Abstract
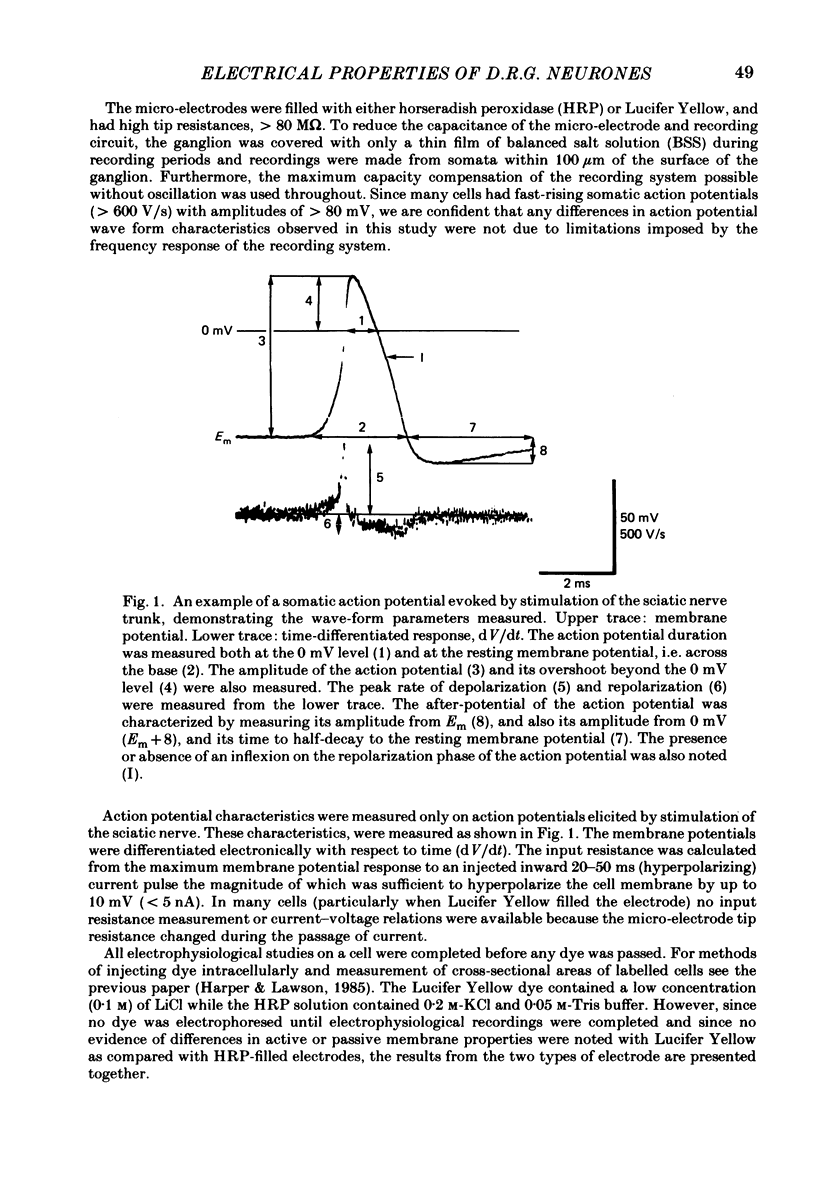
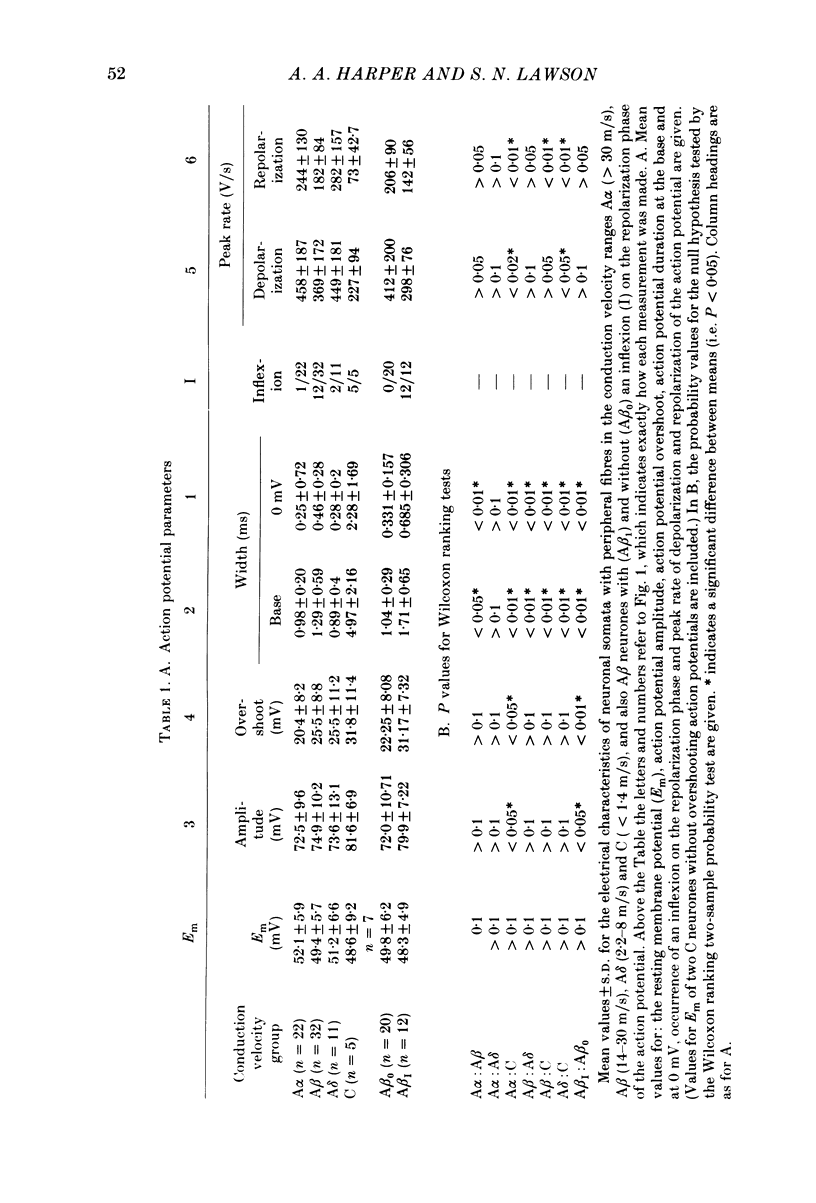
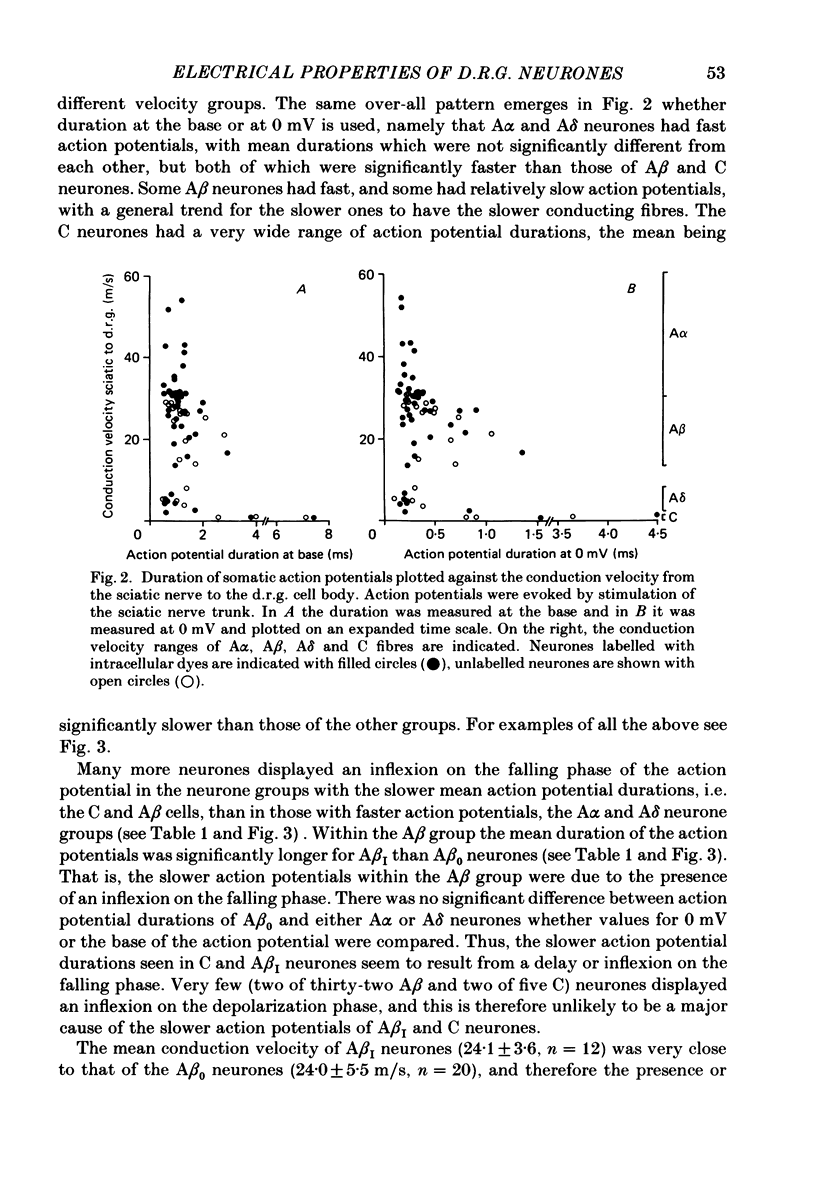
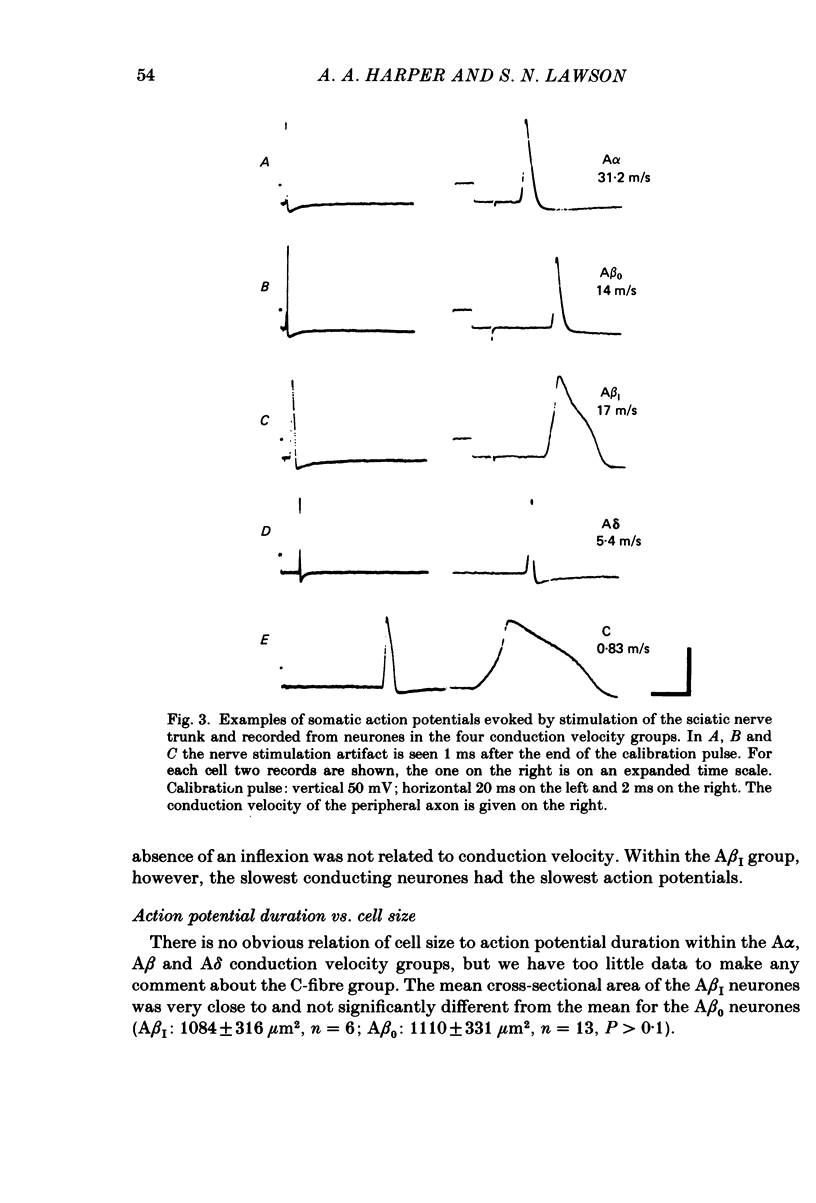
The electrical characteristics of individual rat dorsal root ganglion neurones were studied and related to the peripheral axon conduction velocity and morphological cell type. Neurones were divided into four groups based on the conduction velocity of their peripheral axons (A alpha, 30-55 m/s; A beta, 14-30 m/s; A delta, 2.2-8 m/s and C less than 1.4 m/s). Electrophysiological parameters examined included membrane potential, action potential amplitude and duration, after-potential height and duration, input resistance and the occurrence of time-dependent rectification. The mean duration of the somatic action potentials was found to be characteristic for each of the conduction velocity groupings. However, there was considerable overlap between groups. The fast-conducting (A alpha) and slowly conducting (A delta) myelinated fibres had short-duration action potentials, within the ranges 0.49-1.35 and 0.5-1.7 ms at the base respectively. The A beta and C cells had somatic action potentials with durations in the ranges of 0.6-2.9 and 0.6-7.4 ms respectively. The longer action potential durations could be related to the presence of an inflexion on the repolarizing phase seen in a third of A beta neurones (called A beta I neurones) and in all C neurones. The action potential overshoot was larger in C neurones and A beta I neurones than in the other neurone groups. The mean duration of the after-hyperpolarization was several times greater in C neurones than in A neurones. A delta neurones displayed the shortest and greatest amplitude after-hyperpolarizations. Large, long-lasting after-hyperpolarizations were not limited to neurones displaying an inflexion. The electrophysiological properties of the soma membrane of A delta neurones closely resembled those of A alpha neurones, while in several respects those of C neurones resembled the A beta I neuronal properties. The input resistance was found to be much greater in C than in A cells, although there was no significant difference between specific membrane resistance values calculated for the different groups. A number of A cells exhibited time-dependent rectification.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belmonte C., Gallego R. Membrane properties of cat sensory neurones with chemoreceptor and baroreceptor endings. J Physiol. 1983 Sep;342:603–614. doi: 10.1113/jphysiol.1983.sp014871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Burgess P. R., Perl E. R., Taylor C. B. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971 Jan;34(1):116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Perkel D. H., Norris J. C., Peacock J. H. Electrotonic structure and specific membrane properties of mouse dorsal root ganglion neurons. J Neurophysiol. 1981 Jan;45(1):1–15. doi: 10.1152/jn.1981.45.1.1. [DOI] [PubMed] [Google Scholar]
- Dichter M. A., Fischbach G. D. The action potential of chick dorsal root ganglion neurones maintained in cell culture. J Physiol. 1977 May;267(2):281–298. doi: 10.1113/jphysiol.1977.sp011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R., Eyzaguirre C. Membrane and action potential characteristics of A and C nodose ganglion cells studied in whole ganglia and in tissue slices. J Neurophysiol. 1978 Sep;41(5):1217–1232. doi: 10.1152/jn.1978.41.5.1217. [DOI] [PubMed] [Google Scholar]
- Görke K., Pierau F. K. Spike potentials and membrane properties of dorsal root ganglion cells in pigeons. Pflugers Arch. 1980 Jul;386(1):21–28. doi: 10.1007/BF00584182. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer E. J., Macdonald R. L. Calcium- and sodium-dependent action potentials of mouse spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1982 Apr;47(4):641–655. doi: 10.1152/jn.1982.47.4.641. [DOI] [PubMed] [Google Scholar]
- ITO M. The electrical activity of spinal ganglion cells investigated with intracellular microelectrodes. Jpn J Physiol. 1957 Dec 20;7(4):297–323. doi: 10.2170/jjphysiol.7.297. [DOI] [PubMed] [Google Scholar]
- Jaffe R. A., Sampson S. R. Analysis of passive and active electrophysiologic properties of neurons in mammalian nodose ganglia maintained in vitro. J Neurophysiol. 1976 Jul;39(4):802–815. doi: 10.1152/jn.1976.39.4.802. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Harper A. A., Harper E. I., Garson J. A., Anderton B. H. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol. 1984 Sep 10;228(2):263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- Lawson S. N. The postnatal development of large light and small dark neurons in mouse dorsal root ganglia: a statistical analysis of cell numbers and size. J Neurocytol. 1979 Jun;8(3):275–294. doi: 10.1007/BF01236123. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Yoshida S., Yonezawa T. A Ca- dependent regenerative response in rodent dorsal root ganglion cells cultured in vitro. Brain Res. 1976 Oct 15;115(2):334–338. doi: 10.1016/0006-8993(76)90519-9. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannese E., Gioia M., Carandente O., Ventura R. A quantitative electron microscope study of the perikaryal projections of sensory ganglion neurons. I. Cat and rabbit. J Comp Neurol. 1983 Mar 1;214(3):239–250. doi: 10.1002/cne.902140302. [DOI] [PubMed] [Google Scholar]
- SATO M., AUSTIN G. Intracellular potentials of mammalian dorsal root ganglion cells. J Neurophysiol. 1961 Nov;24:569–582. doi: 10.1152/jn.1961.24.6.569. [DOI] [PubMed] [Google Scholar]
- Scott B. S., Edwards B. A. Electric membrane properties of adult mouse DRG neurons and the effect of culture duration. J Neurobiol. 1980 May;11(3):291–301. doi: 10.1002/neu.480110307. [DOI] [PubMed] [Google Scholar]
- Williams J., Zieglgänsberger W. Mature spinal ganglion cells are not sensitive to opiate receptor mediated actions. Neurosci Lett. 1981 Jan 20;21(2):211–216. doi: 10.1016/0304-3940(81)90384-0. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y., Samejima A. Tetrodotoxin-resistant sodium and calcium components of action potentials in dorsal root ganglion cells of the adult mouse. J Neurophysiol. 1978 Sep;41(5):1096–1106. doi: 10.1152/jn.1978.41.5.1096. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Matsuda Y. Studies on sensory neurons of the mouse with intracellular-recording and horseradish peroxidase-injection techniques. J Neurophysiol. 1979 Jul;42(4):1134–1145. doi: 10.1152/jn.1979.42.4.1134. [DOI] [PubMed] [Google Scholar]


