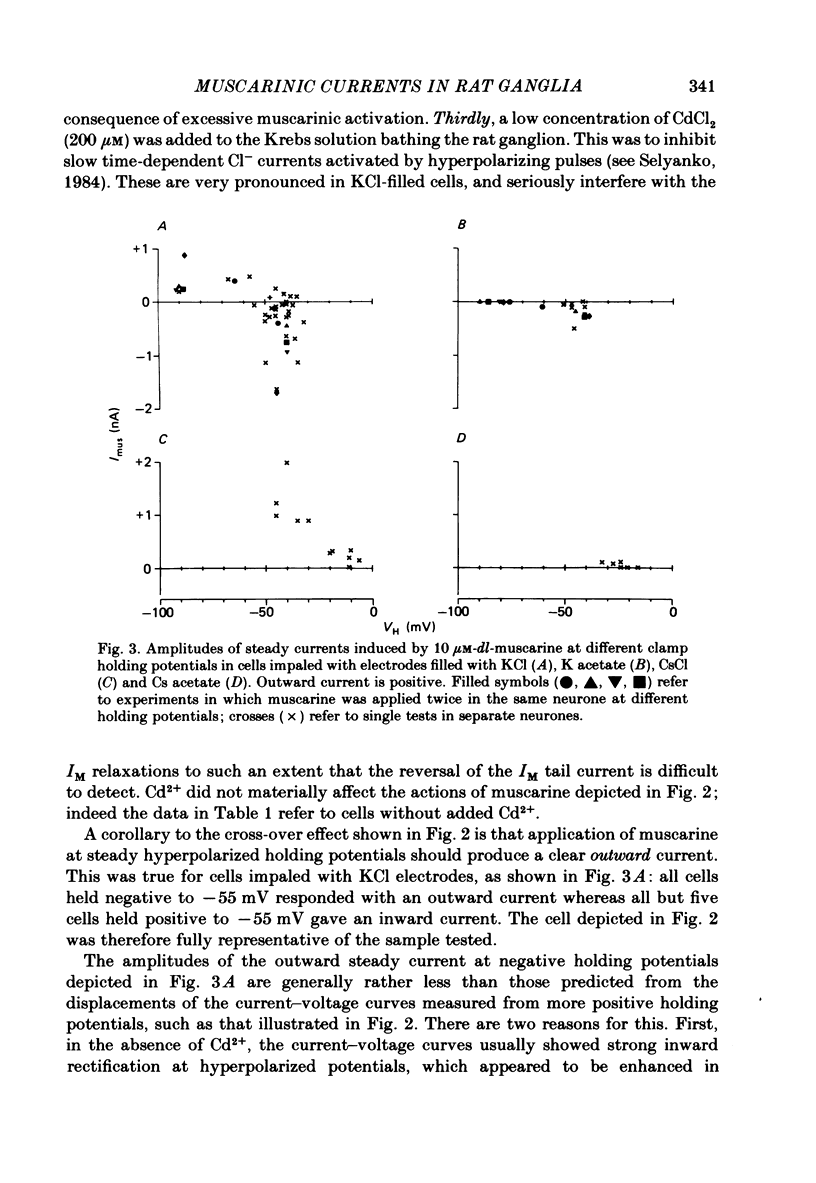
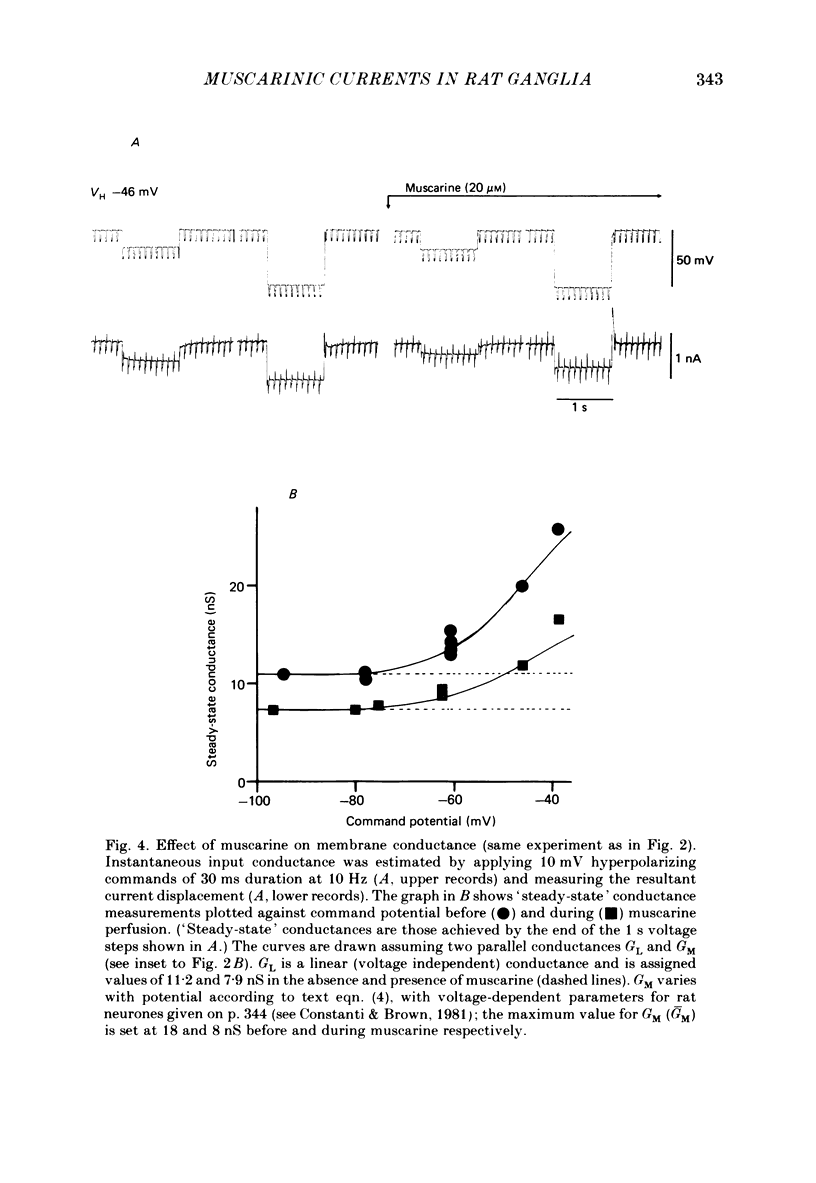
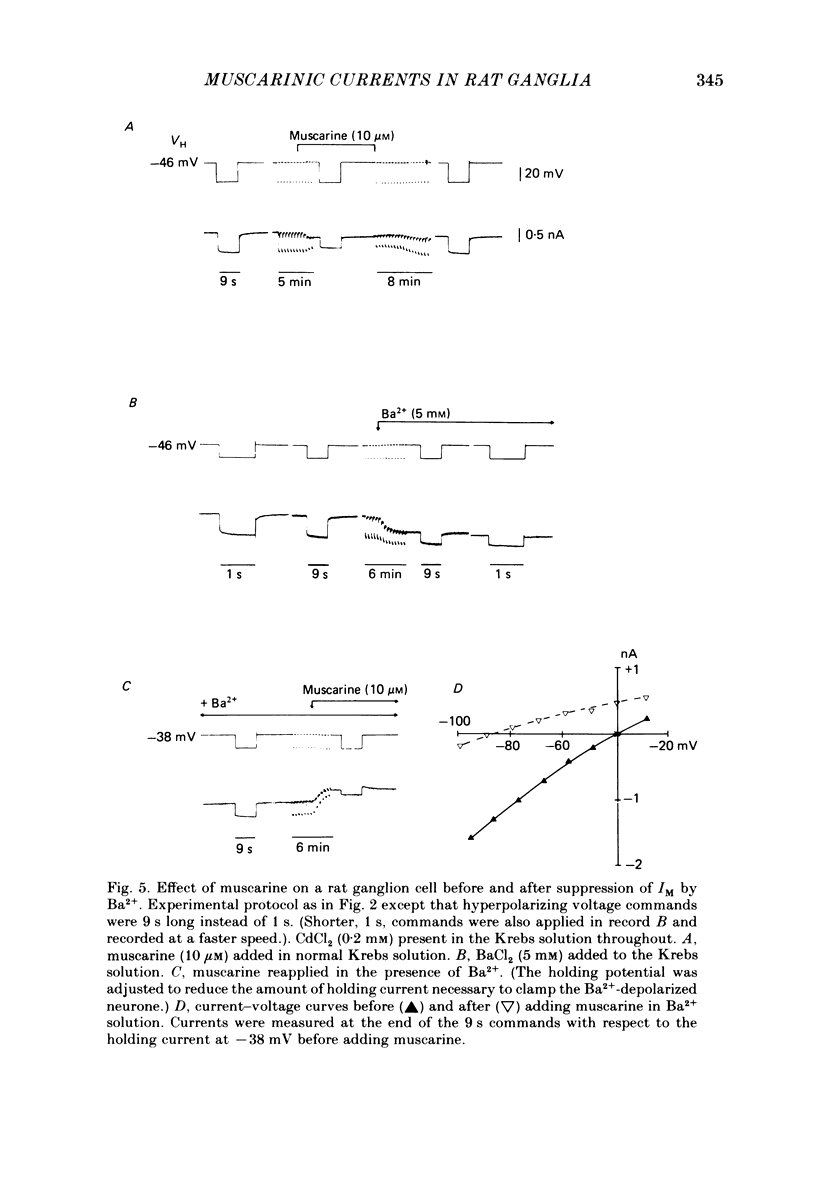
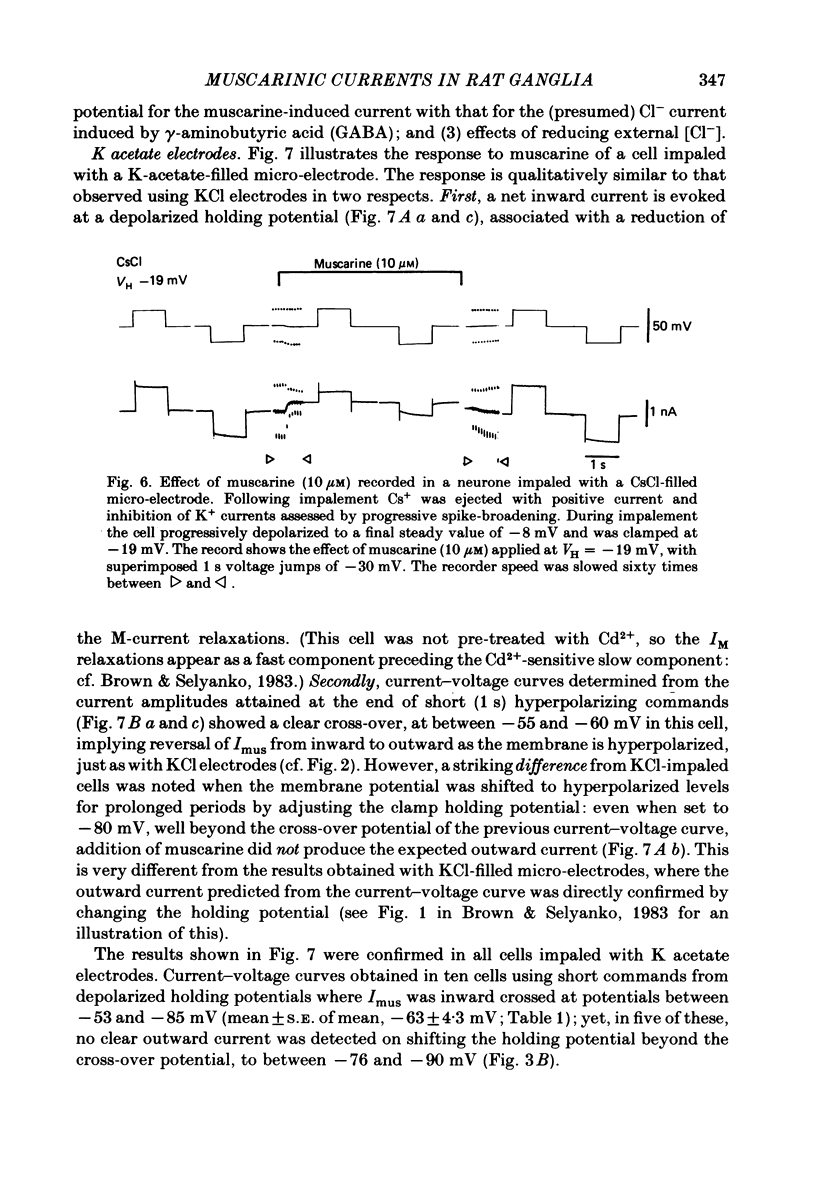
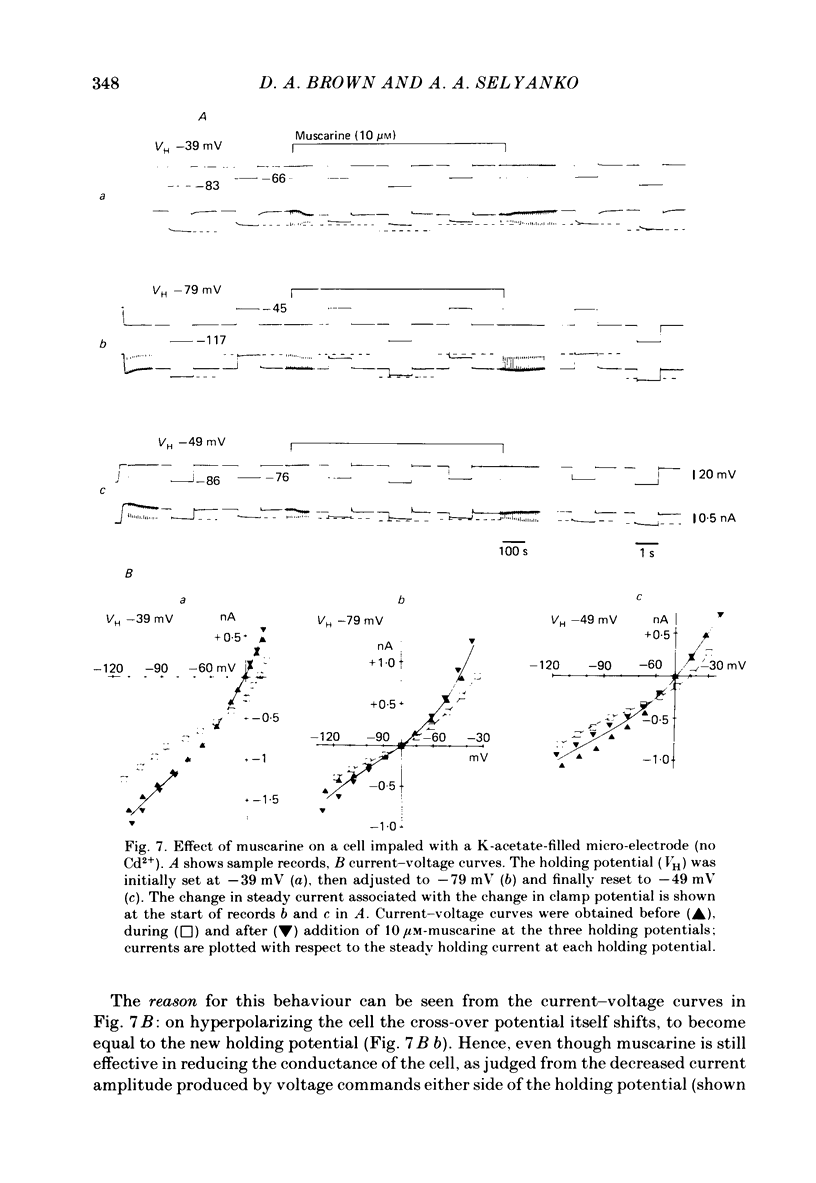
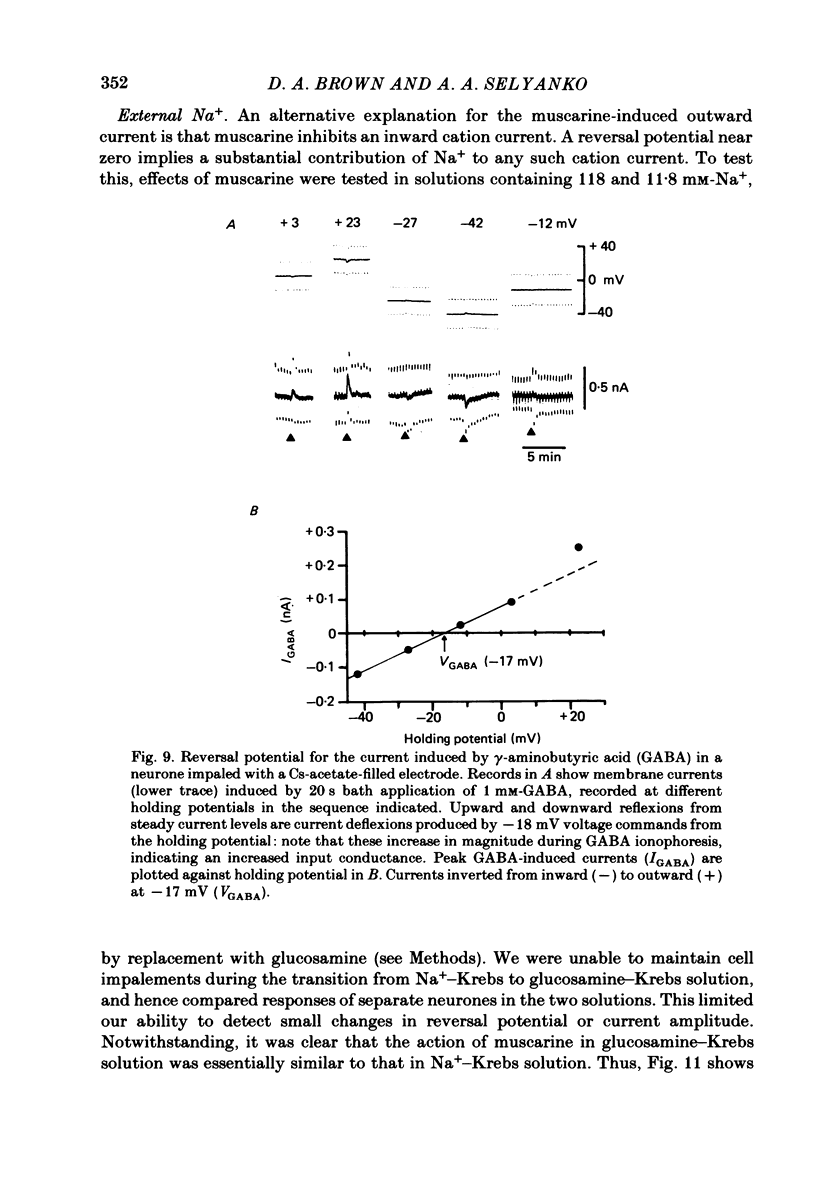
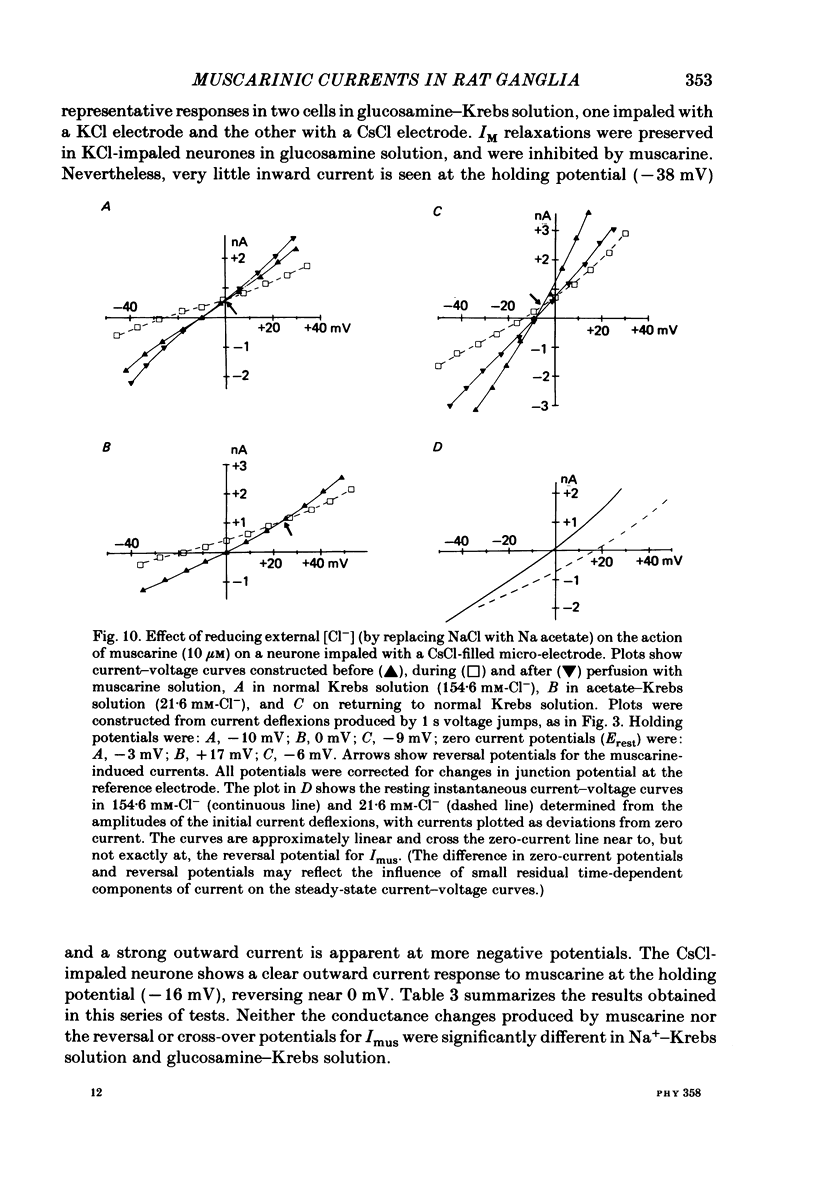
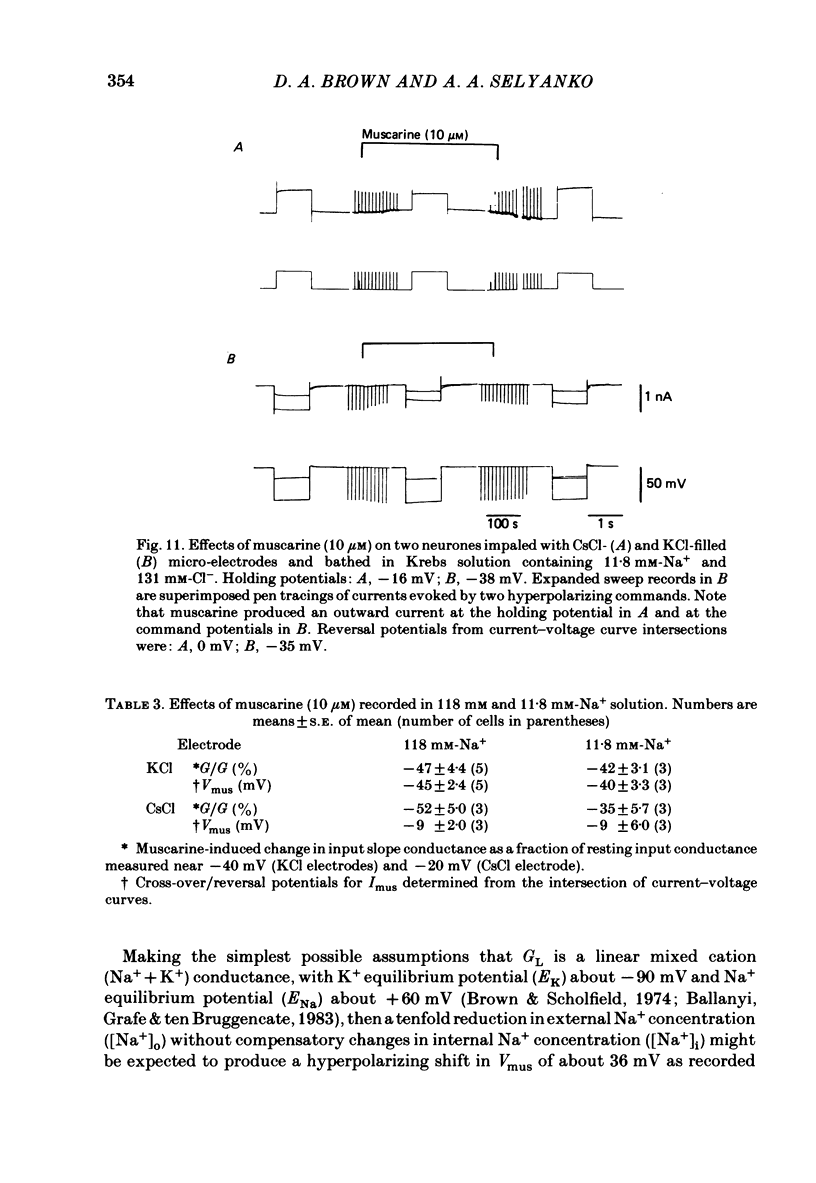
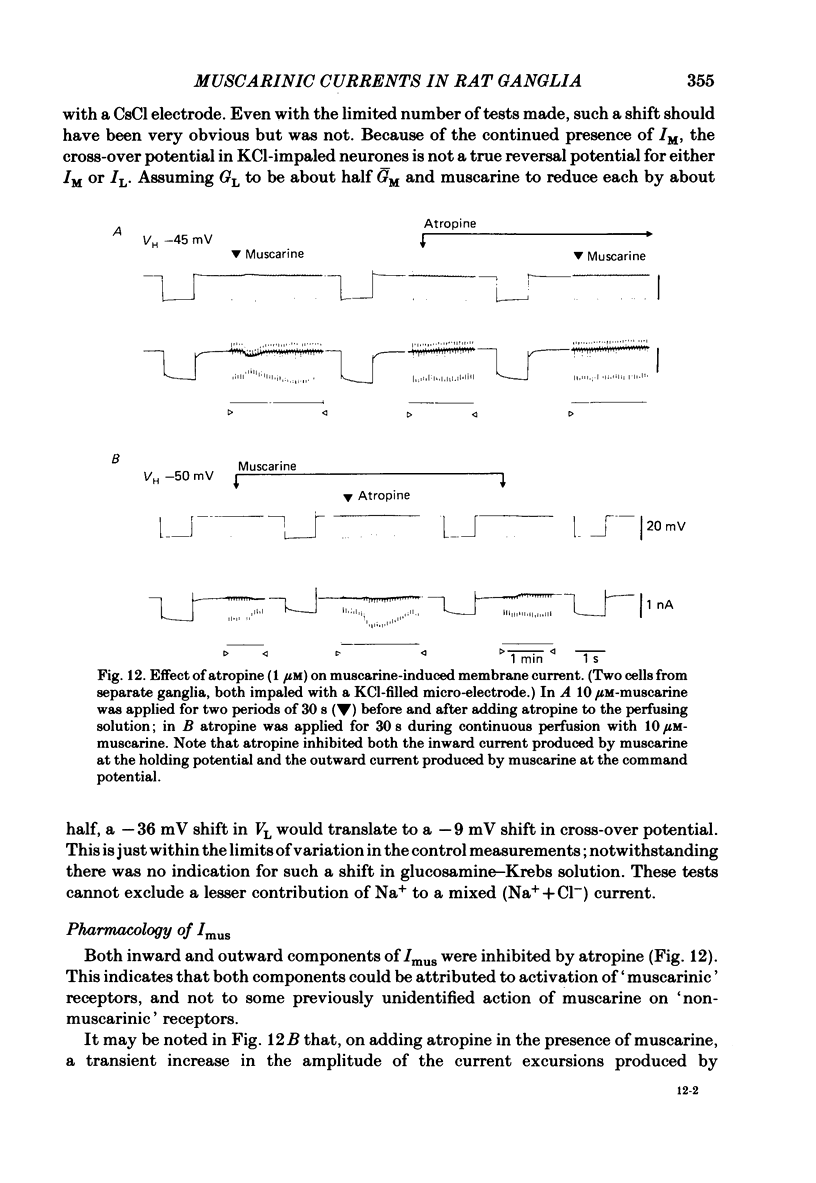
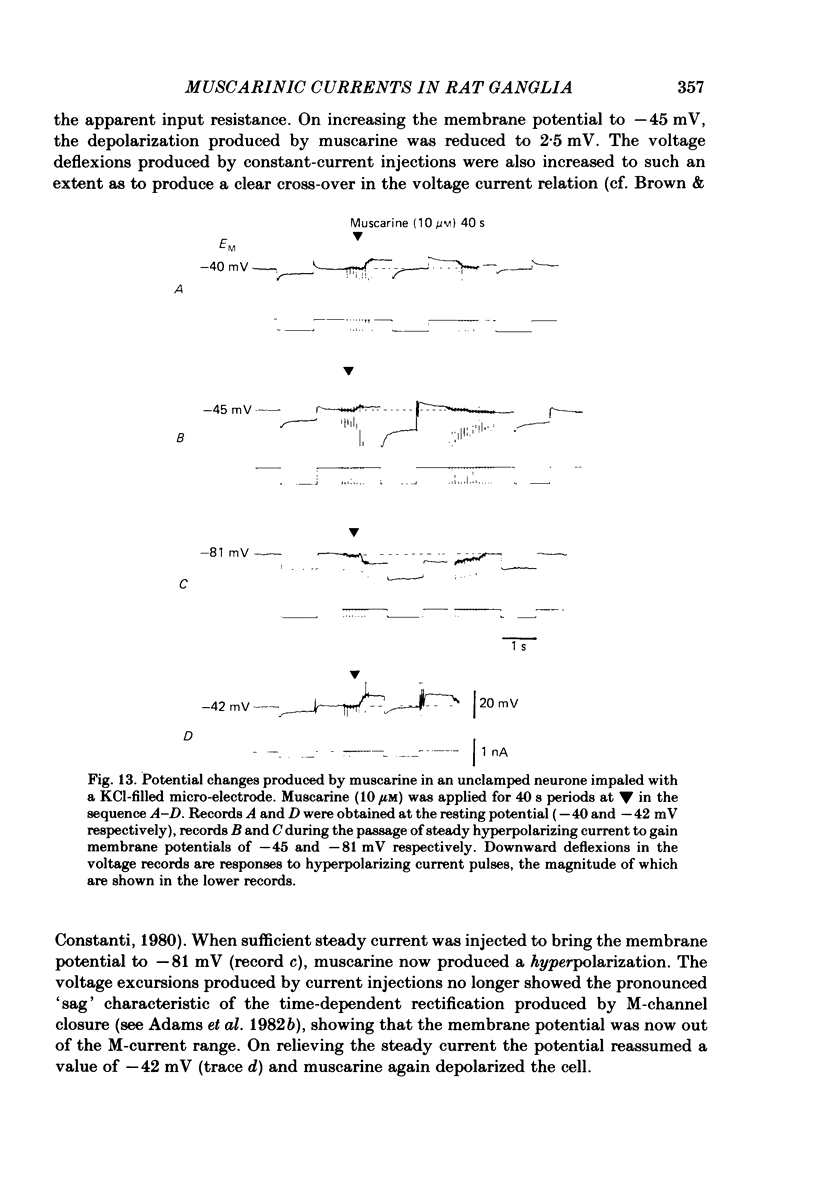
Membrane currents induced by muscarine (Imus) were recorded in voltage-clamped neurones in isolated rat superior cervical ganglia. Two components of Imus were regularly recorded: an inward current resulting from inhibition of the outward K+ current, IM; and an outward current attributable to the reduction of a steady inward current. The presence of these two components caused a 'cross-over' in the current-voltage curves at -50 +/- 3 mV in neurones impaled with KCl-filled micro-electrodes or at -63 +/- 4 mV in neurones impaled with K-acetate-filled electrodes. Both components of Imus were prevented by atropine. Both persisted in Krebs solution containing tetrodotoxin (1 microM), Cd2+ (200 microM) or 0 Ca2+. When IM was inhibited by external Ba2+ or internal Cs+ only the outward component of Imus could be detected. This component reversed at +3 +/- 2 mV in cells impaled with CsCl-filled electrodes or at -20 +/- 3 mV in cells impaled with Cs-acetate-filled electrodes. The reversal potentials agreed with those for the currents induced by gamma-aminobutyric acid (+4 +/- 2 mV and -16 +/- 3 mV with CsCl and Cs acetate electrodes respectively). Replacement of external NaCl with Na acetate (so reducing external Cl- concentration ( [Cl-]o) from 155 to 22 mM) shifted the reversal potential for Imus by +25 and +14.5 mV in two cells impaled with CsCl-filled electrodes. A tenfold reduction of external [Na+] (by glucosamine replacement) did not significantly alter the reversal potential for Imus in KCl or CsCl-impaled cells. Under conditions where IM is already inhibited, the residual outward component of Imus can lead to hyperpolarization and inhibition of neuronal activity in unclamped cells. We conclude that both inward and outward components of Imus result from direct activation of muscarinic receptors on the ganglion cells. The inward component results from IM inhibition. We suggest that the outward component results from inhibition of another, voltage-independent current IX which largely comprises a Cl- current. The inward component induces membrane depolarization and an increased excitability; the outward component can lead to hyperpolarization and reduced excitability.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akasu T., Koketsu K. Modulation of voltage-dependent currents by muscarinic receptor in sympathetic neurones of bullfrog. Neurosci Lett. 1982 Mar 17;29(1):41–45. doi: 10.1016/0304-3940(82)90361-5. [DOI] [PubMed] [Google Scholar]
- Ballanyi K., Grafe P., Reddy M. M., ten Bruggencate G. Different types of potassium transport linked to carbachol and gamma-aminobutyric acid actions in rat sympathetic neurons. Neuroscience. 1984 Jul;12(3):917–927. doi: 10.1016/0306-4522(84)90179-9. [DOI] [PubMed] [Google Scholar]
- Ballanyi K., Grafe P., ten Bruggencate G. Intracellular free sodium and potassium, post-carbachol hyperpolarization, and extracellular potassium-undershoot in rat sympathetic neurones. Neurosci Lett. 1983 Aug 8;38(3):275–279. doi: 10.1016/0304-3940(83)90381-6. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Constanti A. Intracellular observations on the effects of muscarinic agonists on rat sympathetic neurones. Br J Pharmacol. 1980 Dec;70(4):593–608. doi: 10.1111/j.1476-5381.1980.tb09778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Forward A., Marsh S. Antagonist discrimination between ganglionic and ileal muscarinic receptors. Br J Pharmacol. 1980;71(2):362–364. doi: 10.1111/j.1476-5381.1980.tb10948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Changes of intracellular sodium and potassium ion concentrations in isolated rat superior cervical ganglia induced by depolarizing agents. J Physiol. 1974 Oct;242(2):307–319. doi: 10.1113/jphysiol.1974.sp010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. C. Liquid junction potentials and their effect on potential measurements in biological systems. Int Rev Cytol. 1968;24:345–371. doi: 10.1016/s0074-7696(08)61402-3. [DOI] [PubMed] [Google Scholar]
- Chesnoy-Marchais D. Characterization of a chloride conductance activated by hyperpolarization in Aplysia neurones. J Physiol. 1983 Sep;342:277–308. doi: 10.1113/jphysiol.1983.sp014851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Adams P. R., Brown D. A. Who do barium ions imitate acetylcholine? Brain Res. 1981 Feb 9;206(1):244–250. doi: 10.1016/0006-8993(81)90125-6. [DOI] [PubMed] [Google Scholar]
- Constanti A., Brown D. A. M-Currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981 Jul 17;24(3):289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- Galvan M. A transient outward current in rat sympathetic neurones. Neurosci Lett. 1982 Aug 31;31(3):295–300. doi: 10.1016/0304-3940(82)90036-2. [DOI] [PubMed] [Google Scholar]
- Galvan M., Dörge A., Beck F., Rick R. Intracellular electrolyte concentrations in rat sympathetic neurones measured with an electron microprobe. Pflugers Arch. 1984 Mar;400(3):274–279. doi: 10.1007/BF00581559. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982 Dec 27;31(26):2991–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- Hashiguchi T., Kobayashi H., Tosaka T., Libet B. Two muscarinic depolarizing mechanisms in mammalian sympathetic neurons. Brain Res. 1982 Jun 24;242(2):378–382. doi: 10.1016/0006-8993(82)90329-8. [DOI] [PubMed] [Google Scholar]
- Ivanov A. Ia, Skok V. I. Svoistva medlennogo vozbuzhdaiushchego postsinapticheskogo potentsiala v neironakh simpaticheskogo gangliia mlekopitaiushchikh. Neirofiziologiia. 1981;13(4):371–379. [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Actions of noradrenaline and acetylcholine on sympathetic ganglion cells. J Physiol. 1970 Jun;208(2):353–372. doi: 10.1113/jphysiol.1970.sp009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Libet B. Generation of slow postsynaptic potentials without increases in ionic conductance. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1304–1311. doi: 10.1073/pnas.60.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Analysis of the slow excitatory postsynaptic potential in bullfrog sympathetic ganglion cells. Jpn J Physiol. 1976;26(6):651–669. doi: 10.2170/jjphysiol.26.651. [DOI] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Unusual nature of ganglionic slow EPSP studied by a voltage-clamp method. Life Sci. 1969 Jan 1;8(1):33–42. doi: 10.1016/0024-3205(69)90290-2. [DOI] [PubMed] [Google Scholar]
- Sharp A. P., Thomas R. C. The effects of chloride substitution on intracellular pH in crab muscle. J Physiol. 1981 Mar;312:71–80. doi: 10.1113/jphysiol.1981.sp013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]


