Abstract
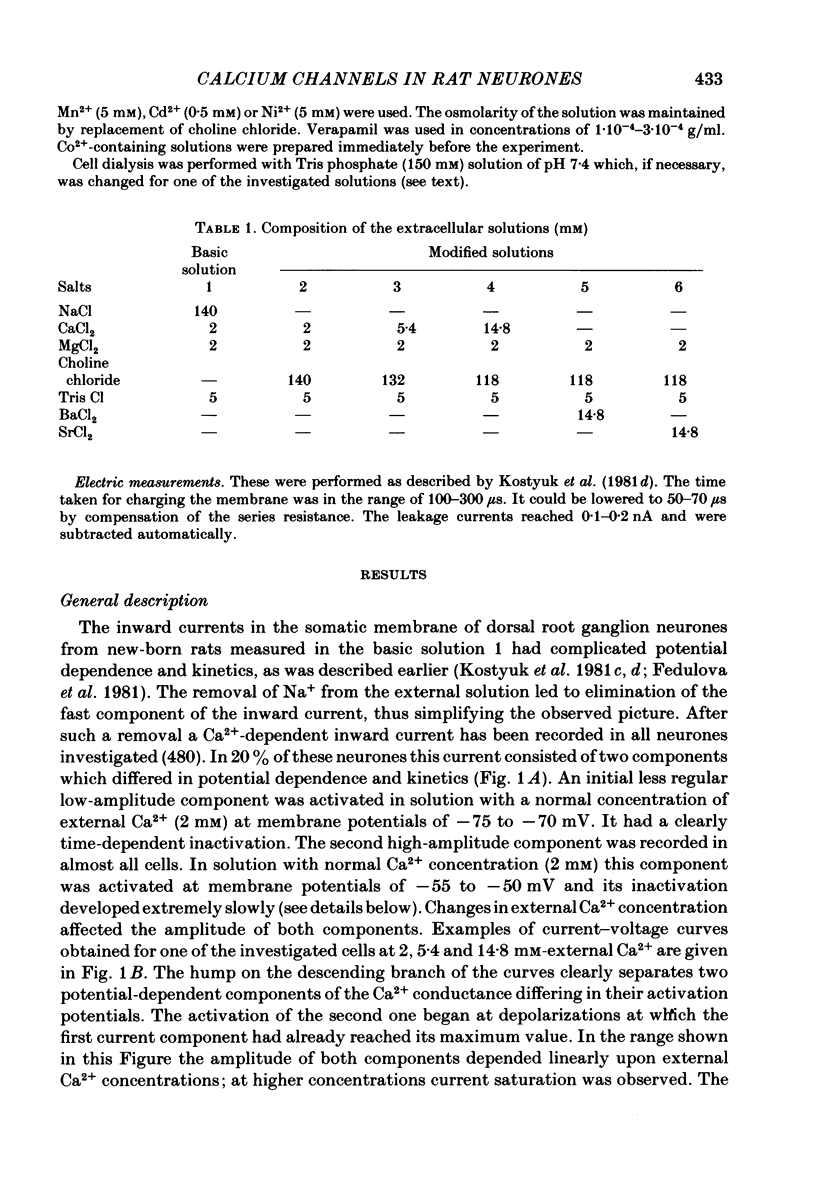
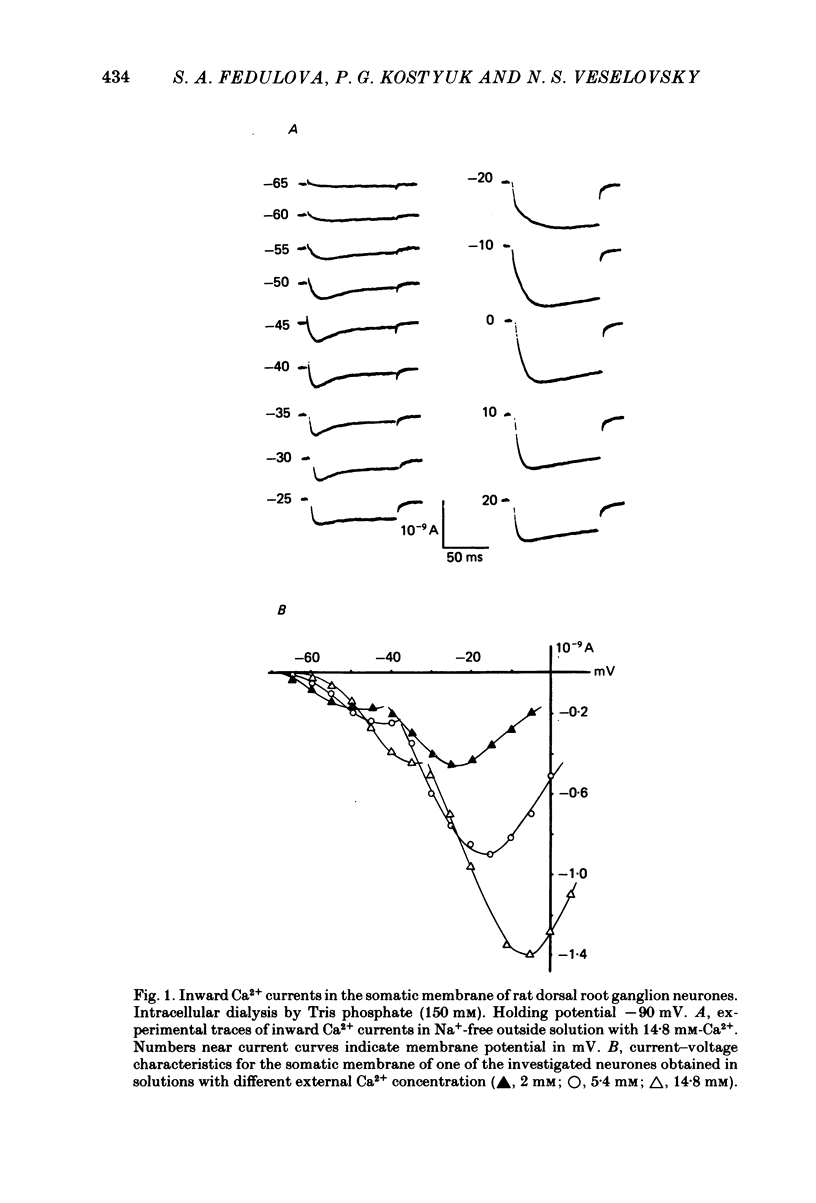
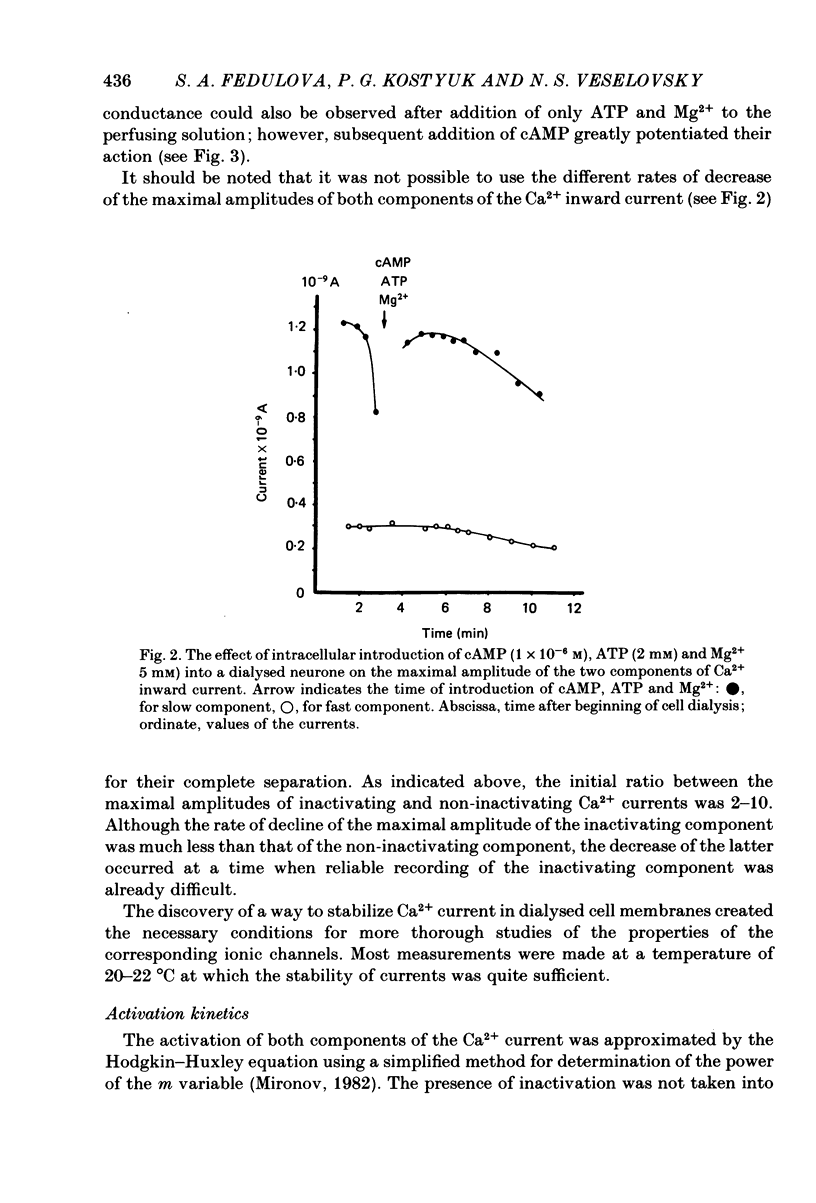
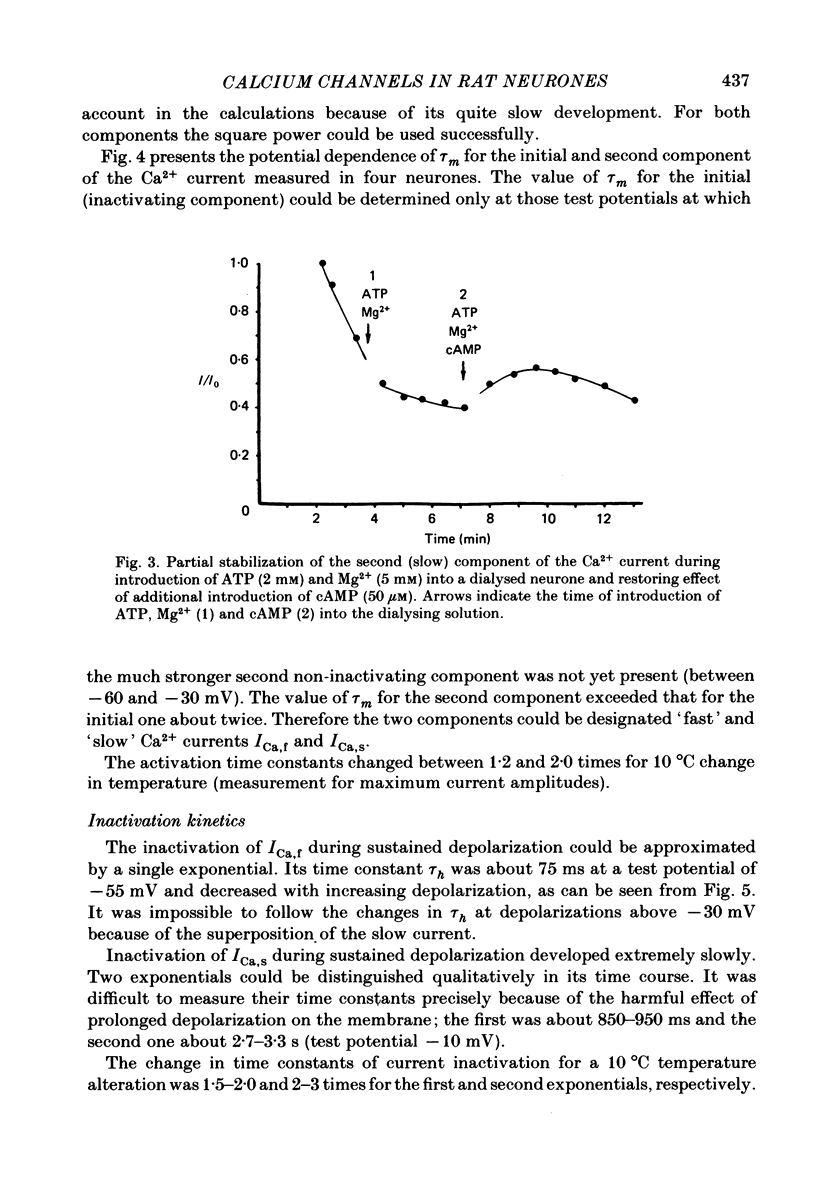
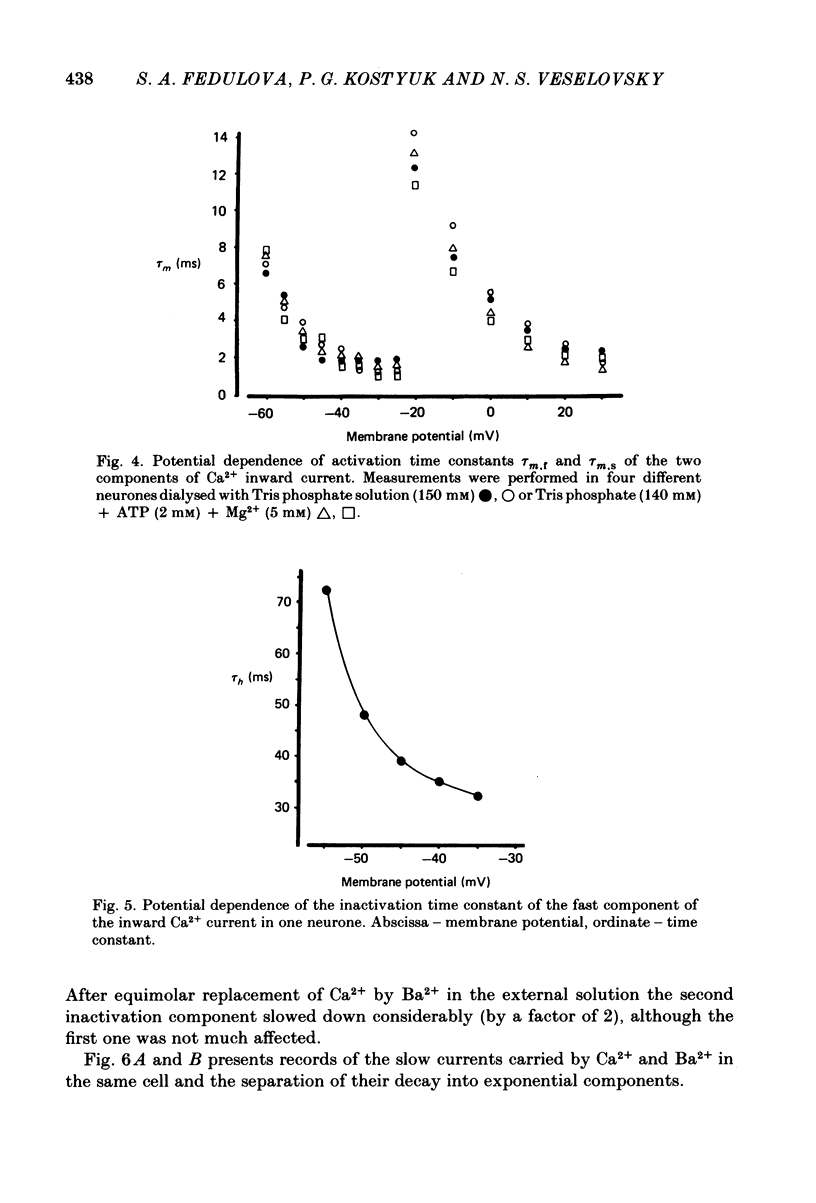
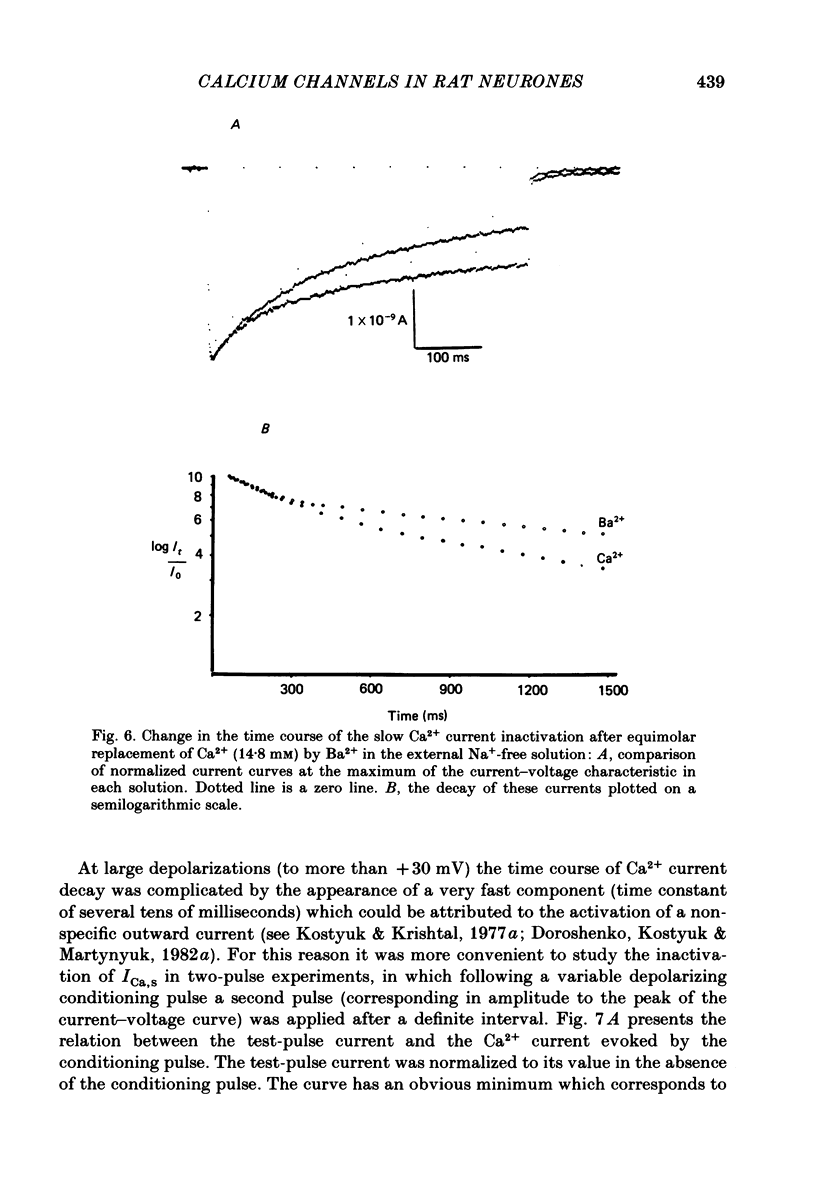
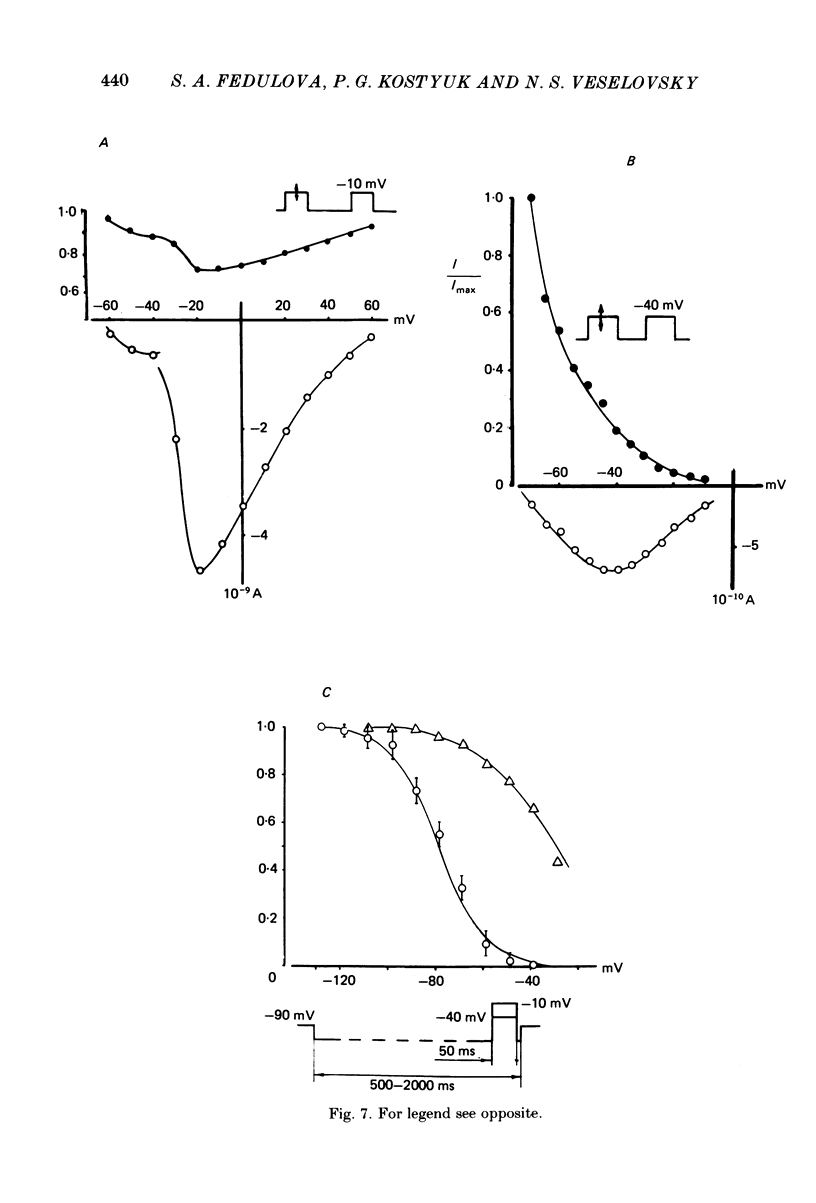
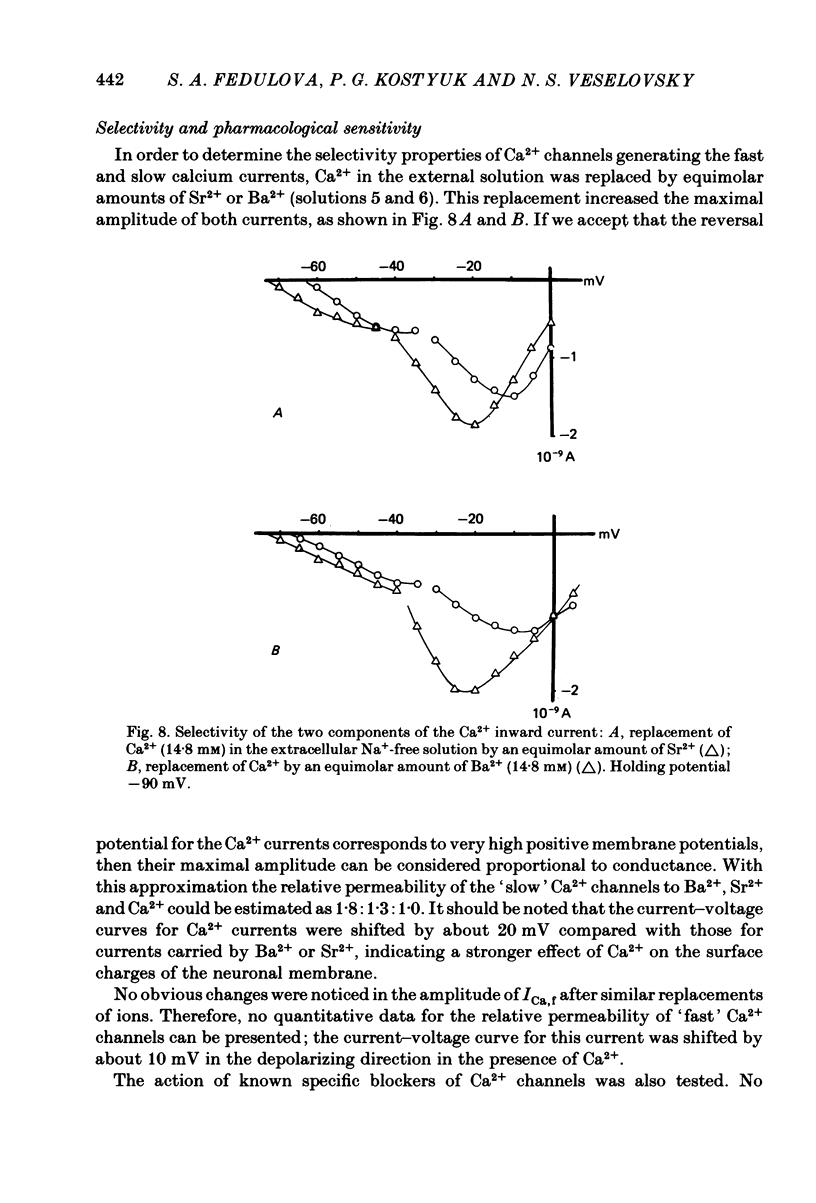
Ca2+ inward currents evoked by membrane depolarization have been studied by the intracellular dialysis technique in the somatic membrane of isolated dorsal root ganglion neurones of new-born rats. In about 20% of the investigated cells a hump has been detected on the descending branch of the current-voltage curve, indicating the presence of two populations of Ca2+ channels differing in their potential-dependent characteristics. An initial less regular component of the Ca2+ current was activated at membrane potentials from -75 to -70 mV. Its amplitude reached 0.2-0.9 nA at 14.6 mM-extracellular Ca2+. The activation kinetics of this component could be approximated by the Hodgkin-Huxley equation using the square of the m variable. tau m varied in the range from 8 to 1 ms at potentials between -60 and -25 mV ('fast' Ca2+ current). The second component of the Ca2+ current was activated at membrane depolarizations to between -55 and -50 mV. It could be recorded in all cells investigated and reached a maximum value of 1-7 nA at the same extracellular Ca2+ concentration. This component decreased rapidly during cell dialysis with saline solutions. The decrease could be slowed down by cooling and accelerated by warming the extracellular solution. Intracellular introduction of 3',5'-cAMP together with ATP and Mg2+ not only prevented the decrease but often restored the maximal current amplitude to its initial level. The activation kinetics of this component could also be approximated by a square function, tau m being in the range 16-2.5 ms at membrane potentials between -20 and +3 mV ('slow' Ca2+ current). The fast Ca2+ current inactivated exponentially at sustained depolarizations in a potential-dependent manner, tau h varying from 76 to 35 ms at potentials between -50 and -30 mV. The inactivation of the slow Ca2+ current studied in double-pulse experiments was current-dependent and developed very slowly (time constant of several hundreds of milliseconds). It slowed down even more at low temperature or after substitution of Ba2+ for Ca2+ in the extracellular solution. Both currents could also be carried by Ba2+ and Sr2+, although the ion-selecting properties of the two types of channels showed quantitative differences. Specific blockers of Ca2+ channels (Co2+, Mn2+, Cd2+, Ni2+ or verapamil) exerted similar effects on them. The existence of metabolically dependent and metabolically independent Ca2+ channels in the neuronal membrane and their possible functional role are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Morimoto K., Tsuda Y., wilson D. L. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. J Physiol. 1981 Nov;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshenko P. A., Kostyuk P. G., Martynyuk A. E. Intracellular metabolism of adenosine 3',5'-cyclic monophosphate and calcium inward current in perfused neurones of Helix pomatia. Neuroscience. 1982;7(9):2125–2134. doi: 10.1016/0306-4522(82)90124-5. [DOI] [PubMed] [Google Scholar]
- Doroshenko P. A., Kostyuk P. G., Martynyuk A. E., Kursky M. D., Vorobetz Z. D. Intracellular protein kinase and calcium inward currents in perfused neurones of the snail Helix pomatia. Neuroscience. 1984 Jan;11(1):263–267. doi: 10.1016/0306-4522(84)90229-x. [DOI] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Calcium channels in the somatic membrane of the rat dorsal root ganglion neurons, effect of cAMP. Brain Res. 1981 Jun 9;214(1):210–214. doi: 10.1016/0006-8993(81)90457-1. [DOI] [PubMed] [Google Scholar]
- Fishman M. C., Spector I. Potassium current suppression by quinidine reveals additional calcium currents in neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5245–5249. doi: 10.1073/pnas.78.8.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda J., Kameyama M., Yamaguchi K. Breakdown of cytoskeletal filaments selectively reduces Na and Ca spikes in cultured mammal neurones. Nature. 1981 Nov 5;294(5836):82–85. doi: 10.1038/294082a0. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K. R., Strumwasser F., Nairn A. C., Walter U., Wilson F. D., Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A. Effects of calcium and calcium-chelating agents on the inward and outward current in the membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):569–580. doi: 10.1113/jphysiol.1977.sp011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Calcium inward current and related charge movements in the membrane of snail neurones. J Physiol. 1981 Jan;310:403–421. doi: 10.1113/jphysiol.1981.sp013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Effect of internal fluoride and phosphate on membrane currents during intracellular dialysis of nerve cells. Nature. 1975 Oct 23;257(5528):691–693. doi: 10.1038/257691a0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-II. Calcium currents. Neuroscience. 1981;6(12):2431–2437. doi: 10.1016/0306-4522(81)90089-0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-I. Sodium currents. Neuroscience. 1981;6(12):2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Matsumoto G., Sakai H. Restoration of membrane excitability of squid giant axons by reagents activating tyrosine-tubulin ligase. J Membr Biol. 1979 Oct 5;50(1):15–22. doi: 10.1007/BF01868785. [DOI] [PubMed] [Google Scholar]
- Mironov S. L. Opredelenie pokazatelia stepeni peremennoi aktivatsii ionnykh kanalov dlia modeli Khodzhkina--Khaksli. Neirofiziologiia. 1982;14(2):207–208. [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K., Randić M. Electrophysiological properties of rat spinal dorsal horn neurones in vitro: calcium-dependent action potentials. J Physiol. 1983 Jan;334:141–153. doi: 10.1113/jphysiol.1983.sp014485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. Influx of calcium, strontium, and barium in presynaptic nerve endings. J Gen Physiol. 1982 Jun;79(6):1065–1087. doi: 10.1085/jgp.79.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B. Calcium current inactivation in identified neurones of Helix aspersa. J Physiol. 1981 Dec;321:273–285. doi: 10.1113/jphysiol.1981.sp013983. [DOI] [PMC free article] [PubMed] [Google Scholar]


