Abstract
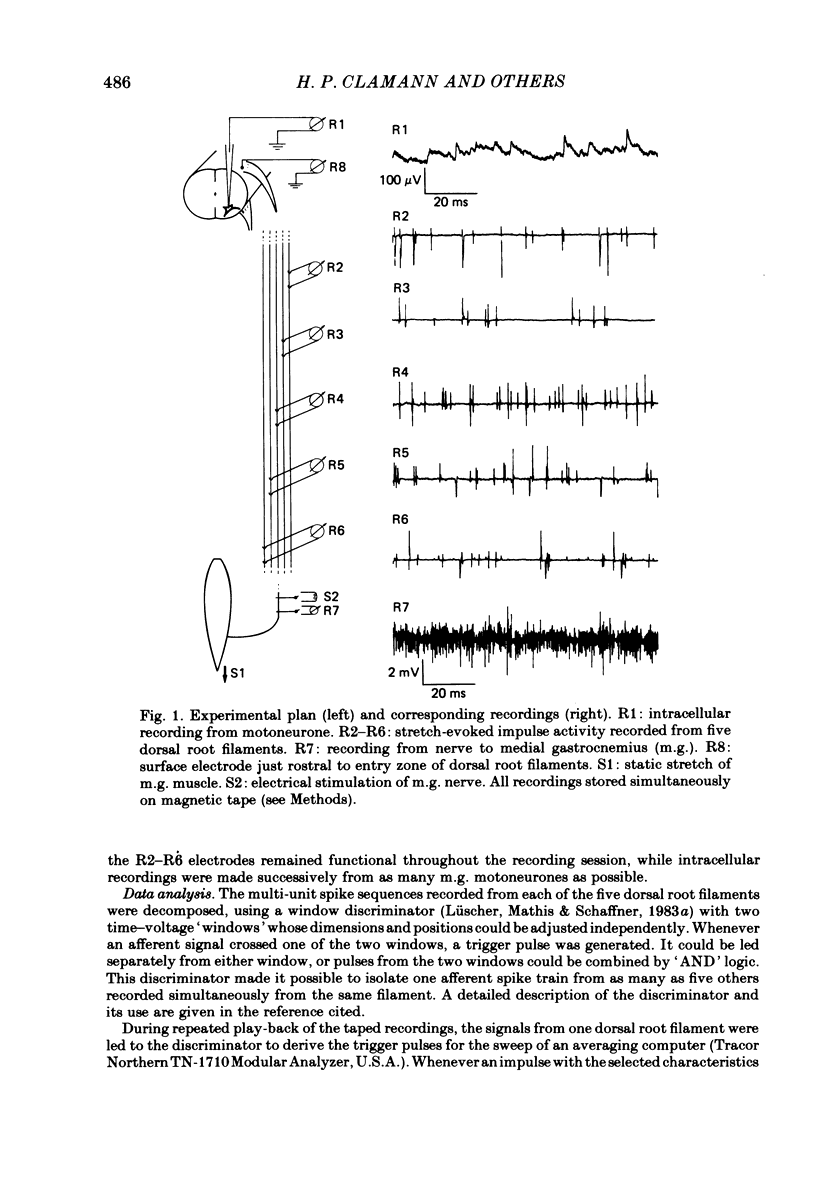
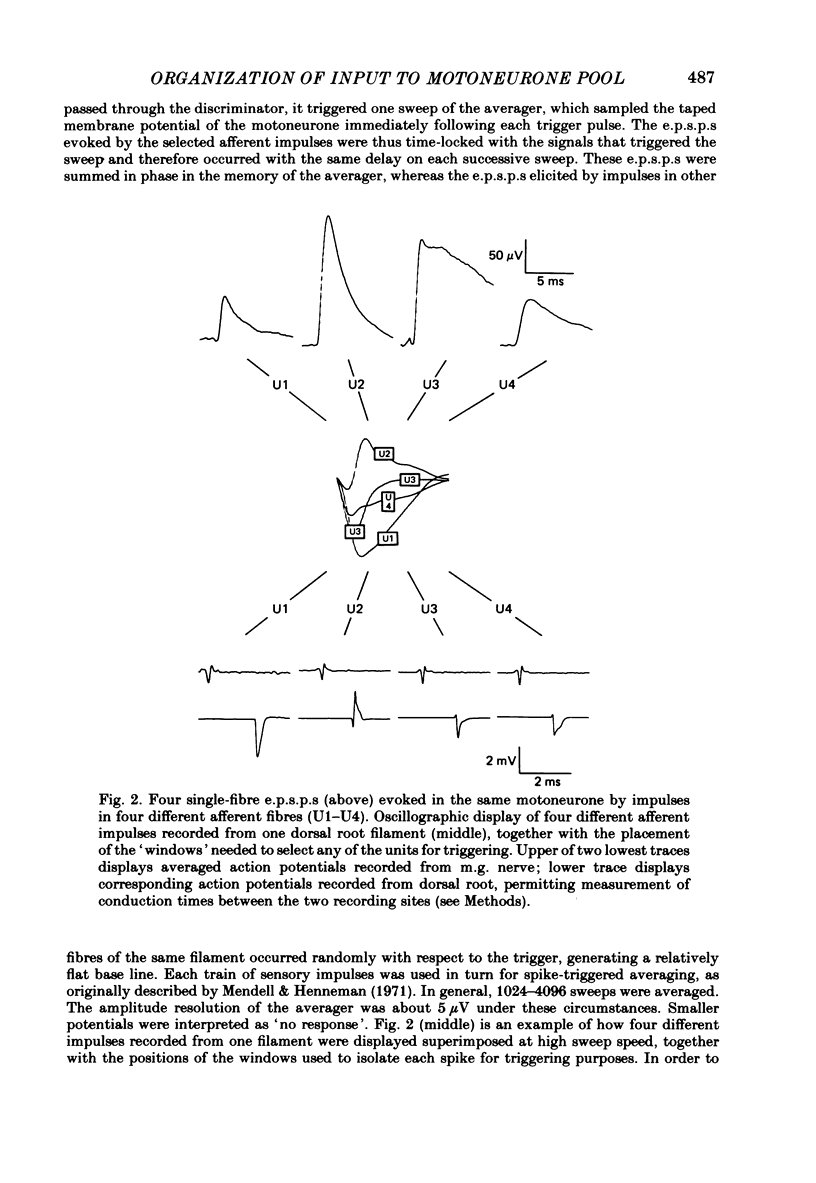
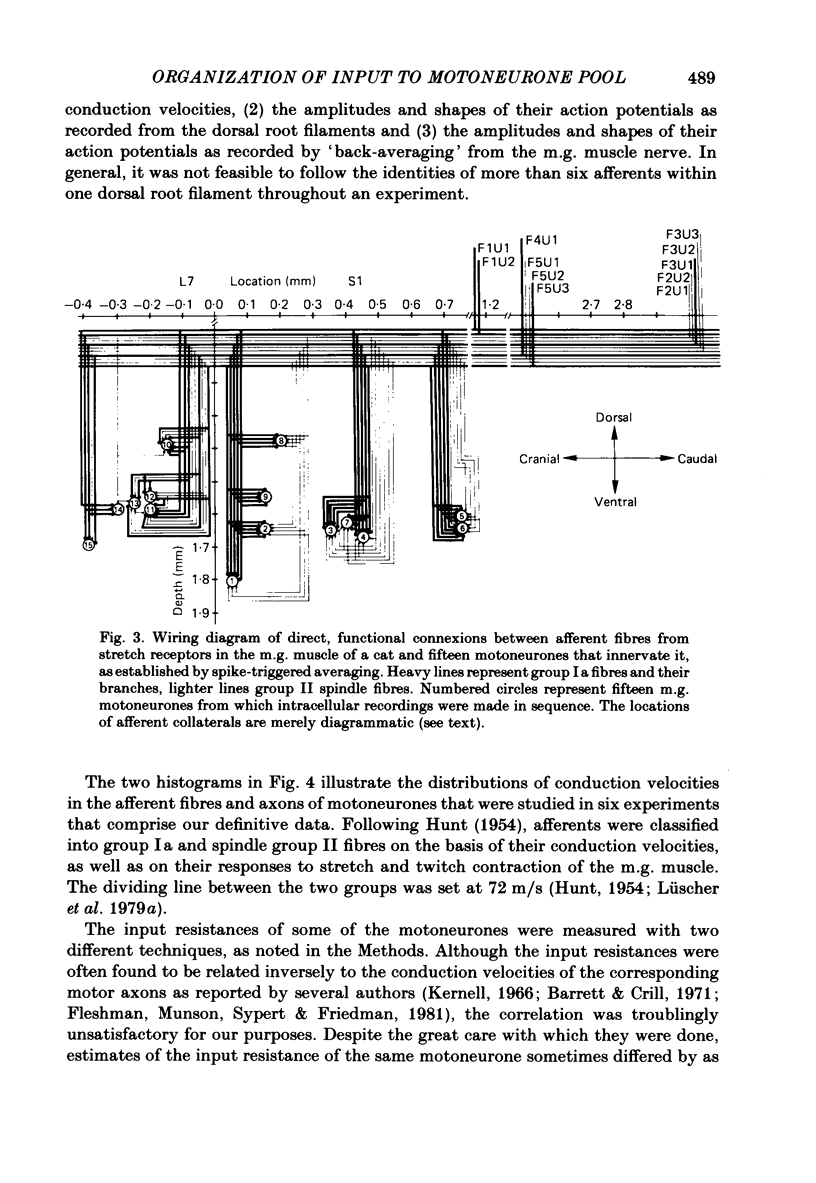
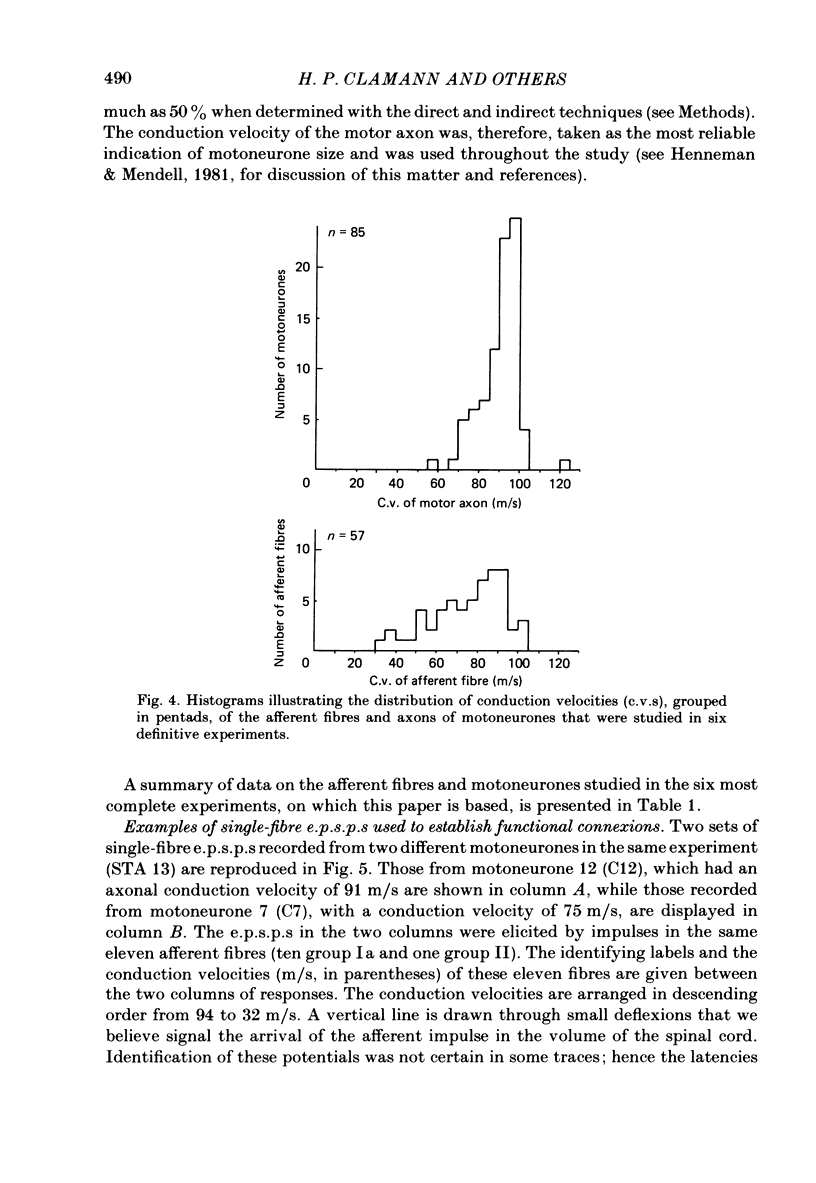
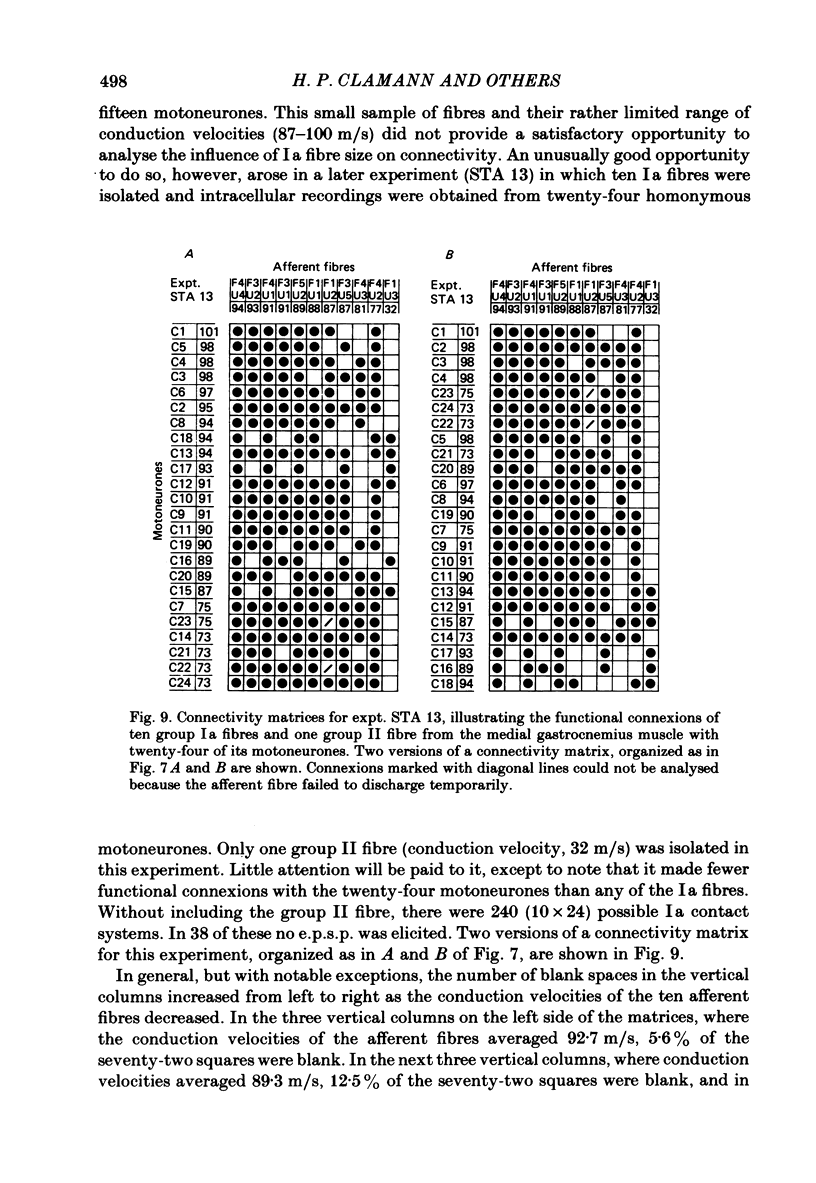
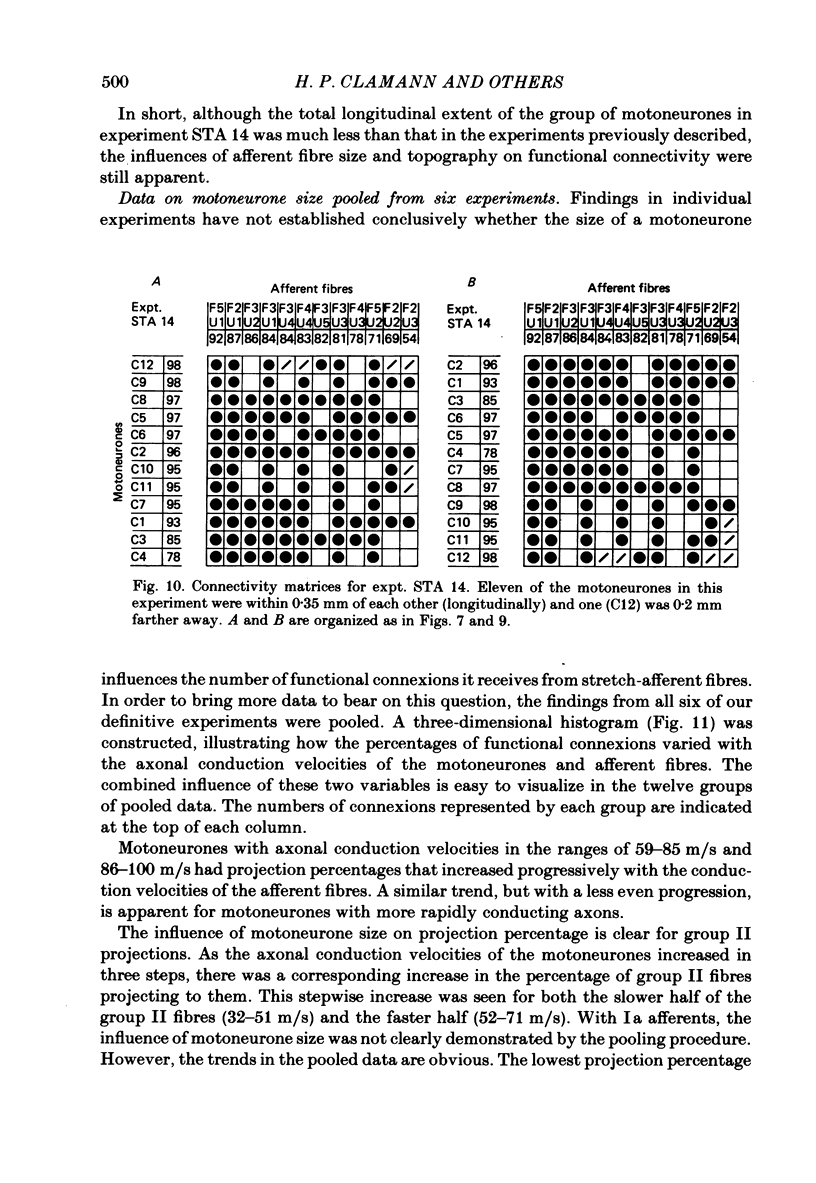
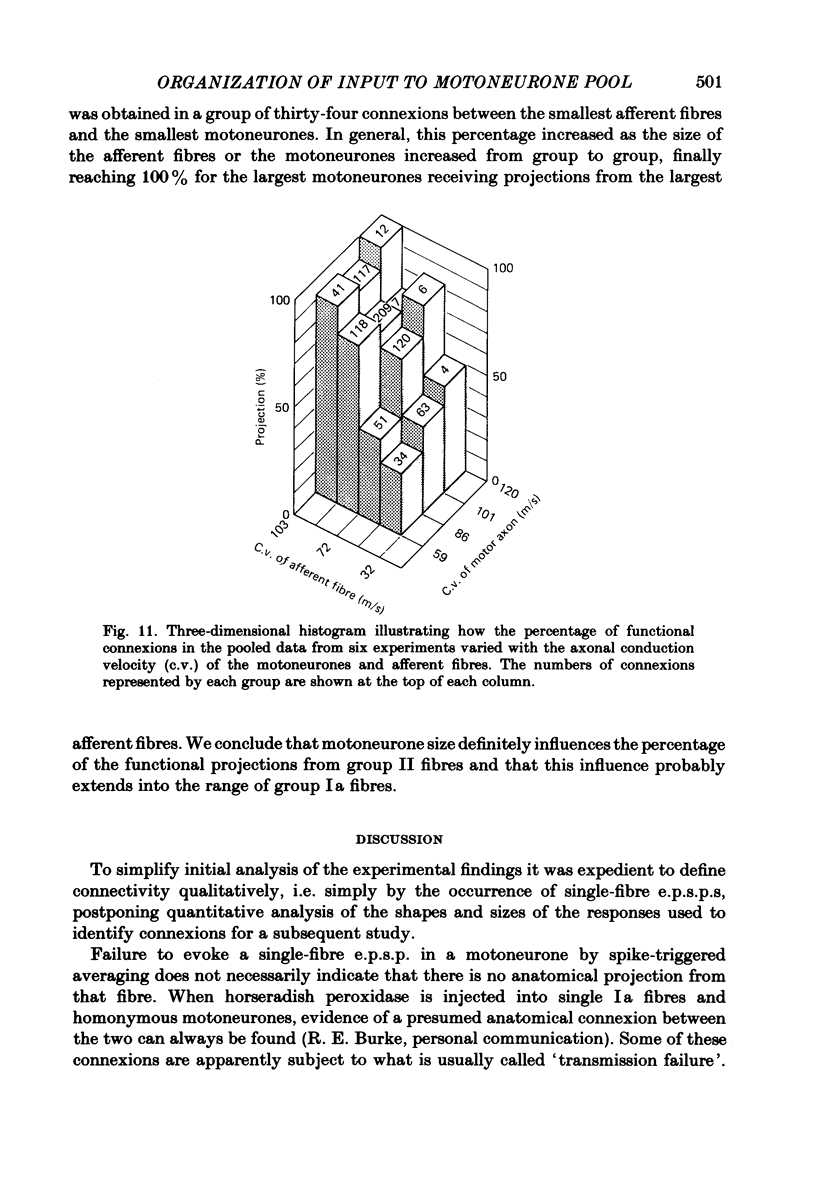
A greatly expanded version of spike-triggered averaging (Mendell & Henneman, 1971), performed off-line on tape-recorded signals, was utilized to determine the presence or absence of functional connexions between stretch-afferent fibres and homonymous motoneurones. As many as 264 possible connexions between eleven Ia or spindle group II fibres and twenty-four motoneurones were studied in each single, acute experiment. Morphological and topographical factors influencing functional connectivity were analysed with the aid of wiring diagrams and connectivity matrices. In all experiments the greater the conduction velocity (i.e. diameter) of a Ia or group II fibre, the higher was the probability of its having functional connexions with homonymous motoneurones. The greater the longitudinal distance between the spinal entry points of Ia fibres and the location of a motoneurone, the less was the same probability. The influence of axonal conduction velocity of motoneurones on functional connectivity was apparent in some experiments, but not in others. In pooled data large motoneurones received functional connexions from a higher percentage of group II fibres than did small cells. The projection percentage reached 100 only when both Ia fibres and motoneurones were large, suggesting that motoneurone size influences the probability of functional connexions from group Ia as well as group II fibres. On a cell-to-cell level, connectivity apparently does not follow strict, deterministic rules. The results raise the question of how probabilistic connexions between afferent fibres and motoneurones give rise to deterministic outputs from the whole pool.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. N., Crill W. E. Specific membrane resistivity of dye-injected cat motoneurons. Brain Res. 1971 May 21;28(3):556–561. doi: 10.1016/0006-8993(71)90066-7. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat's lumbosacral spinal cord. J Physiol. 1981;313:121–140. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Non-quantal fluctuations and transmission failures in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):689–704. doi: 10.1113/jphysiol.1976.sp011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Statistical fluctuations in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):665–688. doi: 10.1113/jphysiol.1976.sp011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W., Friedman W. A. Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1326–1338. doi: 10.1152/jn.1981.46.6.1326. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E. Relation between size of neurons and their susceptibility to discharge. Science. 1957 Dec 27;126(3287):1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E., Lüscher H. R., Mathis J. Simultaneously active and inactive synapses of single Ia fibres on cat spinal motoneurones. J Physiol. 1984 Jul;352:147–161. doi: 10.1113/jphysiol.1984.sp015283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965 May;28(3):599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Redman S. J., Wong K. Post-tetanic potentiation and facilitation of synaptic potentials evoked in cat spinal motoneurones. J Physiol. 1981 Dec;321:97–109. doi: 10.1113/jphysiol.1981.sp013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles J. F. Central terminations of muscle afferents on motoneurones in the cat spinal cord. J Physiol. 1976 Oct;262(1):91–117. doi: 10.1113/jphysiol.1976.sp011587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N., Mannen H., Hongo T., Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979 Jul 15;186(2):189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. Modifications to synaptic transmission at group Ia synapses on cat spinal motoneurones by 4-aminopyridine. J Physiol. 1981 Dec;321:111–126. doi: 10.1113/jphysiol.1981.sp013974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D. Input resistance, electrical excitability, and size of ventral horn cells in cat spinal cord. Science. 1966 Jun 17;152(3729):1637–1640. doi: 10.1126/science.152.3729.1637. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Mathis J., Henneman E. Wiring diagrams of functional connectivity in monosynaptic reflex arcs of the spinal cord. Neurosci Lett. 1984 Mar 23;45(2):217–222. doi: 10.1016/0304-3940(84)90102-2. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Mathis J., Schaffner H. A dual time--voltage window discriminator for multiunit nerve spike decomposition. J Neurosci Methods. 1983 Feb;7(2):99–105. doi: 10.1016/0165-0270(83)90072-9. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Fetz E., Henneman E. Postsynatpic population potentials recorded from ventral roots perfused with isotonic sucrose: connections of groups Ia and II spindle afferent fibers with large populations of motoneurons. J Neurophysiol. 1979 Jul;42(4):1146–1164. doi: 10.1152/jn.1979.42.4.1146. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Composite EPSPs in motoneurons of different sizes before and during PTP: implications for transmission failure and its relief in Ia projections. J Neurophysiol. 1983 Jan;49(1):269–289. doi: 10.1152/jn.1983.49.1.269. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. How the size of motoneurones determines their susceptibility to discharge. Nature. 1979 Dec 20;282(5741):859–861. doi: 10.1038/282859a0. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. Topographic distribution of terminals of Ia and group II fibers in spinal cord, as revealed by postsynaptic population potentials. J Neurophysiol. 1980 Apr;43(4):968–985. doi: 10.1152/jn.1980.43.4.968. [DOI] [PubMed] [Google Scholar]
- Lüscher H., Ruenzel P. W., Henneman E. Effects of impulse frequency, PTP, and temperature on responses elicited in large populations of motoneurons by impulses in single Ia-fibers. J Neurophysiol. 1983 Nov;50(5):1045–1058. doi: 10.1152/jn.1983.50.5.1045. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Modifiability of spinal synapses. Physiol Rev. 1984 Jan;64(1):260–324. doi: 10.1152/physrev.1984.64.1.260. [DOI] [PubMed] [Google Scholar]
- Nelson S. G., Collatos T. C., Niechaj A., Mendell L. M. Immediate increase in Ia-motoneuron synaptic transmission caudal to spinal cord transection. J Neurophysiol. 1979 May;42(3):655–664. doi: 10.1152/jn.1979.42.3.655. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Terminal patterns in cat spinal cord. 3. Primary afferent collaterals. Brain Res. 1969 May;13(3):417–443. doi: 10.1016/0006-8993(69)90258-3. [DOI] [PubMed] [Google Scholar]
- Scott J. G., Mendell L. M. Individual EPSPs produced by single triceps surae Ia afferent fibers in homonymous and heteronymous motoneurons. J Neurophysiol. 1976 Jul;39(4):679–692. doi: 10.1152/jn.1976.39.4.679. [DOI] [PubMed] [Google Scholar]
- Westbury D. R. A comparison of the structures of alpha and gamma-spinal motoneurones of the cat. J Physiol. 1982 Apr;325:79–91. doi: 10.1113/jphysiol.1982.sp014137. [DOI] [PMC free article] [PubMed] [Google Scholar]


