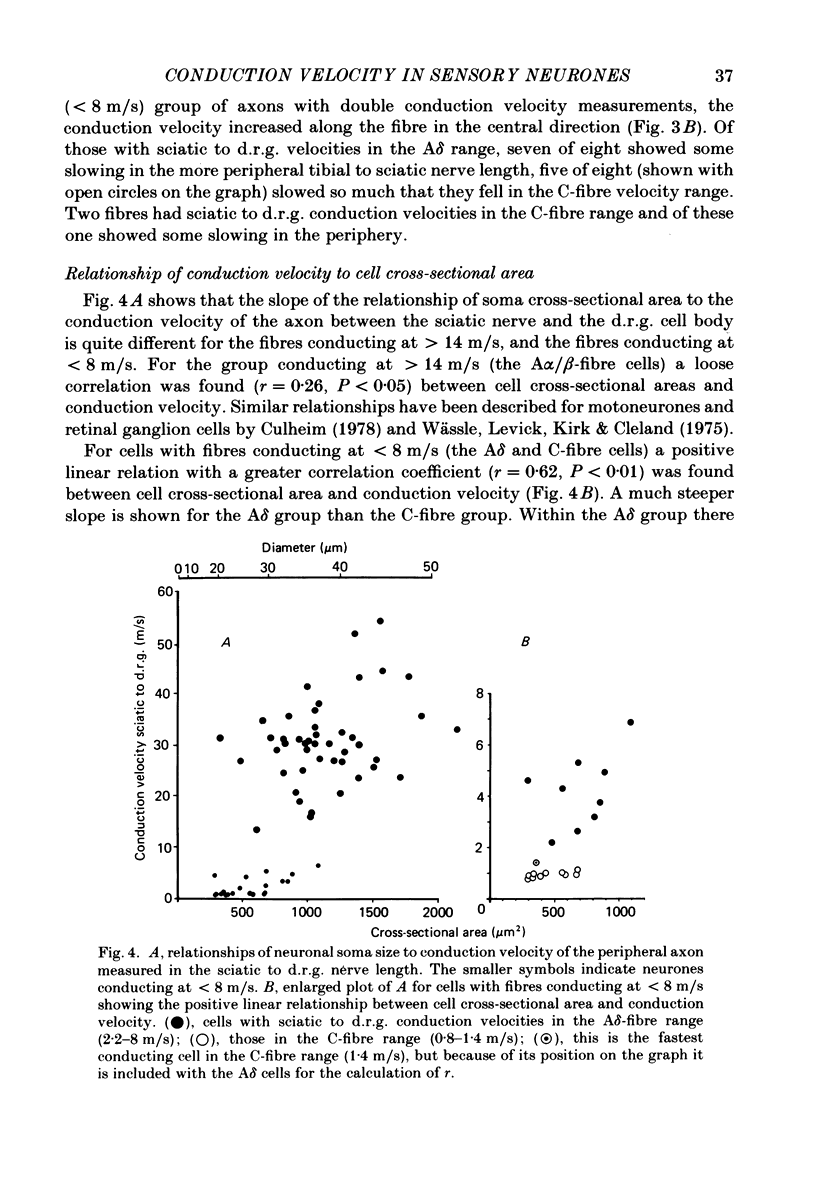
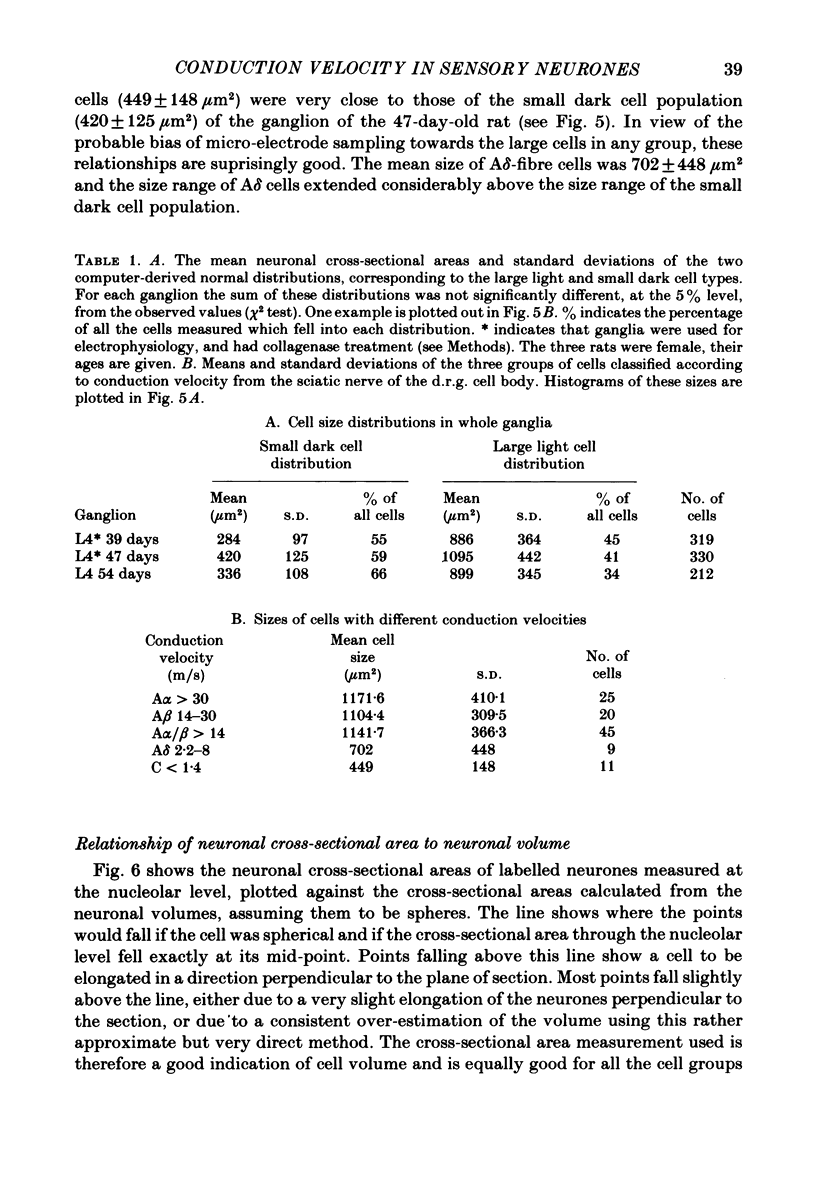
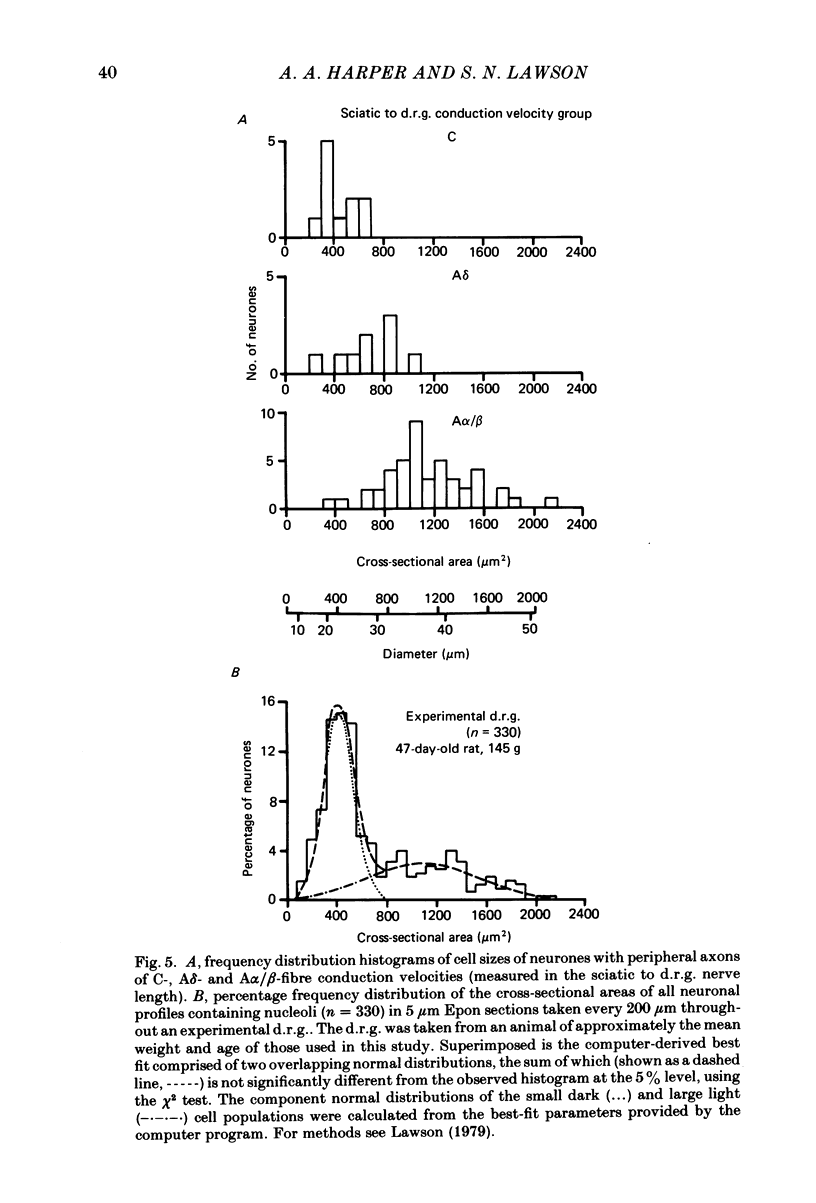
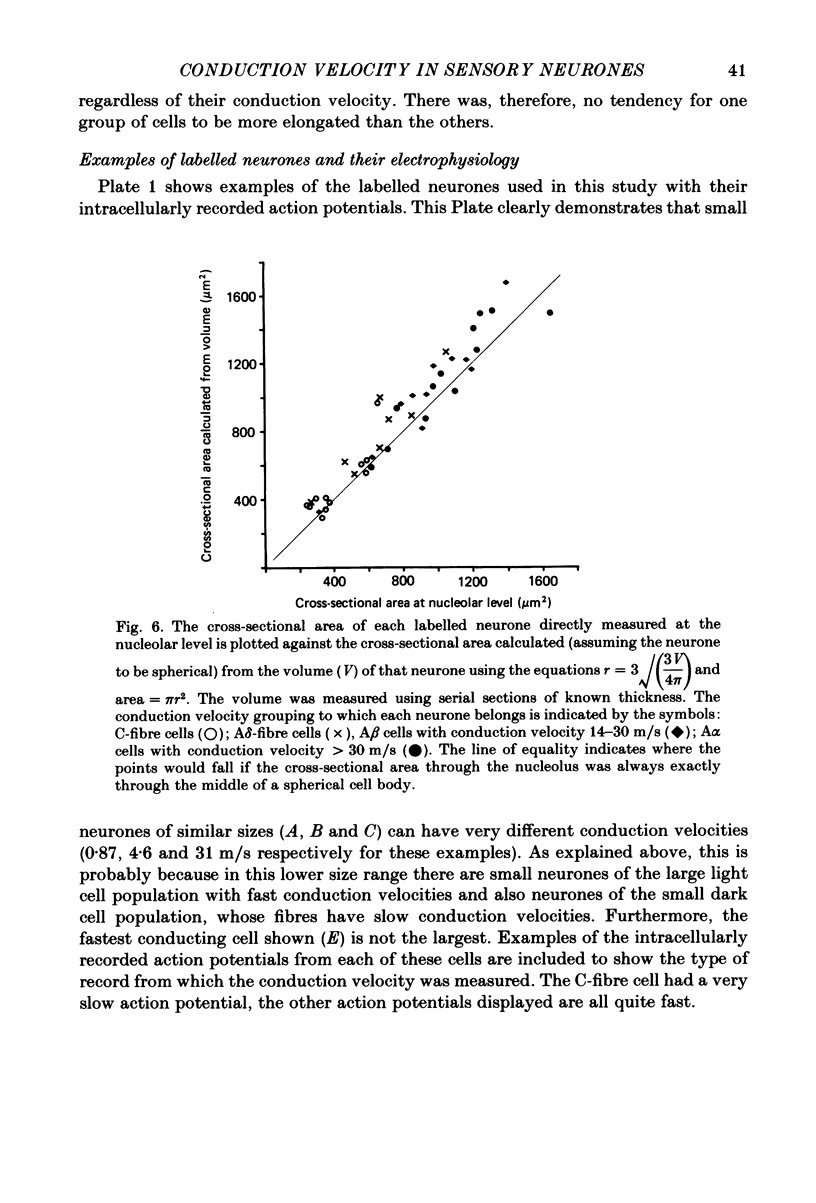
Abstract
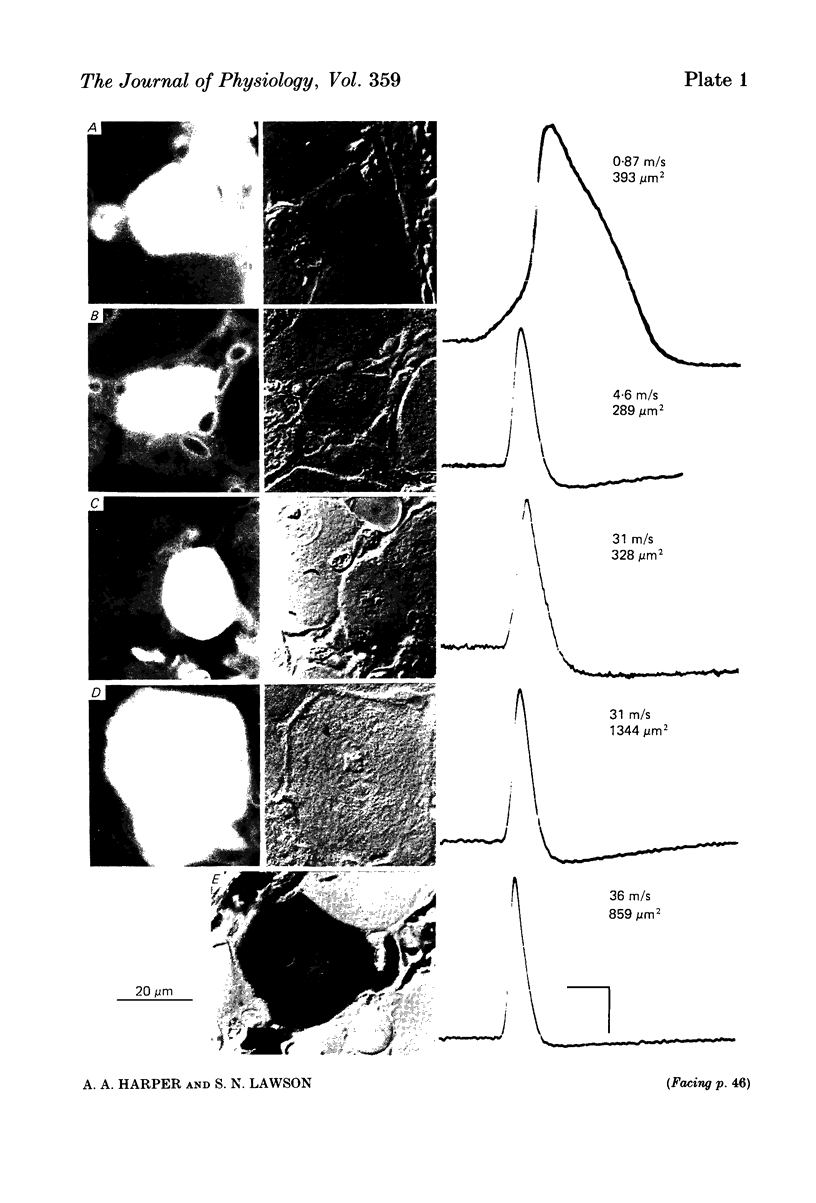
Combining intracellular recording and dye-injection techniques permitted direct correlation of neuronal soma size with peripheral nerve conduction velocity in individual neurones of the L4 dorsal root ganglion (d.r.g.) of the anaesthetized 5-8-week-old rat. The conduction velocities fell into two main groups; those greater than 14 m/s (A alpha and beta fibres) and those less than 8 m/s (A delta and C fibres). Fibres with conduction velocities in the A delta range (2.2-8 m/s) in the sciatic nerve between the sciatic notch and the neuronal soma in the d.r.g. often conducted more slowly, that is in the C-fibre range (less than 1.4 m/s), in the periphery from the tibial nerve to the sciatic notch. For the fast-conducting myelinated afferents, there was a loose positive correlation between cell size and the conduction velocity of the peripheral axon, whereas a clearer positive correlation existed between neuronal cell size and axonal conduction velocity both for A delta- and for C-fibre afferents. The relationship of the cell cross-sectional area (measured at the nucleolar level), to the cell volume for each neuronal soma was similar for the different conduction velocity groups. The somata of the fast-conducting myelinated A alpha and A beta fibres had a similar mean and range of cross-sectional areas to those of the large light cell population. The somata with A delta and C fibres were of a more uniform size and were restricted to the smaller cells within the ganglia. The mean and range of cross-sectional areas of the C cells was similar to those of the small dark cell population. A delta somata had a larger mean and range of cell sizes than those of the small dark cell population. The relationships of peripheral axon type to the morphological cell types are discussed.
Full text
PDF


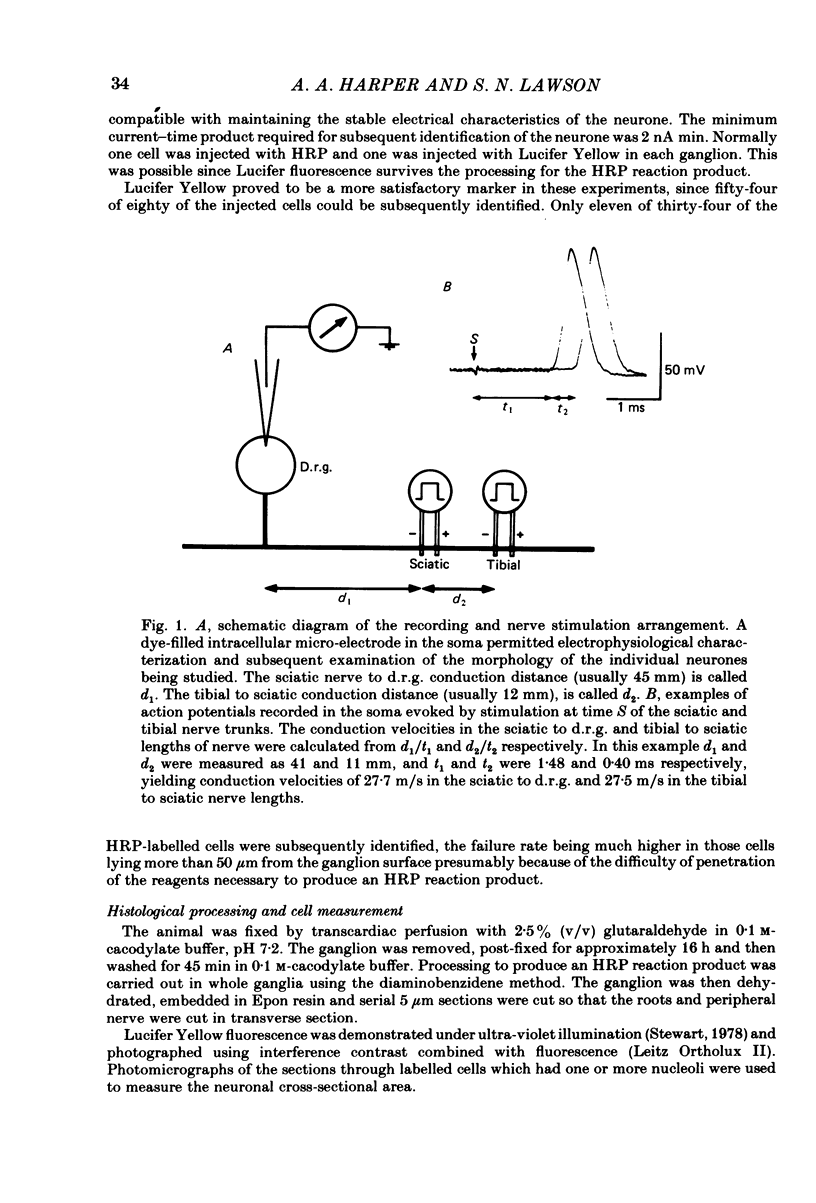
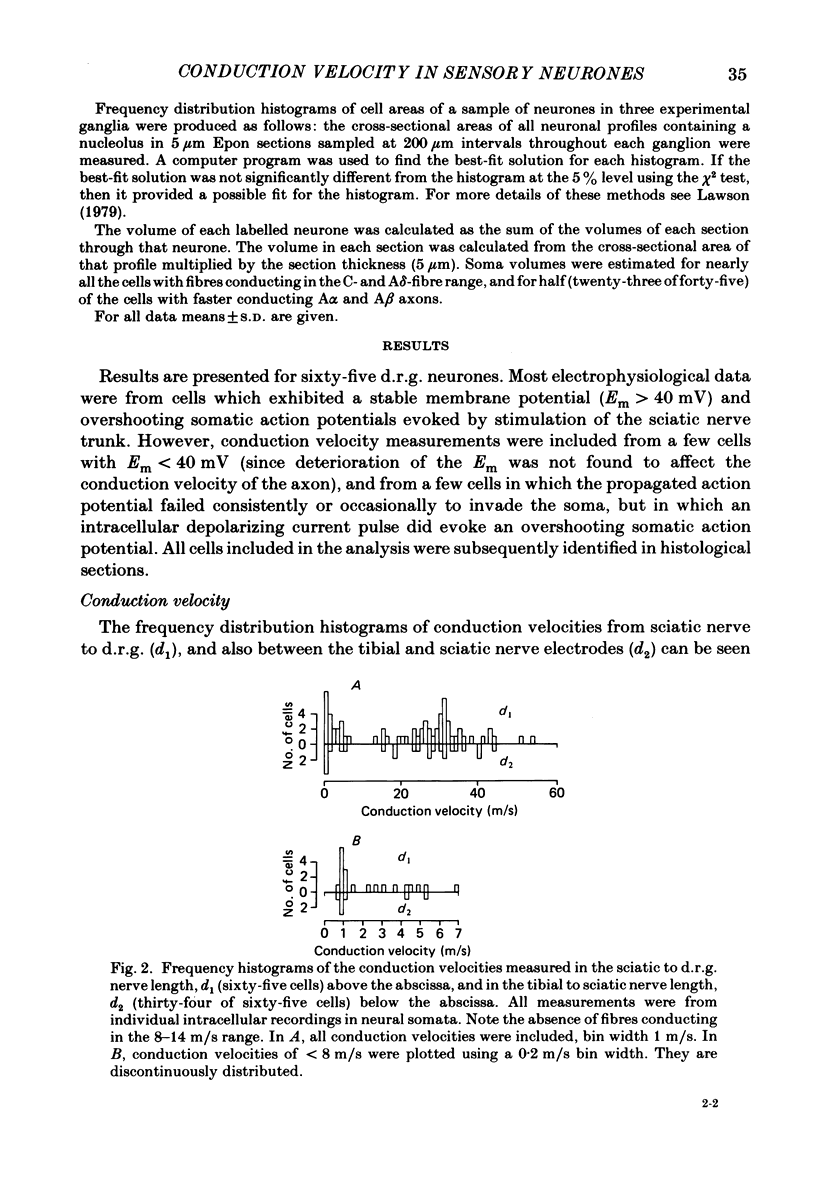
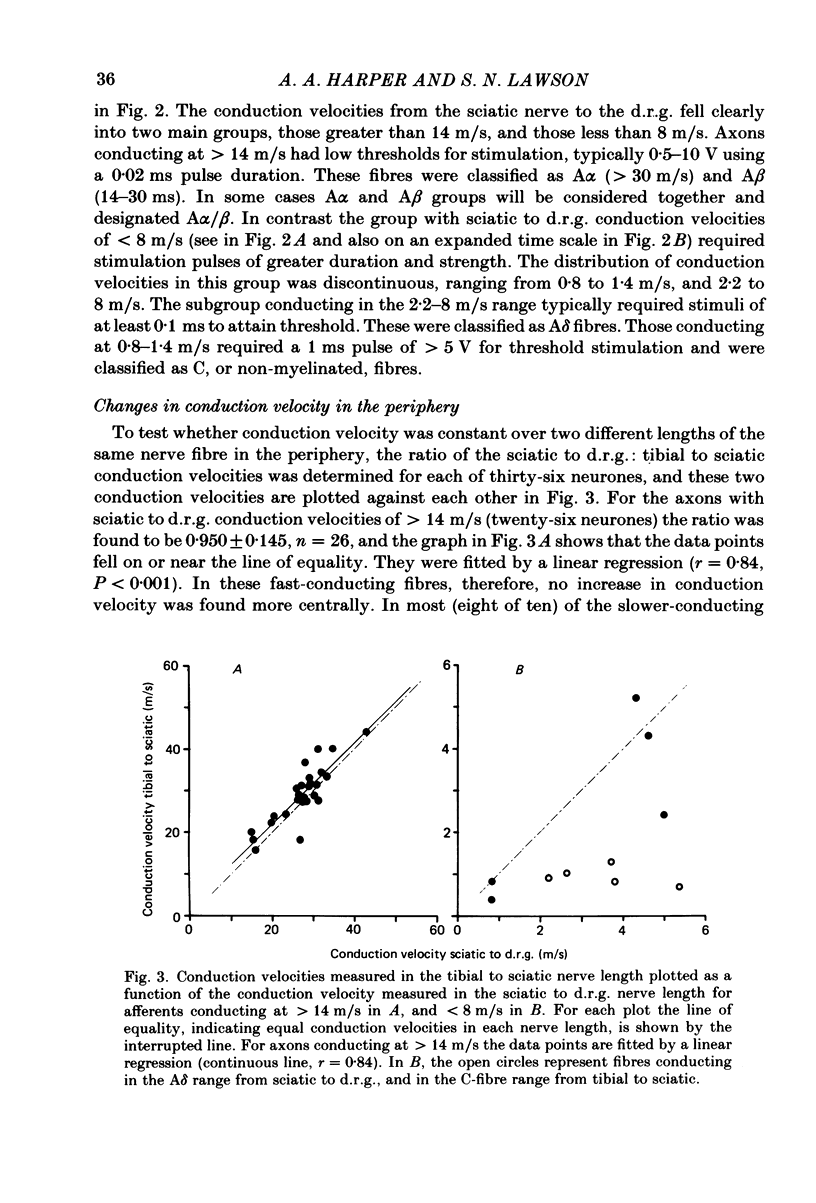






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRREN J. E., WALL P. D. Age changes in conduction velocity, refractory period, number of fibers, connective tissue space and blood vessels in sciatic nerve of rats. J Comp Neurol. 1956 Feb;104(1):1–16. doi: 10.1002/cne.901040102. [DOI] [PubMed] [Google Scholar]
- DUN F. T. The delay and blockage of sensory impulses in the dorsal root ganglion. J Physiol. 1955 Feb 28;127(2):252–264. doi: 10.1113/jphysiol.1955.sp005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce I. R., Keen P. An ultrastructural classification of the neuronal cell bodies of the rat dorsal root ganglion using zinc iodide-osmium impregnation. Cell Tissue Res. 1977 Dec 13;185(2):263–277. doi: 10.1007/BF00220670. [DOI] [PubMed] [Google Scholar]
- Duclaux R., Mei N., Ranieri F. Conduction velocity along the afferent vagal dendrites: a new type of fibre. J Physiol. 1976 Sep;260(2):487–495. doi: 10.1113/jphysiol.1976.sp011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A. P., Lambert E. H. Age changes in conduction velocity of unmyelinated fibers. J Comp Neurol. 1973 Feb 15;147(4):547–552. doi: 10.1002/cne.901470408. [DOI] [PubMed] [Google Scholar]
- Horch K. W., Tuckett R. P., Burgess P. R. A key to the classification of cutaneous mechanoreceptors. J Invest Dermatol. 1977 Jul;69(1):75–82. doi: 10.1111/1523-1747.ep12497887. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elde R., Johansson O., Luft R., Nilsson G., Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience. 1976;1(2):131–136. doi: 10.1016/0306-4522(76)90008-7. [DOI] [PubMed] [Google Scholar]
- IGGO A. The electrophysiological identification of single nerve fibres, with particular reference to the slowest-conducting vagal afferent fibres in the cat. J Physiol. 1958 Jun 18;142(1):110–126. doi: 10.1113/jphysiol.1958.sp006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Ogawa H. Primate cutaneous thermal nociceptors. J Physiol. 1971 Jul;216(2):77P–78P. [PubMed] [Google Scholar]
- Lawson S. N., Biscoe T. J. Development of mouse dorsal root ganglia: an autoradiographic and quantitative study. J Neurocytol. 1979 Jun;8(3):265–274. doi: 10.1007/BF01236122. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Caddy K. W., Biscoe T. J. Development of rat dorsal root ganglion neurones. Studies of cell birthdays and changes in mean cell diameter. Cell Tissue Res. 1974;153(3):399–413. doi: 10.1007/BF00229167. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Harper A. A., Harper E. I., Garson J. A., Anderton B. H. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol. 1984 Sep 10;228(2):263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- Lawson S. N. The postnatal development of large light and small dark neurons in mouse dorsal root ganglia: a statistical analysis of cell numbers and size. J Neurocytol. 1979 Jun;8(3):275–294. doi: 10.1007/BF01236123. [DOI] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Hunt S. P. Fluoride-resistant acid phosphatase-containing neurones in dorsal root ganglia are separate from those containing substance P or somatostatin. Neuroscience. 1982 Jan;7(1):89–97. doi: 10.1016/0306-4522(82)90155-5. [DOI] [PubMed] [Google Scholar]
- Spencer P. S., Raine C. S., Wiśniewski H. Axon diameter and myelin thickness. Unusual relationships in dorsal root ganglia. Anat Rec. 1973 Jun;176(2):225–243. doi: 10.1002/ar.1091760209. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Wässle H., Levick W. R., Kirk D. L., Cleland B. G. Axonal conduction velocity and perikaryal size. Exp Neurol. 1975 Oct;49(1 Pt 1):246–251. doi: 10.1016/0014-4886(75)90208-3. [DOI] [PubMed] [Google Scholar]
- Yamadori T. A light and electron microscopic study on the postnatal development of spinal ganglia in rats. Kaibogaku Zasshi. 1970 Aug 1;45(4):191–205. [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y. Studies on sensory neurons of the mouse with intracellular-recording and horseradish peroxidase-injection techniques. J Neurophysiol. 1979 Jul;42(4):1134–1145. doi: 10.1152/jn.1979.42.4.1134. [DOI] [PubMed] [Google Scholar]
- Zenker W., Högl E. The prebifurcation section of the axon of the rat spinal ganglion cell. Cell Tissue Res. 1976 Jan 27;165(3):345–363. doi: 10.1007/BF00222438. [DOI] [PubMed] [Google Scholar]