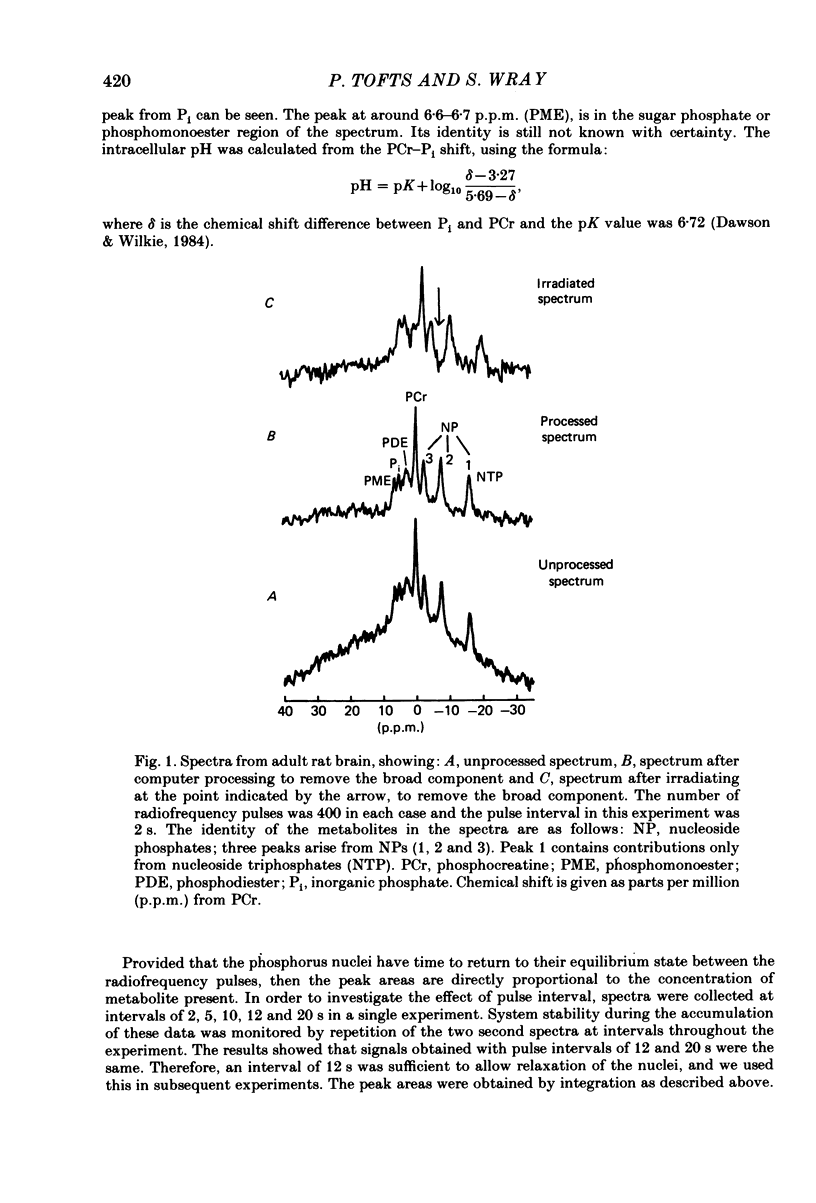
Abstract
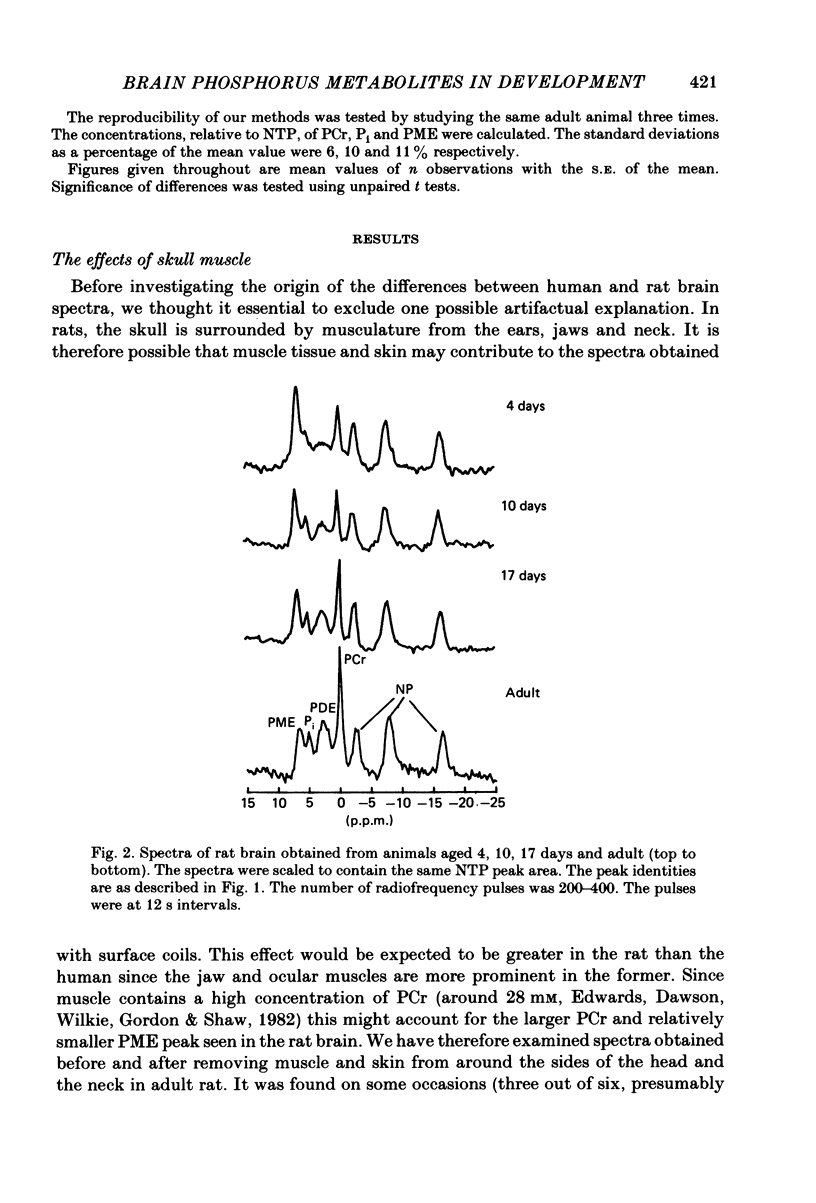
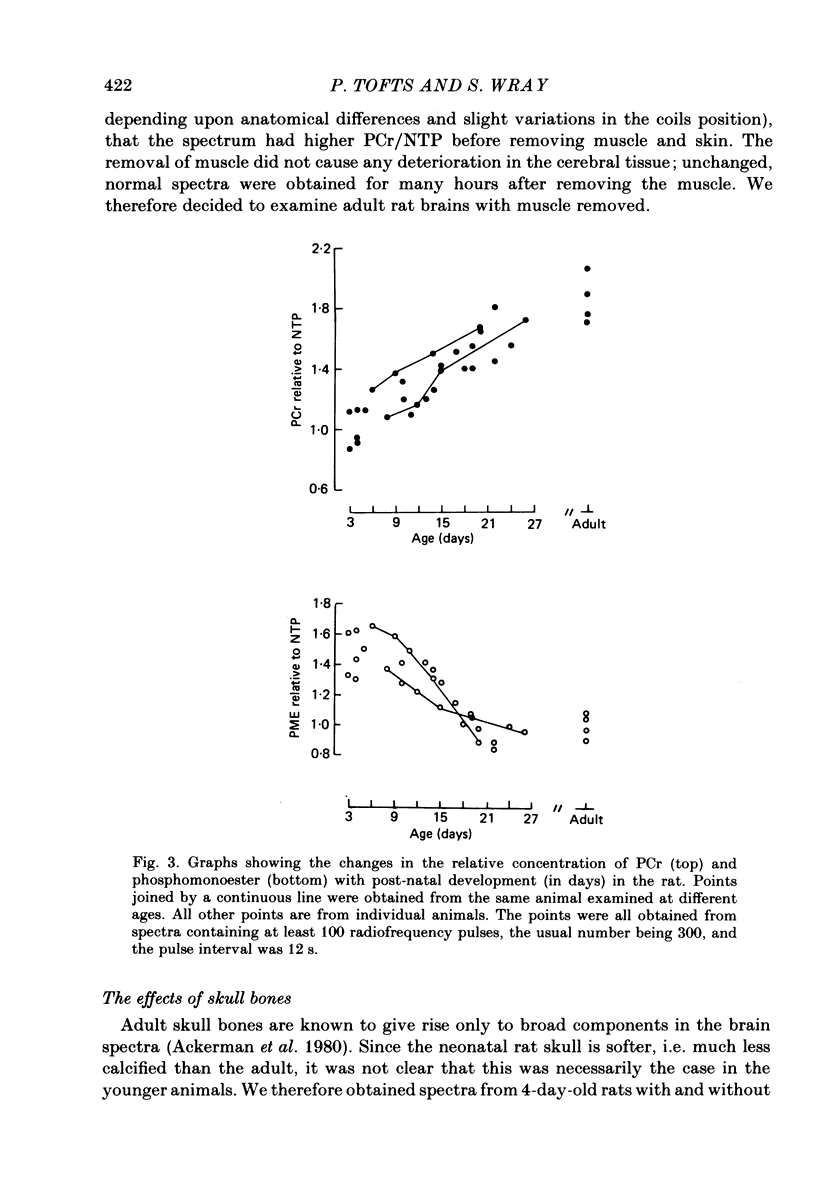
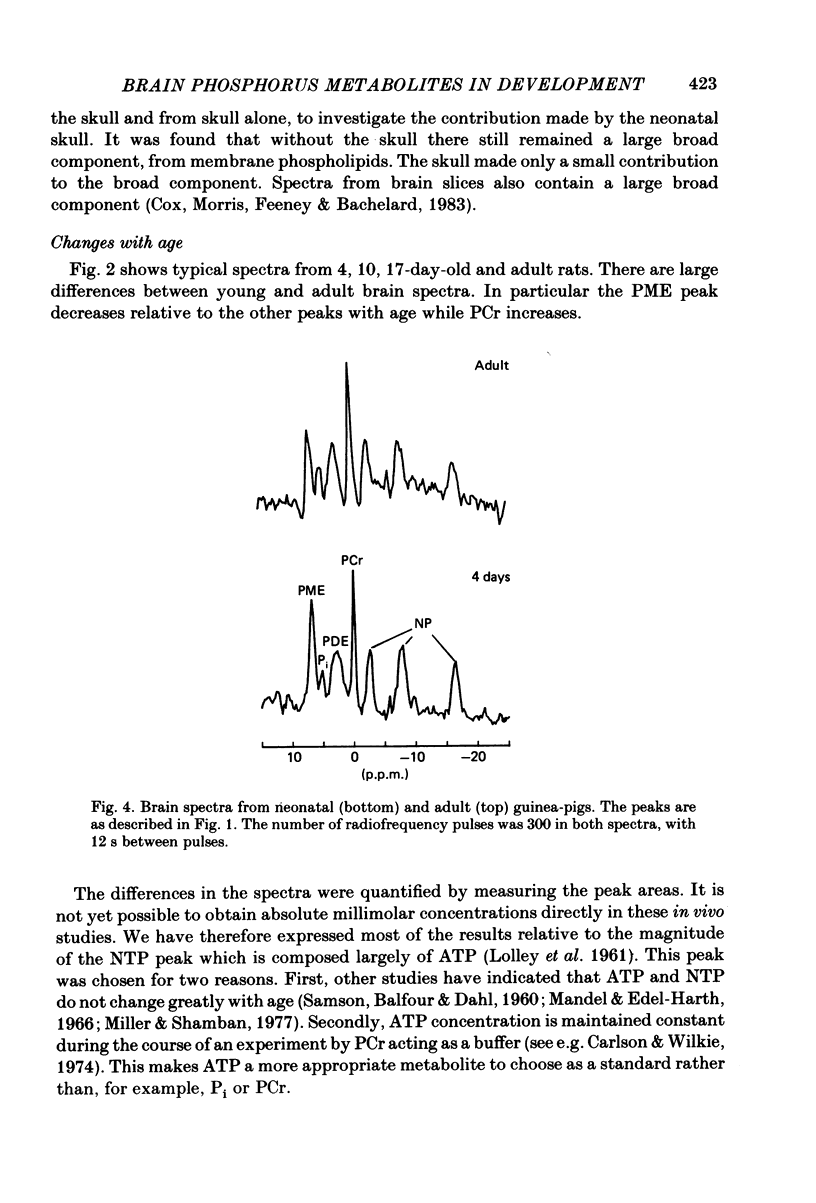
Changes in brain phosphorus metabolites during the post-natal development of the rat and in neonatal and adult guinea-pigs have been studied in vivo using 31P nuclear magnetic resonance spectroscopy (n.m.r.s.). The brain spectra showed clear differences with age, particularly during the first 3 weeks post-partum. The spectra from 4-day-old rats resembled those of new-born human infants. We suggest that the differences between human and animal brains seen in previously published spectra arise because of an age difference rather than a species difference. The phosphocreatine (PCr) to nucleoside triphosphate (NTP) ratio increased from around 1.0 in 3-day-old rats to 1.8 in adult animals. The adult ratio is larger than that previously reported from in vitro chemical analyses. An unknown compound in the phosphomonoester (PME) region of the spectra predominated in young animals, but decreased in concentration relative to NTP with age and reached adult values by around 2 weeks post-partum. Neonatal guinea-pigs, which are much more developed at birth than the rat, had a significantly greater PCr/NTP ratio than the neonatal rat, but their brain spectra also contained the large PME peak. The intracellular pH of cerebral tissue was estimated to be 7.21 +/- 0.02 and did not show any change with age. The changes we find in the phosphorus compounds in the brain may be of importance in post-natal development, and the possible functional significance of these results is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Agrawal H. C., Davis J. M., Himwich W. A. Postnatal changes in free amino acid pool of rat brain. J Neurochem. 1966 Jul;13(7):607–615. doi: 10.1111/j.1471-4159.1966.tb11957.x. [DOI] [PubMed] [Google Scholar]
- Cady E. B., Costello A. M., Dawson M. J., Delpy D. T., Hope P. L., Reynolds E. O., Tofts P. S., Wilkie D. R. Non-invasive investigation of cerebral metabolism in newborn infants by phosphorus nuclear magnetic resonance spectroscopy. Lancet. 1983 May 14;1(8333):1059–1062. doi: 10.1016/s0140-6736(83)91906-2. [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Westerberg E., Siesjö B. K. The metabolism of purine and pyrimidine nucleotides in rat cortex during insulin-induced hypoglycemia and recovery. J Neurochem. 1981 Jan;36(1):179–189. doi: 10.1111/j.1471-4159.1981.tb02393.x. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Morris P. G., Feeney J., Bachelard H. S. 31P-n.m.r. studies on cerebral energy metabolism under conditions of hypoglycaemia and hypoxia in vitro. Biochem J. 1983 May 15;212(2):365–370. doi: 10.1042/bj2120365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpy D. T., Gordon R. E., Hope P. L., Parker D., Reynolds E. O., Shaw D., Whitehead M. D. Noninvasive investigation of cerebral ischemia by phosphorus nuclear magnetic resonance. Pediatrics. 1982 Aug;70(2):310–313. [PubMed] [Google Scholar]
- Duffy T. E., Kohle S. J., Vannucci R. C. Carbohydrate and energy metabolism in perinatal rat brain: relation to survival in anoxia. J Neurochem. 1975 Feb;24(2):271–276. doi: 10.1111/j.1471-4159.1975.tb11875.x. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Dawson M. J., Wilkie D. R., Gordon R. E., Shaw D. Clinical use of nuclear magnetic resonance in the investigation of myopathy. Lancet. 1982 Mar 27;1(8274):725–731. doi: 10.1016/s0140-6736(82)92635-6. [DOI] [PubMed] [Google Scholar]
- FERRARI V., HARKNESS R. D. Free amino-acids in liver and blood after partial hepatectomy in normal and adrenalectomized rats. J Physiol. 1954 Jun 28;124(3):443–463. doi: 10.1113/jphysiol.1954.sp005120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glonek T., Kopp S. J., Kot E., Pettegrew J. W., Harrison W. H., Cohen M. M. P-31 nuclear magnetic resonance analysis of brain: the perchloric acid extract spectrum. J Neurochem. 1982 Nov;39(5):1210–1219. doi: 10.1111/j.1471-4159.1982.tb12557.x. [DOI] [PubMed] [Google Scholar]
- Griffiths J. R., Cady E., Edwards R. H., McCready V. R., Wilkie D. R., Wiltshaw E. 31P-NMR studies of a human tumour in situ. Lancet. 1983 Jun 25;1(8339):1435–1436. doi: 10.1016/s0140-6736(83)92375-9. [DOI] [PubMed] [Google Scholar]
- Hope P. L., Costello A. M., Cady E. B., Delpy D. T., Tofts P. S., Chu A., Hamilton P. A., Reynolds E. O., Wilkie D. R. Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet. 1984 Aug 18;2(8399):366–370. doi: 10.1016/s0140-6736(84)90539-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V. THE RELATIONSHIPS BETWEEN SUBSTRATES AND ENZYMES OF GLYCOLYSIS IN BRAIN. J Biol Chem. 1964 Jan;239:31–42. [PubMed] [Google Scholar]
- Lust W. D., Passonneau J. V., Veech R. L. Cyclic adenosine monphosphate, metabolites, and phosphorylase in neural tissue: a comparison a methods of fixation. Science. 1973 Jul 20;181(4096):280–282. doi: 10.1126/science.181.4096.280. [DOI] [PubMed] [Google Scholar]
- Mandel P., Edel-Harth S. Free nucleotides in the rat brain during post-natal development. J Neurochem. 1966 Jul;13(7):591–595. doi: 10.1111/j.1471-4159.1966.tb11955.x. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Shamban A. A comparison of methods for stopping intermediary metabolism of developing rat brain. J Neurochem. 1977 Jun;28(6):1327–1334. doi: 10.1111/j.1471-4159.1977.tb12328.x. [DOI] [PubMed] [Google Scholar]
- Ozand P. T., Stevenson J. H., Tildon J. T., Cornblath M. The effects of hyperketonemia on glycolytic intermediates in the developing rat brian. J Neurochem. 1975 Jul;25(1):61–65. doi: 10.1111/j.1471-4159.1975.tb07694.x. [DOI] [PubMed] [Google Scholar]
- Pettegrew J. W., Minshew N. J., Diehl J., Smith T., Kopp S. J., Glonek T. Anatomical considerations for interpreting topical P-31 NMR. Lancet. 1983 Oct 15;2(8355):913–913. doi: 10.1016/s0140-6736(83)90896-6. [DOI] [PubMed] [Google Scholar]
- SAMSON F. E., Jr, BALFOUR W. M., DAHL N. A. Rate of cerebral ATP utilization in rats. Am J Physiol. 1960 Jan;198:213–216. doi: 10.1152/ajplegacy.1960.198.1.213. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Briggs R. W., Radda G. K. 31p NMR saturation transfer measurements of the steady state rates of creatine kinase and ATP synthetase in the rat brain. FEBS Lett. 1982 Apr 19;140(2):289–292. doi: 10.1016/0014-5793(82)80916-2. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., du Boulay G. H., Duchen L. W., Radda G. A 31P nuclear magnetic resonance in vivo study of cerebral ischaemia in the gerbil. J Cereb Blood Flow Metab. 1982 Sep;2(3):299–306. doi: 10.1038/jcbfm.1982.31. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Harris R. L., Veloso D., Veech E. H. Freeze-blowing: a new technique for the study of brain in vivo. J Neurochem. 1973 Jan;20(1):183–188. doi: 10.1111/j.1471-4159.1973.tb12115.x. [DOI] [PubMed] [Google Scholar]


