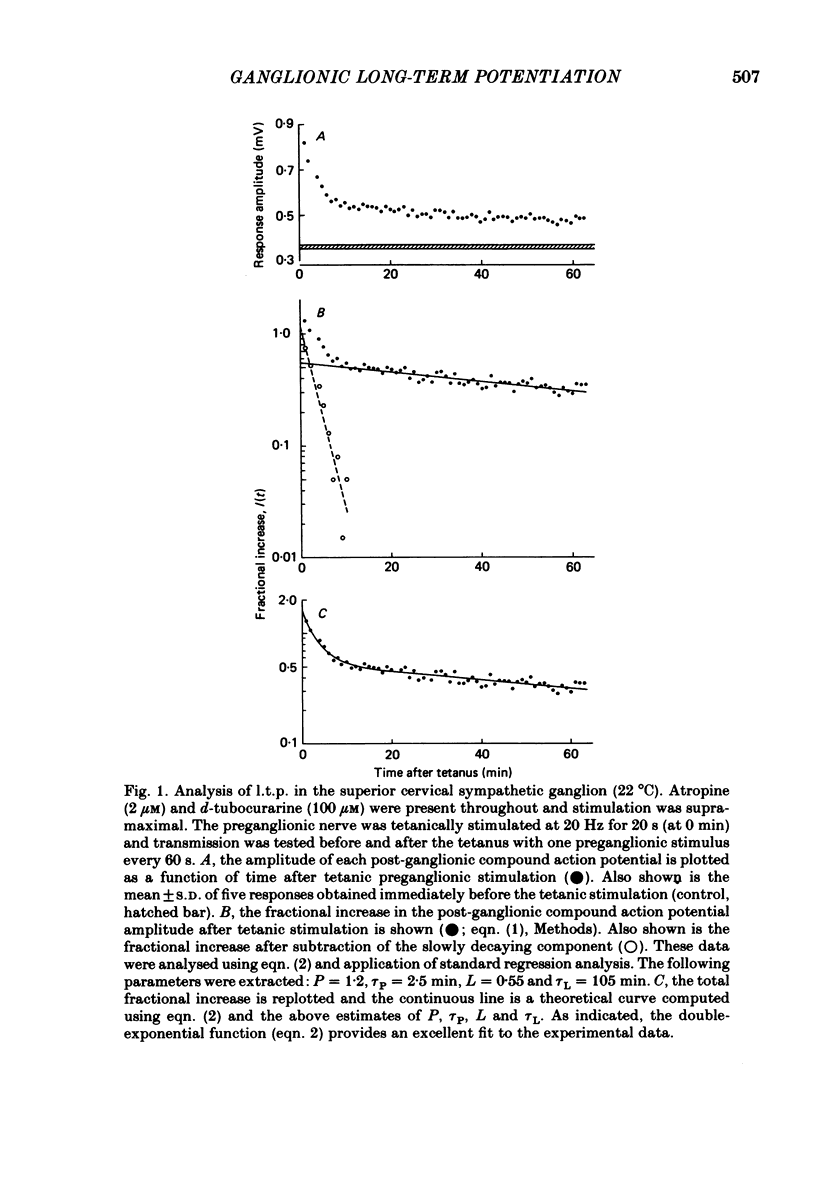
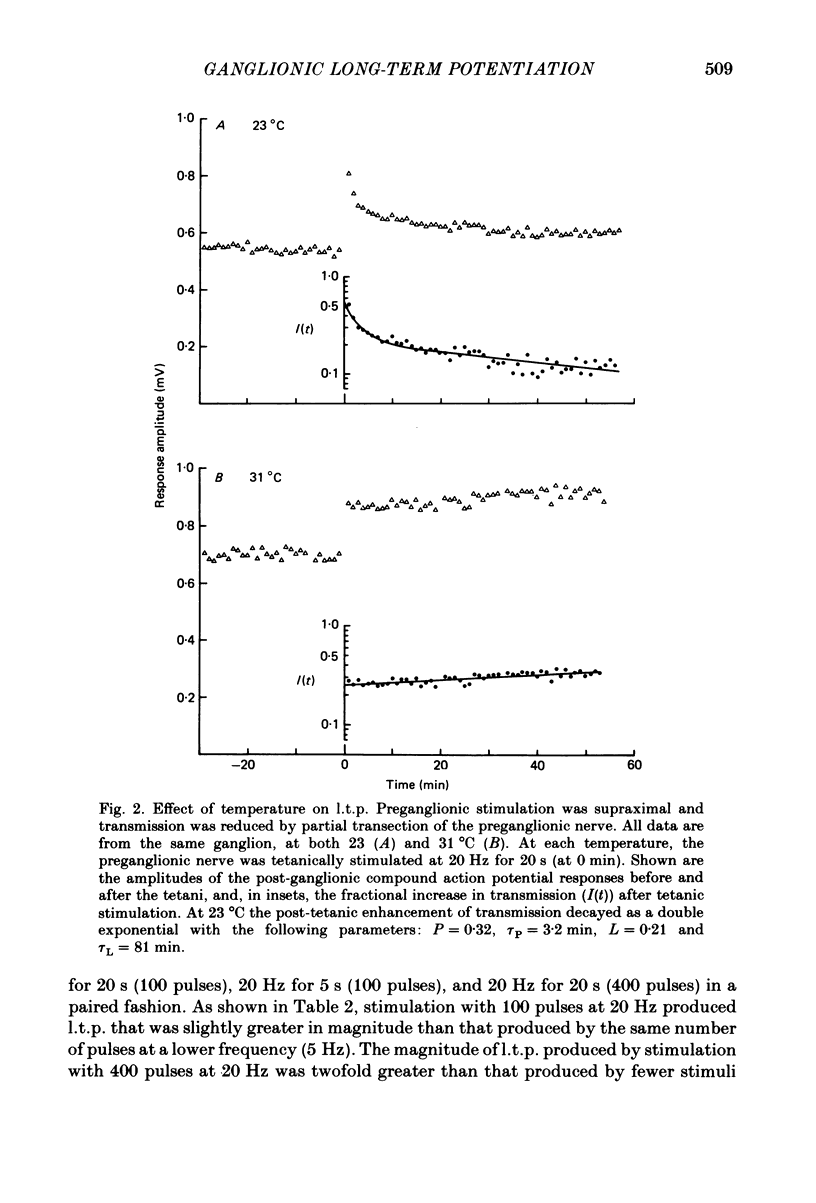
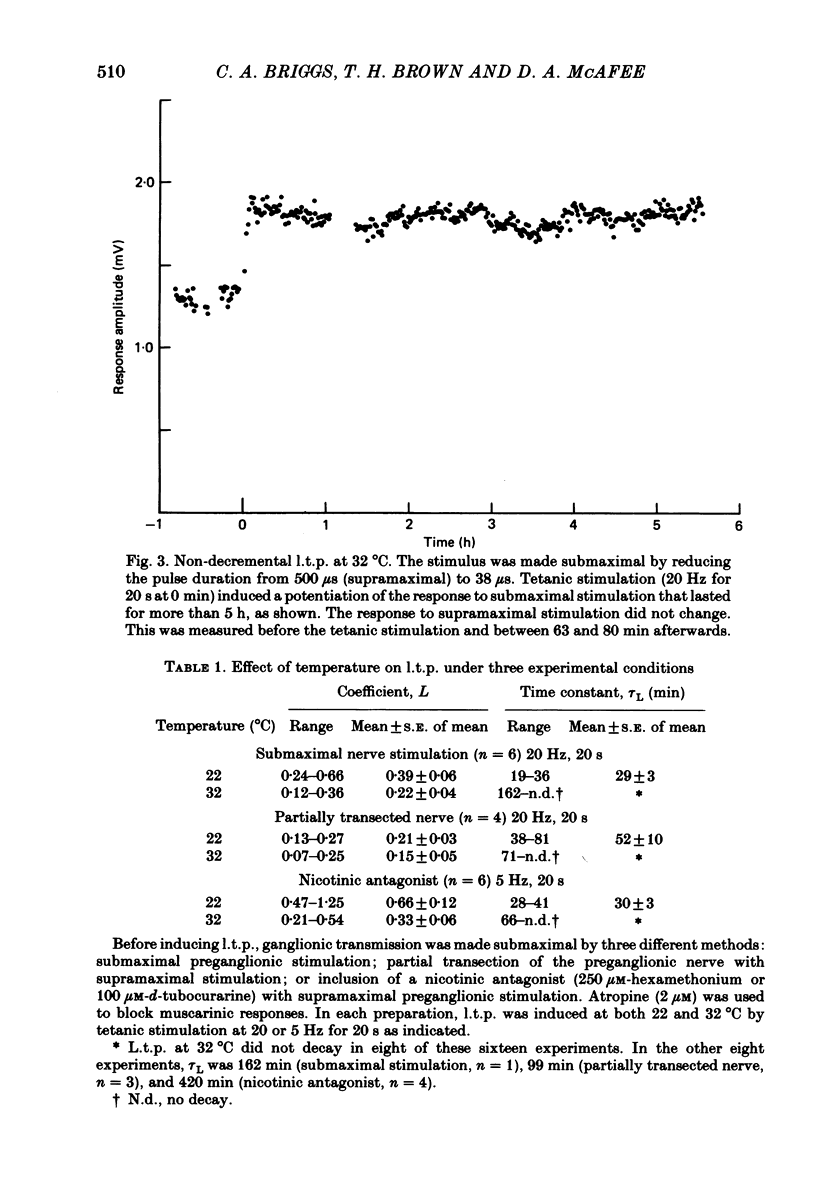
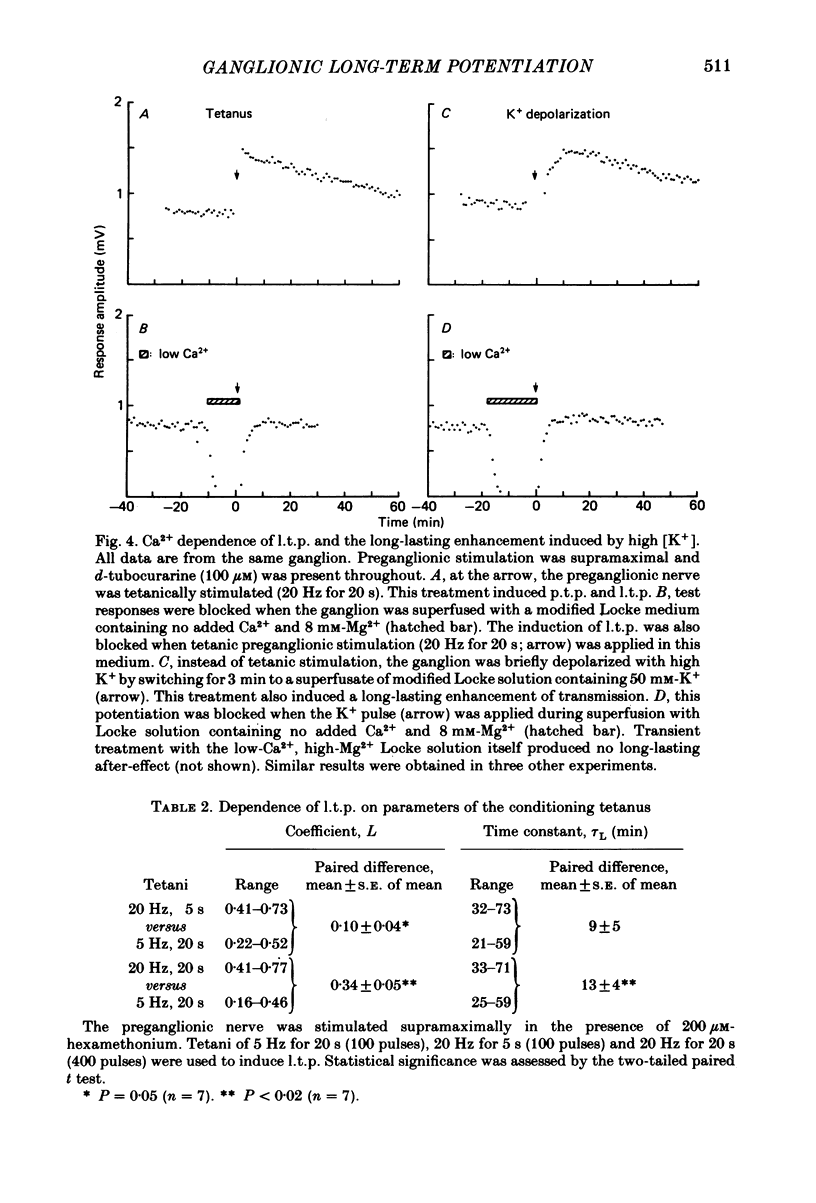
Abstract
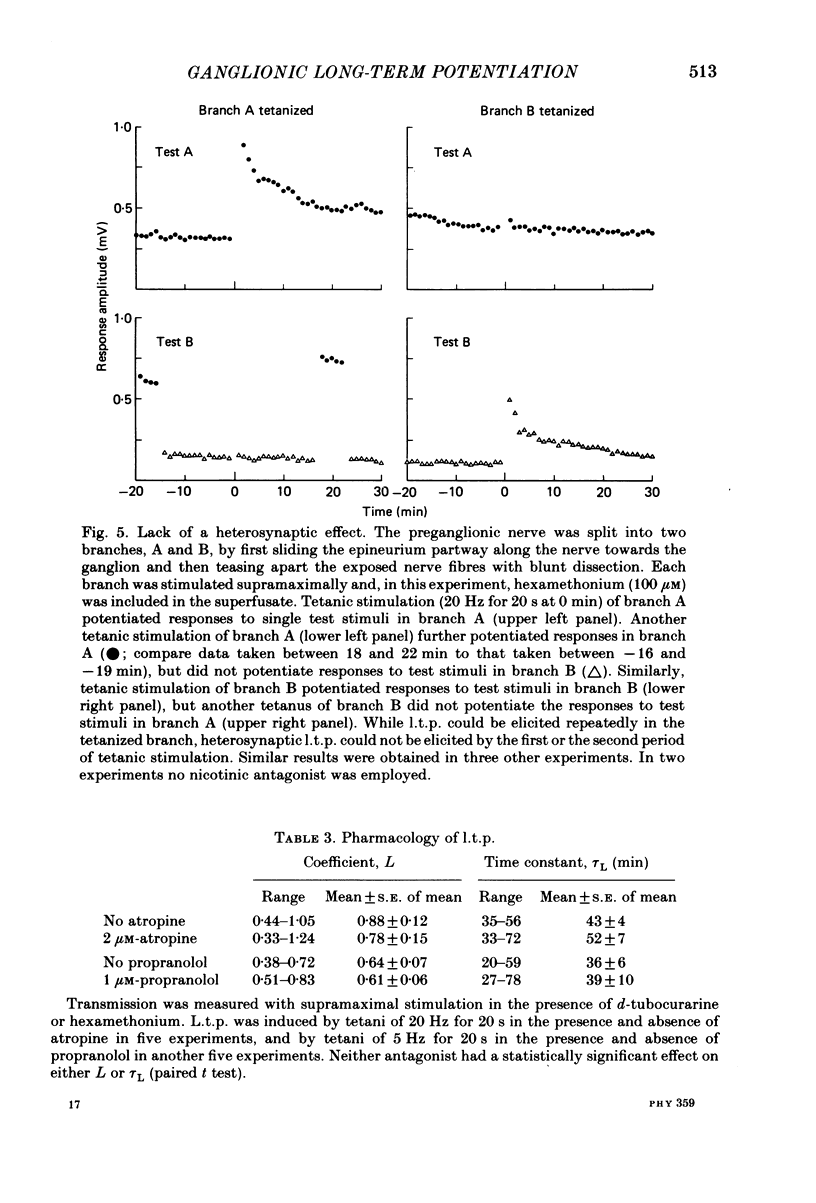
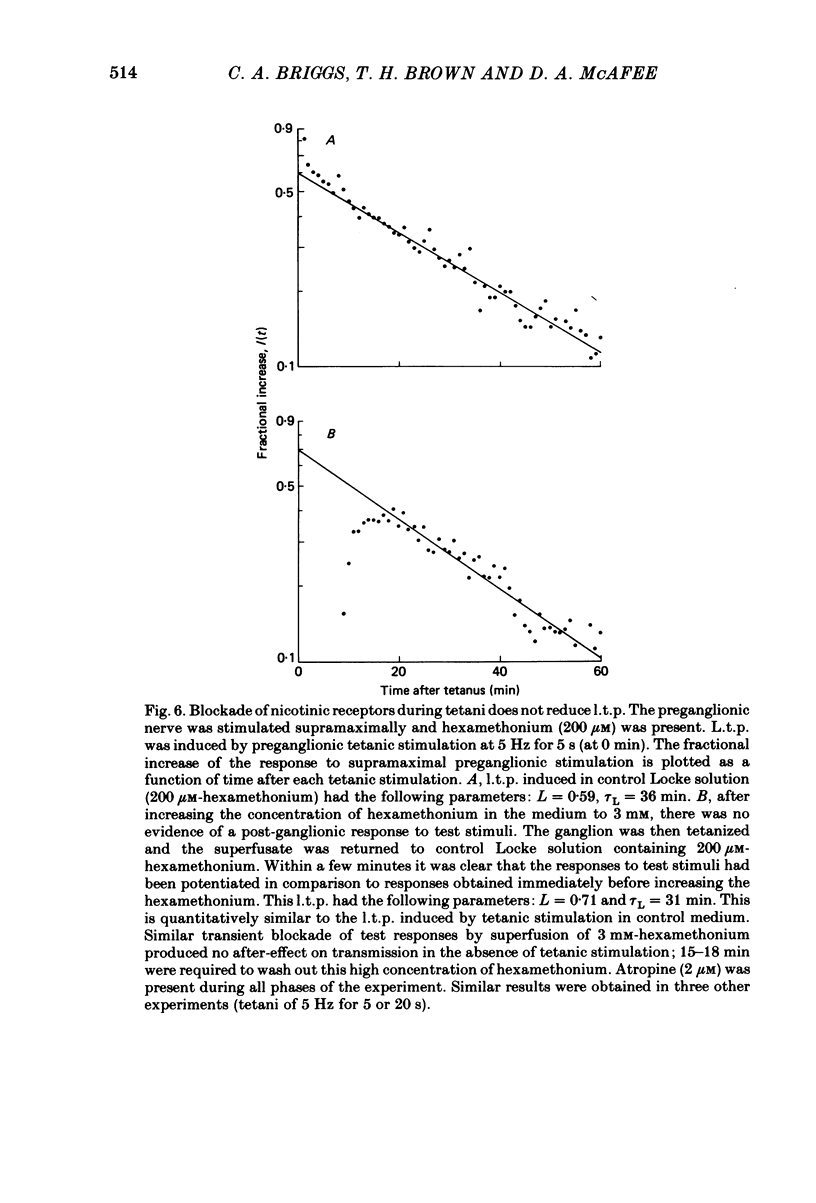
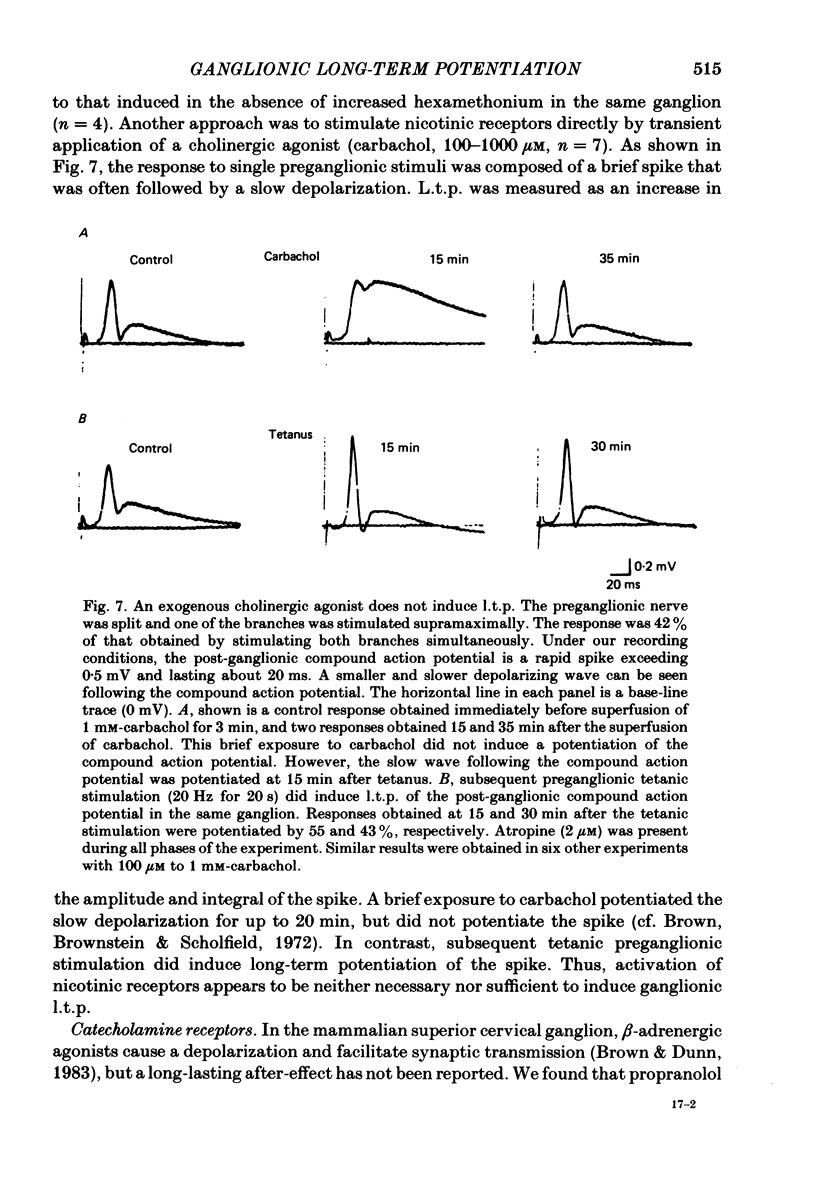
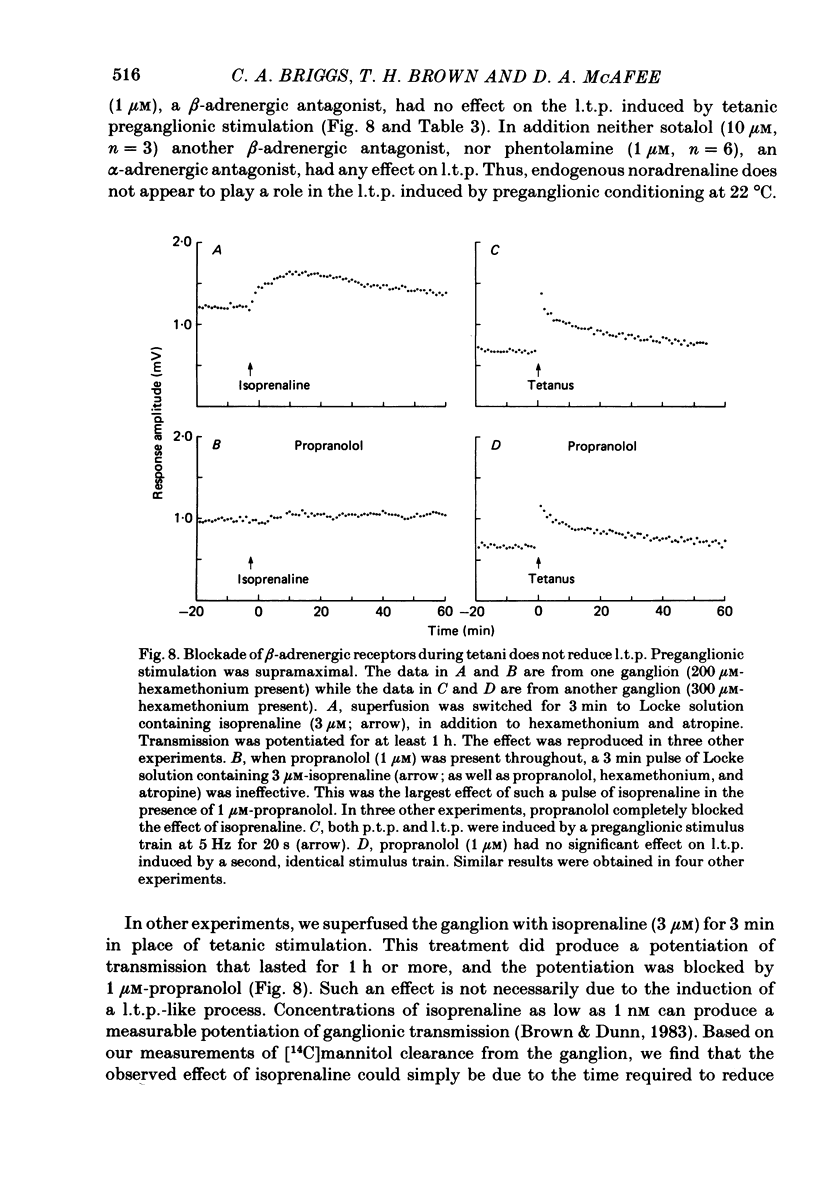
Brief tetanic stimulation of the preganglionic nerve induced a persistent potentiation of nicotinic synaptic transmission in the rat superior cervical sympathetic ganglion. Quantitative measurements of the post-tetanic increase in synaptic efficacy revealed two distinct time courses. The early, rapidly decaying component, termed post-tetanic potentiation (p.t.p.), had a decay time constant of 2-3 min, as reported elsewhere. The duration of the more persistent component, called long-term potentiation (l.t.p.), was extremely temperature dependent, lasting much longer at 32 degrees C than at 22 degrees C. In half of the experiments performed at 32 degrees C, l.t.p. showed no detectable decay over the course of 1 h or more after a brief tetanic stimulation. Other experiments were conducted at 22 degrees C. The induction of l.t.p. was dependent on the extracellular [Ca2+]. Transient elevation of the extracellular [K+] also produced a long-term enhancement of synaptic efficacy, and this effect was also Ca2+ dependent. The tetani that were effective in inducing l.t.p. (5-20 Hz for 5-20 s) were well within the physiological range of preganglionic activity. The magnitude and time course were related to frequency and duration of stimulation. The occurrence of l.t.p. was restricted to those preganglionic fibres that were tetanically stimulated. This lack of heterosynaptic or generalized effects was demonstrated by splitting the preganglionic nerve into two branches that could be independently tested and conditioned. Physiological activation of muscarinic or nicotinic receptors apparently does not play an essential role in causing ganglionic l.t.p., which is expressed as an enhancement of nicotinic transmission. A muscarinic antagonist (2 microM-atropine) did not block l.t.p. Preganglionic stimulation induced l.t.p. even when a high concentration of a nicotinic antagonist (3 mM-hexamethonium) was present during the tetanic stimulation. Furthermore, bath application of a cholinergic agonist (100-1000 microM-carbachol) could not substitute for tetanic stimulation in provoking l.t.p. Activation of adrenergic receptors also appeared not to play an essential role. Neither a beta-adrenergic antagonist (10 microM-sotolol or 1 microM-propranolol) nor an alpha-adrenergic antagonist (1 microM-phentolamine) had any significant effect on the magnitude or duration of l.t.p. The results indicate that ganglionic l.t.p. is a Ca2+- and temperature-dependent process that can be created independently of the activation of nicotinic, muscarinic or adrenergic receptors.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashe J. H., Libet B. Modulation of slow postsynaptic potentials by dopamine, in rabbit sympathetic ganglion. Brain Res. 1981 Jul 27;217(1):93–106. doi: 10.1016/0006-8993(81)90187-6. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G., Brown T. H. Associative long-term potentiation in hippocampal slices. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7347–7351. doi: 10.1073/pnas.80.23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Goddard G. V., Riives M. Reduction of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J Physiol. 1983 Jan;334:475–491. doi: 10.1113/jphysiol.1983.sp014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Brownstein M. J., Scholfield C. N. Origin of the after-hyperpolarization that follows removal of depolarizing agents from the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1972 Apr;44(4):651–671. doi: 10.1111/j.1476-5381.1972.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Dunn P. M. Depolarization of rat isolated superior cervical ganglia mediated by beta 2-adrenoceptors. Br J Pharmacol. 1983 Jun;79(2):429–439. doi: 10.1111/j.1476-5381.1983.tb11016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. H., McAfee D. A. Long-term synaptic potentiation in the superior cervical ganglion. Science. 1982 Mar 12;215(4538):1411–1413. doi: 10.1126/science.6278593. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Dunant Y., Dolivo M. Plasticity of synaptic functions in the exised sympathetic ganglion of the rat. Brain Res. 1968 Aug 26;10(2):271–273. doi: 10.1016/0006-8993(68)90134-0. [DOI] [PubMed] [Google Scholar]
- Dunant Y. Presynaptic spike and excitatory postsynaptic potential in sympathetic ganglion: their modifications by pharmacological agents. Prog Brain Res. 1969;31:131–139. doi: 10.1016/S0079-6123(08)63234-3. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V., Roberson N. L., Worth T. Modulation of long-term potentiation: effects of adrenergic and neuroleptic drugs. Pharmacol Biochem Behav. 1982 Dec;17(6):1257–1264. doi: 10.1016/0091-3057(82)90130-7. [DOI] [PubMed] [Google Scholar]
- Eccles J. C. Calcium in long-term potentiation as a model for memory. Neuroscience. 1983 Dec;10(4):1071–1081. doi: 10.1016/0306-4522(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Gerren R. A., Weinberger N. M. Long term potentiation in the magnocellular medial geniculate nucleus of the anesthetized cat. Brain Res. 1983 Apr 11;265(1):138–142. doi: 10.1016/0006-8993(83)91344-6. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Johnston D. Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science. 1984 Oct 19;226(4672):350–352. doi: 10.1126/science.6091272. [DOI] [PubMed] [Google Scholar]
- IGGO A., VOGT M. Preganglionic sympathetic activity in normal and in reserpine-treated cats. J Physiol. 1960 Jan;150:114–133. doi: 10.1113/jphysiol.1960.sp006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Sundlöf G., Wallin B. G. Discharge patterns of sympathetic neurons supplying skeletal muscle and skin in man and cat. J Auton Nerv Syst. 1983 Mar-Apr;7(3-4):239–256. doi: 10.1016/0165-1838(83)90077-2. [DOI] [PubMed] [Google Scholar]
- Kuba K., Kato E., Kumamoto E., Koketsu K., Hirai K. Sustained potentiation of transmitter release by adrenaline and dibutyryl cyclic AMP in sympathetic ganglia. Nature. 1981 Jun 25;291(5817):654–656. doi: 10.1038/291654a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Kumamoto E., Kuba K. Independence of presynaptic bimodal actions of adrenaline in sympathetic ganglia. Brain Res. 1983 Apr 18;265(2):344–347. doi: 10.1016/0006-8993(83)90354-2. [DOI] [PubMed] [Google Scholar]
- Kumamoto E., Kuba K. Sustained rise in ACh sensitivity of a sympathetic ganglion cell induced by postsynaptic electrical activities. Nature. 1983 Sep 8;305(5930):145–146. doi: 10.1038/305145a0. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G., POSTERNAK J. M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952 Mar;15(2):91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- Lee K. S. Sustained enhancement of evoked potentials following brief, high-frequency stimulation of the cerebral cortex in vitro. Brain Res. 1982 May 13;239(2):617–623. doi: 10.1016/0006-8993(82)90538-8. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979 Oct 19;175(2):233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Libet B., Kobayashi H., Tanaka T. Synaptic coupling into the production and storage of a neuronal memory trace. Nature. 1975 Nov 13;258(5531):155–157. doi: 10.1038/258155a0. [DOI] [PubMed] [Google Scholar]
- Libet B., Tosaka T. Dopamine as a synaptic transmitter and modulator in sympathetic ganglia: a different mode of synaptic action. Proc Natl Acad Sci U S A. 1970 Oct;67(2):667–673. doi: 10.1073/pnas.67.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Halpain S., Baudry M. Effects of high-frequency synaptic stimulation on glumate receptor binding studied with a modified in vitro hippocampal slice preparation. Brain Res. 1982 Jul 22;244(1):101–111. doi: 10.1016/0006-8993(82)90908-8. [DOI] [PubMed] [Google Scholar]
- Lynch G., Larson J., Kelso S., Barrionuevo G., Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983 Oct 20;305(5936):719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- Neuman R. S., Harley C. W. Long-lasting potentiation of the dentate gyrus population spike by norepinephrine. Brain Res. 1983 Aug 22;273(1):162–165. doi: 10.1016/0006-8993(83)91106-x. [DOI] [PubMed] [Google Scholar]
- Njå A., Purves D. Specific innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Jan;264(2):565–583. doi: 10.1113/jphysiol.1977.sp011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R. J., Milgram N. W., Hafner S. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983 Feb 7;260(2):217–231. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Malthe-Sørenssen D. Increased resting and evoked release of transmitter following repetitive electrical tetanization in hippocampus: a biochemical correlate to long-lasting synaptic potentiation. Brain Res. 1981 Mar 16;208(2):436–441. doi: 10.1016/0006-8993(81)90573-4. [DOI] [PubMed] [Google Scholar]
- Teyler T. J., Discenna P. Long-term potentiation as a candidate mnemonic device. Brain Res. 1984 Mar;319(1):15–28. doi: 10.1016/0165-0173(84)90027-4. [DOI] [PubMed] [Google Scholar]
- Voronin L. L. Long-term potentiation in the hippocampus. Neuroscience. 1983 Dec;10(4):1051–1069. doi: 10.1016/0306-4522(83)90099-4. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L., Horn J. P., McAfee D. A., Yarowsky P. J. Facilitation, augmentation, and potentiation of synaptic transmission at the superior cervical ganglion of the rabbit. J Gen Physiol. 1980 Aug;76(2):213–231. doi: 10.1085/jgp.76.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]



