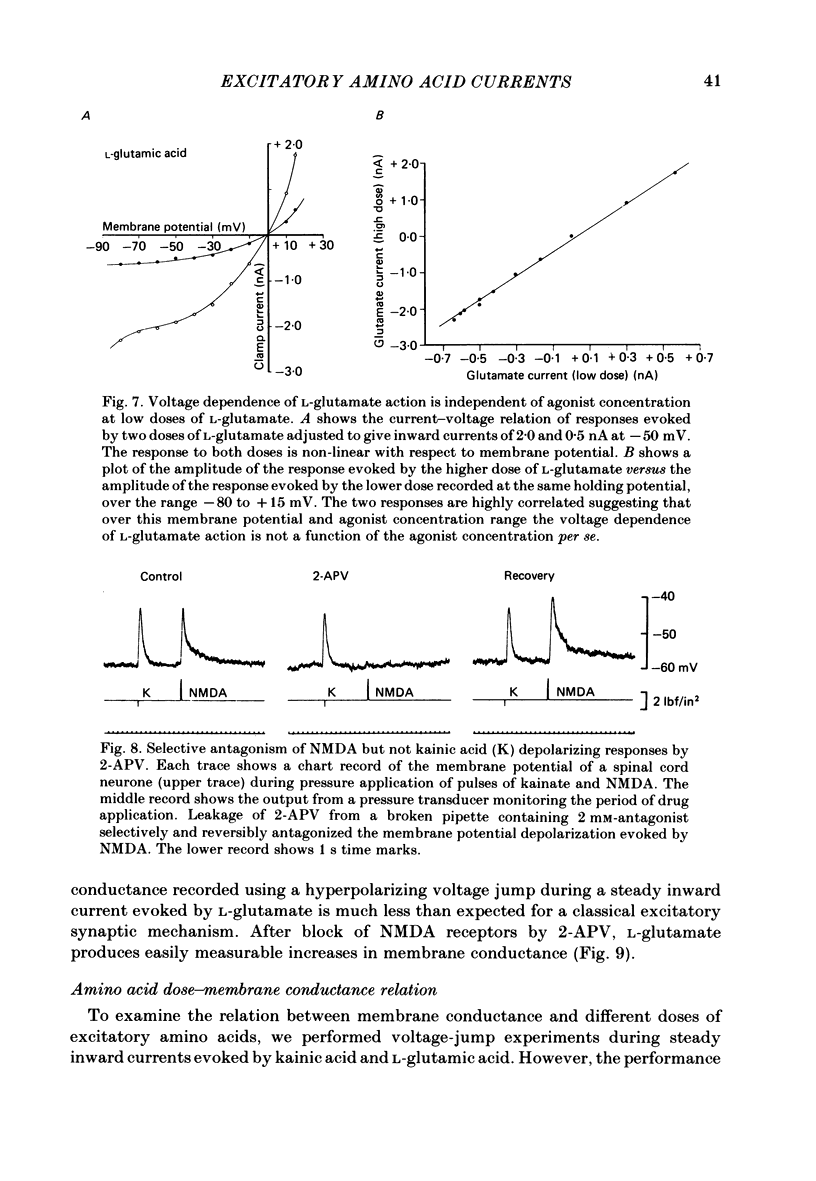
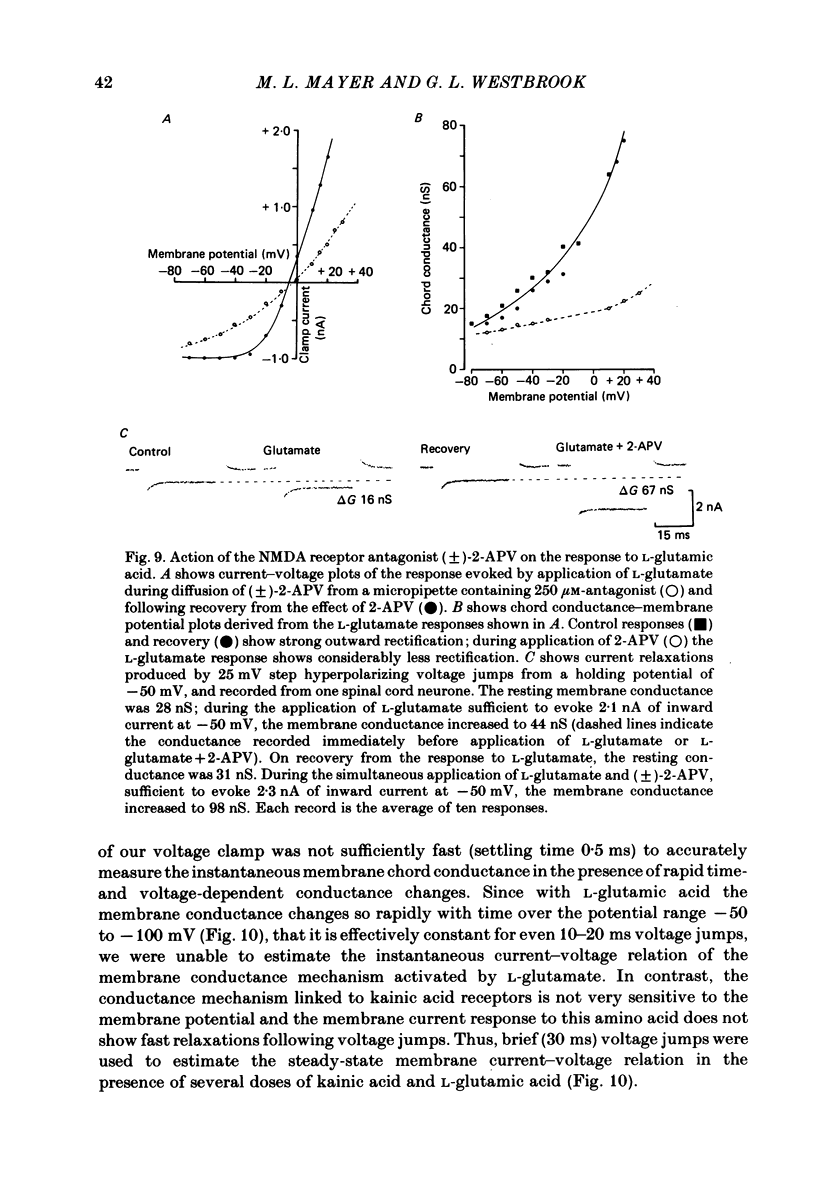
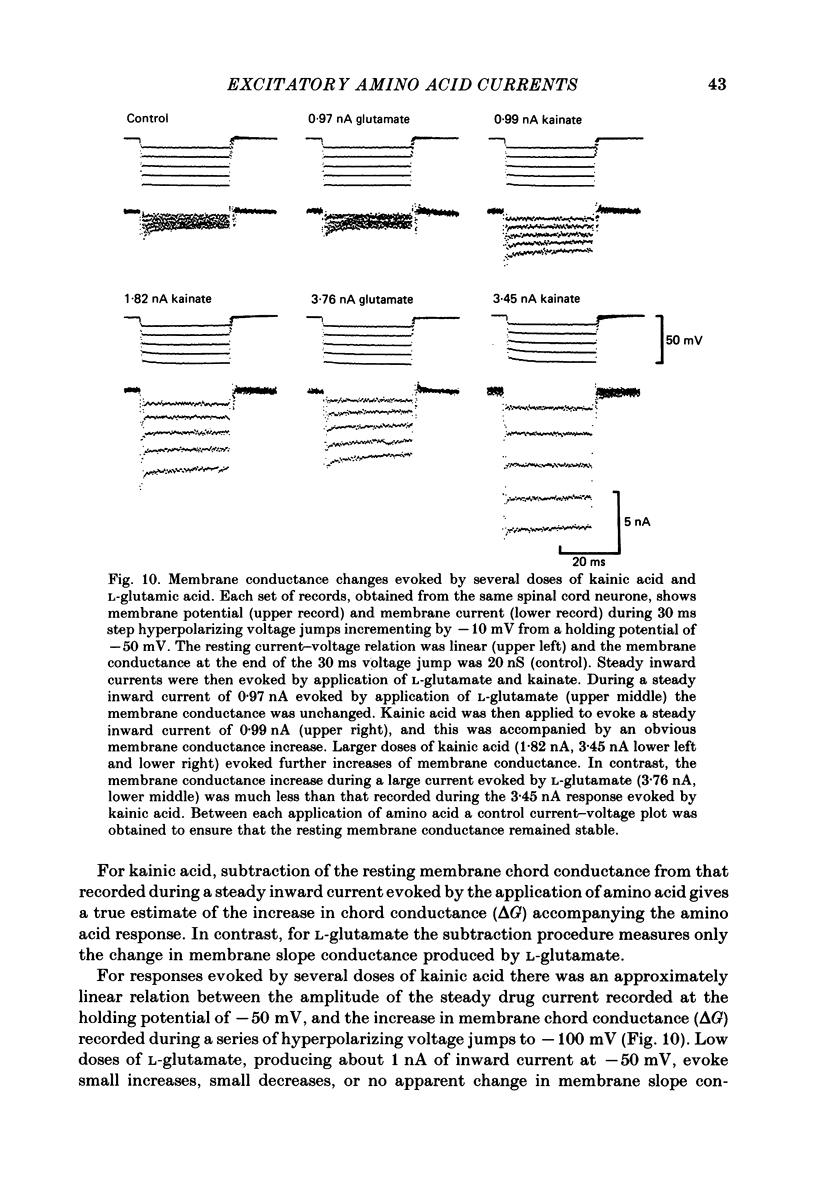
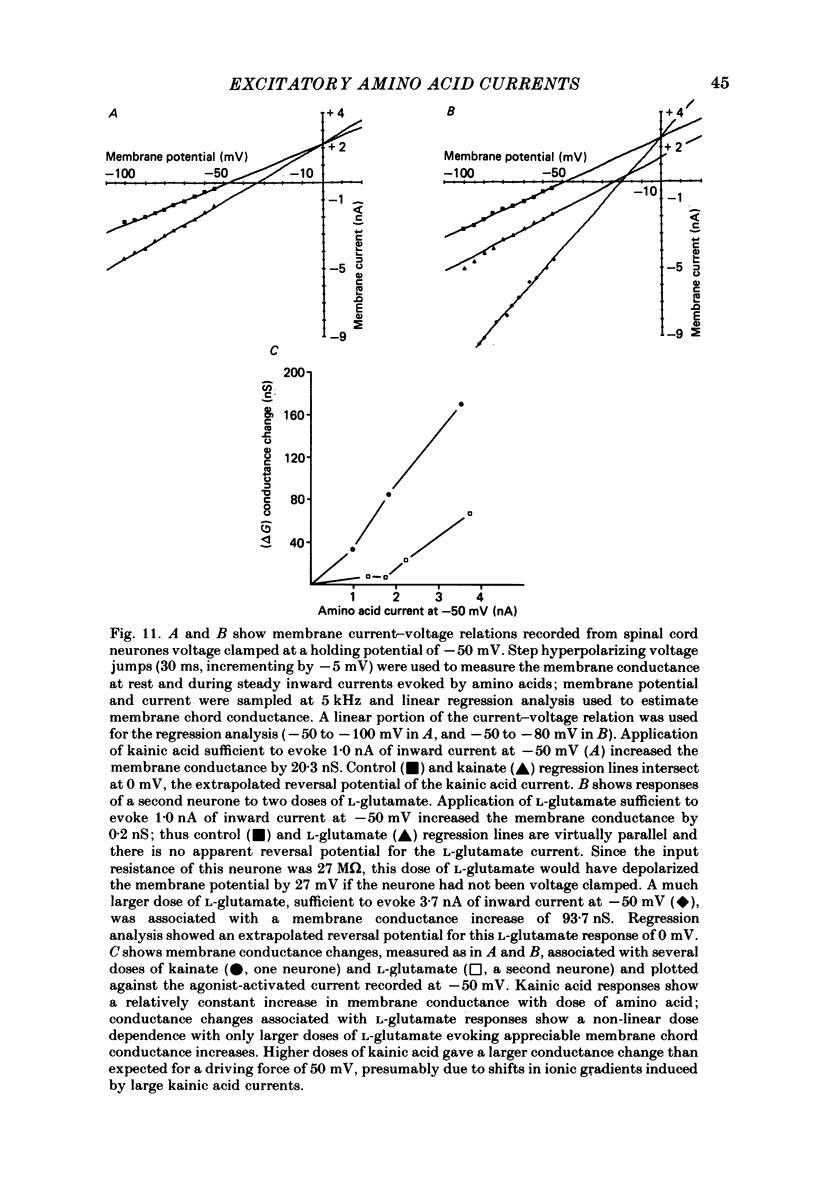
Abstract
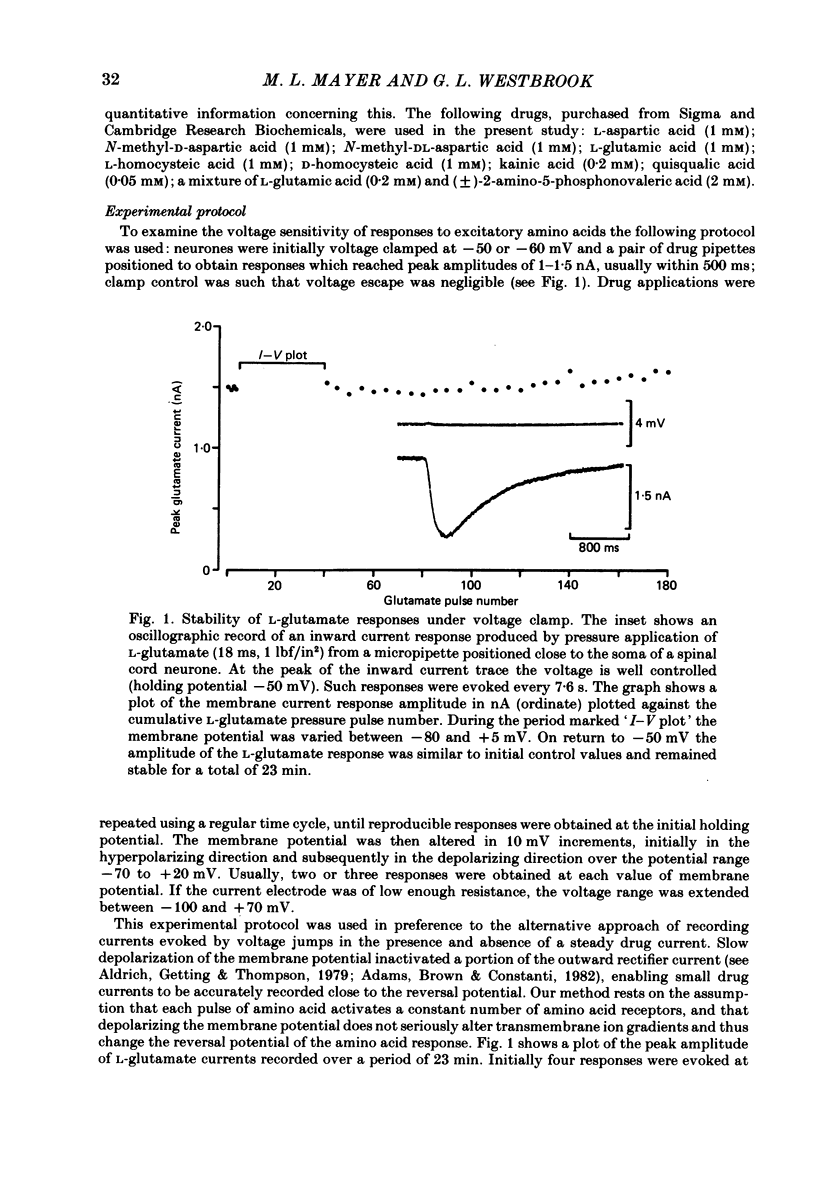
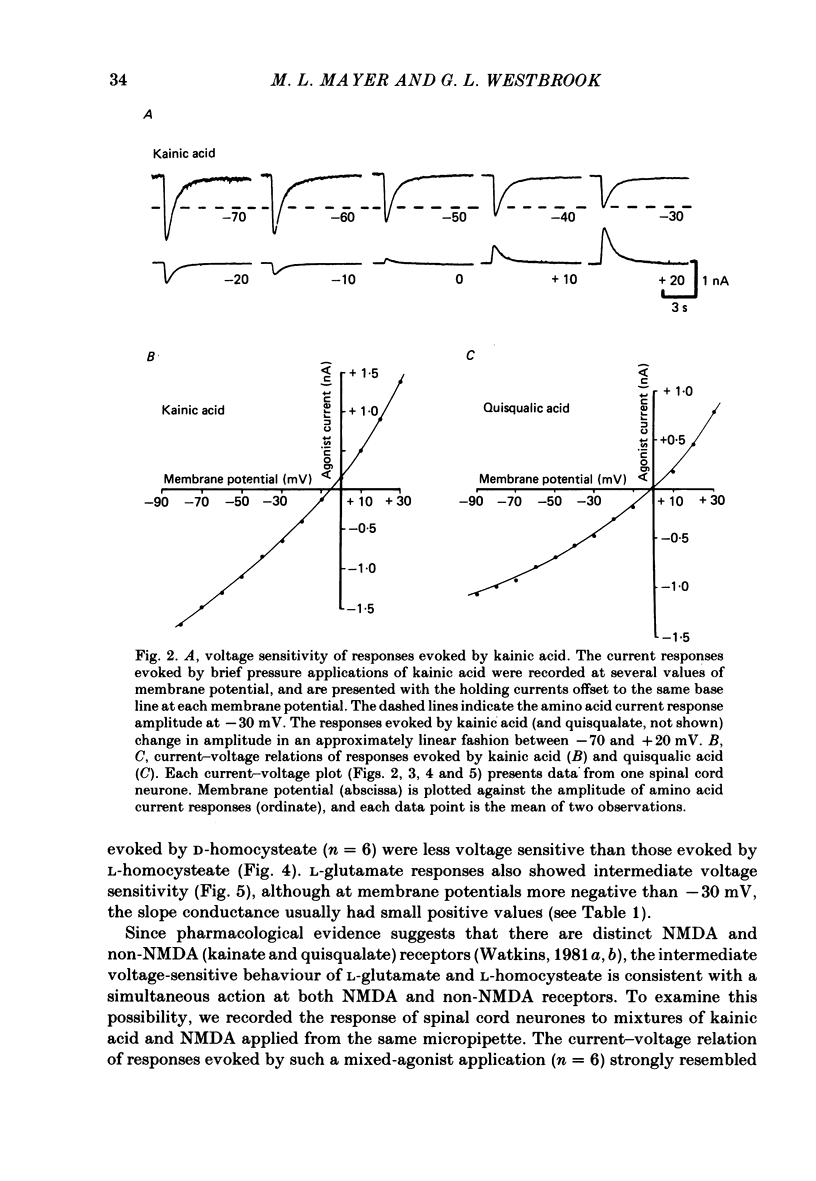
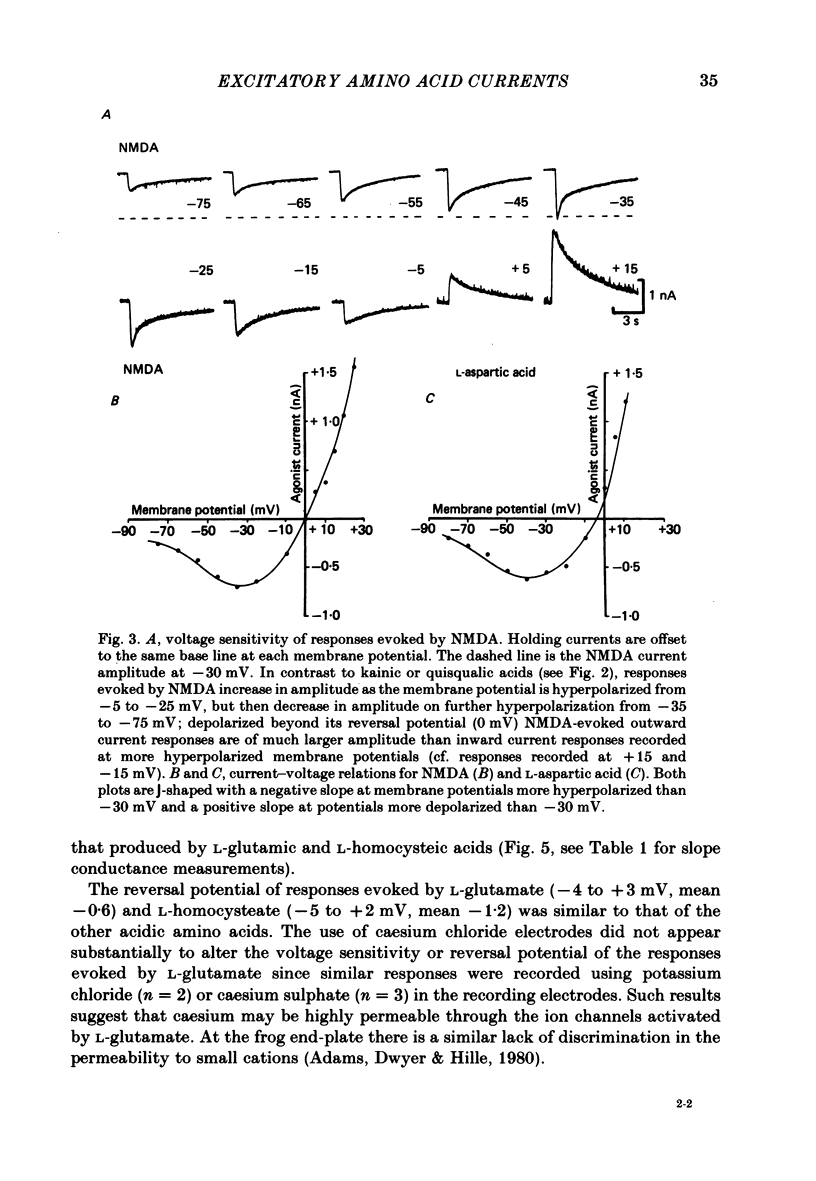
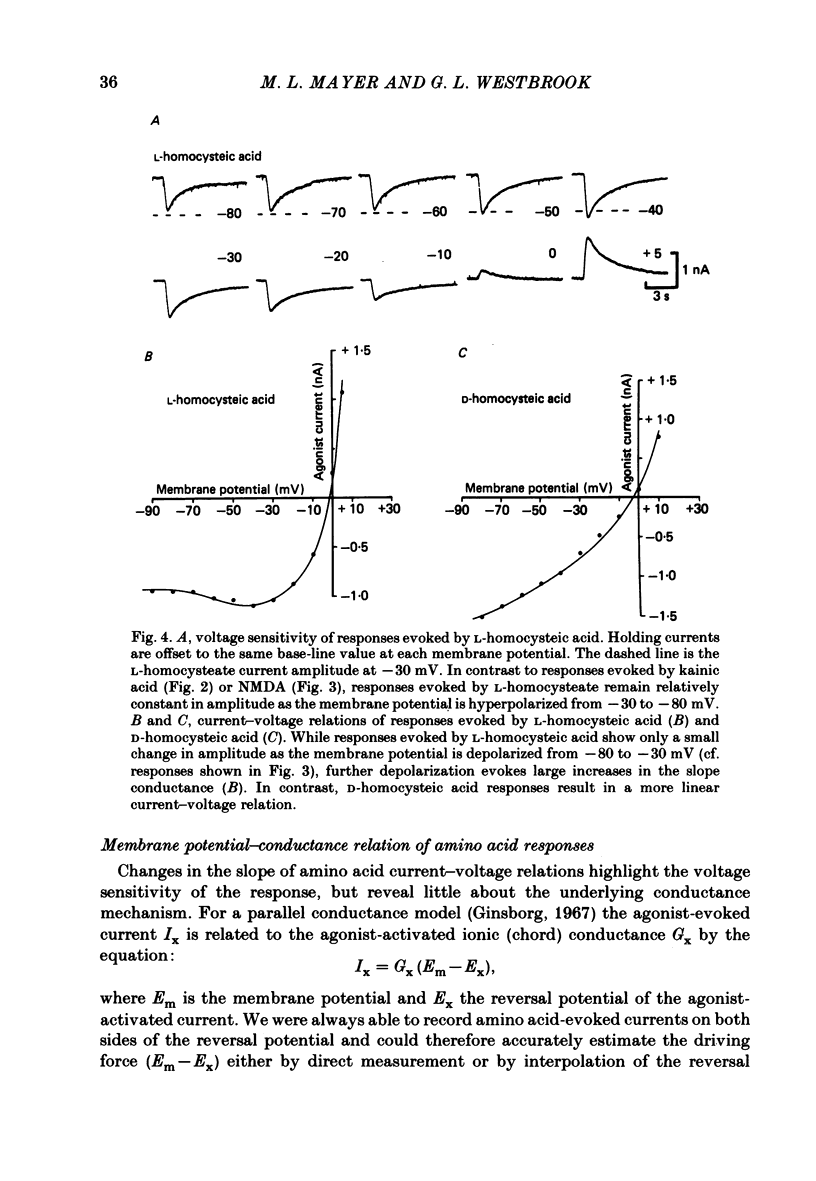
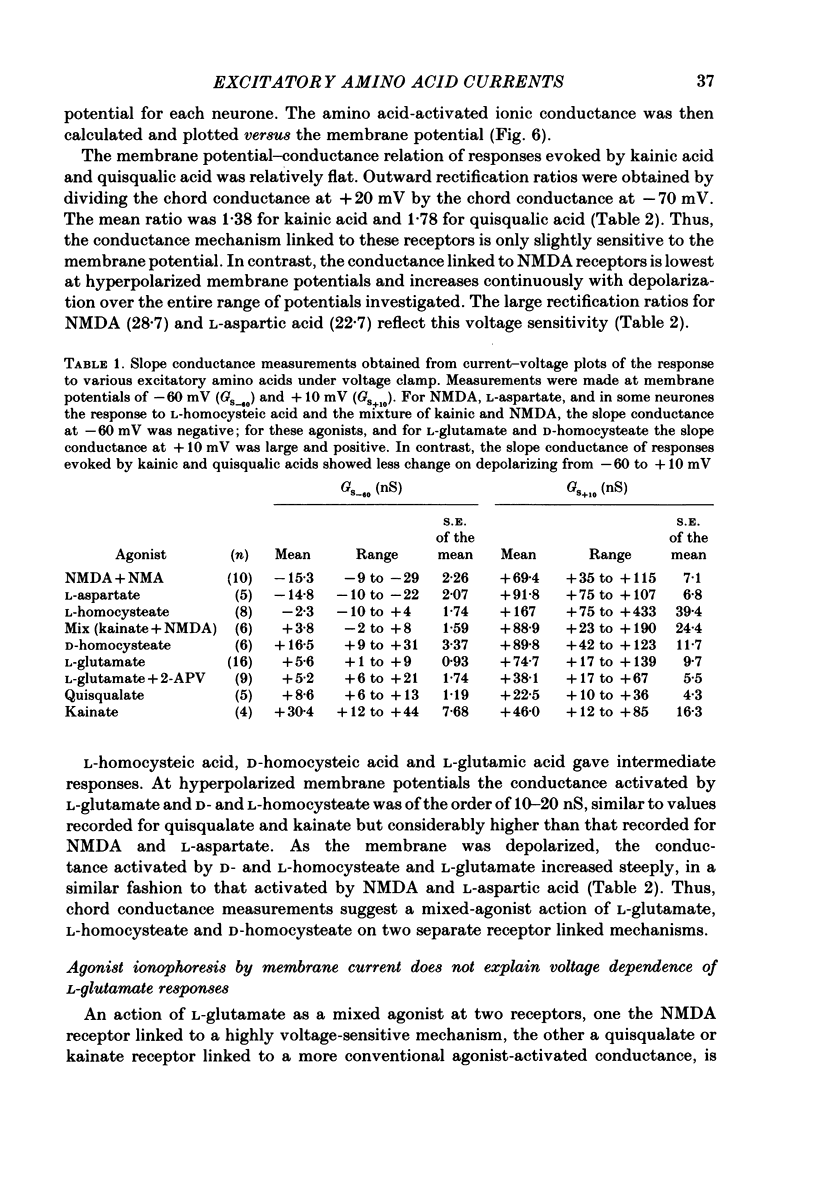
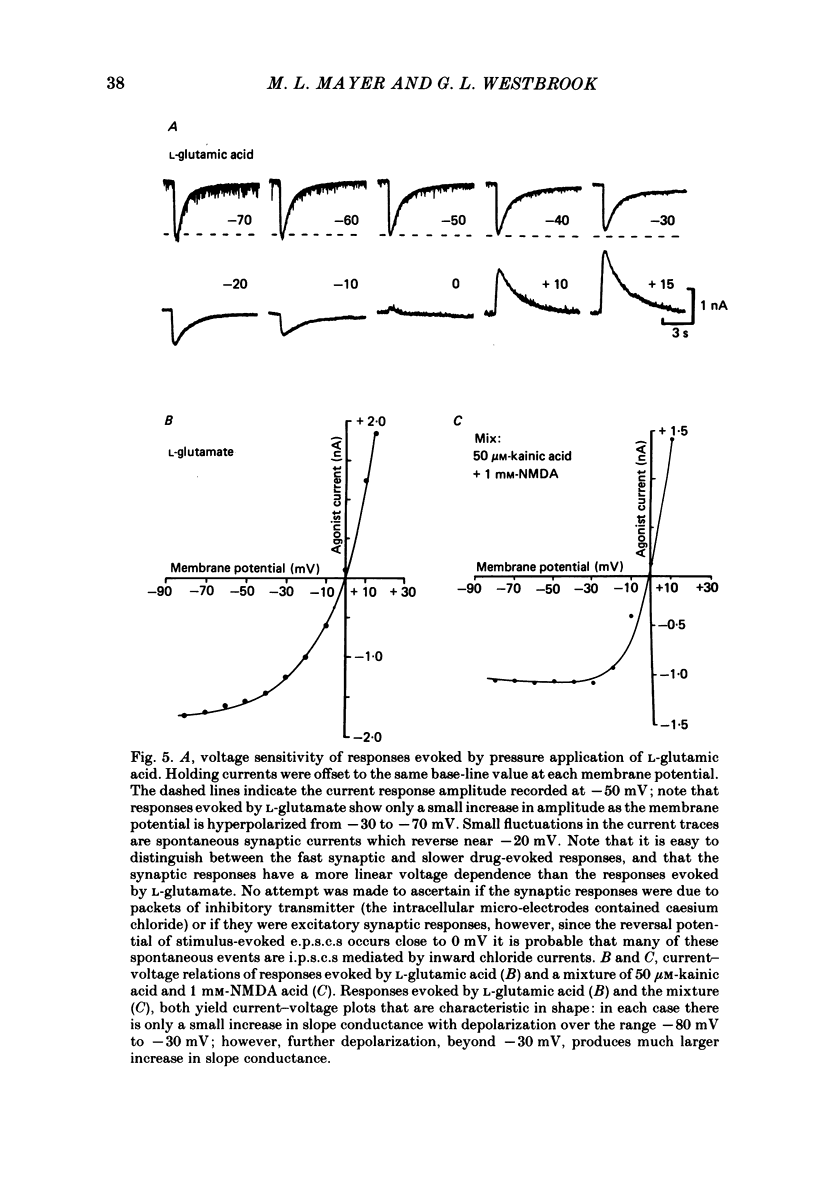
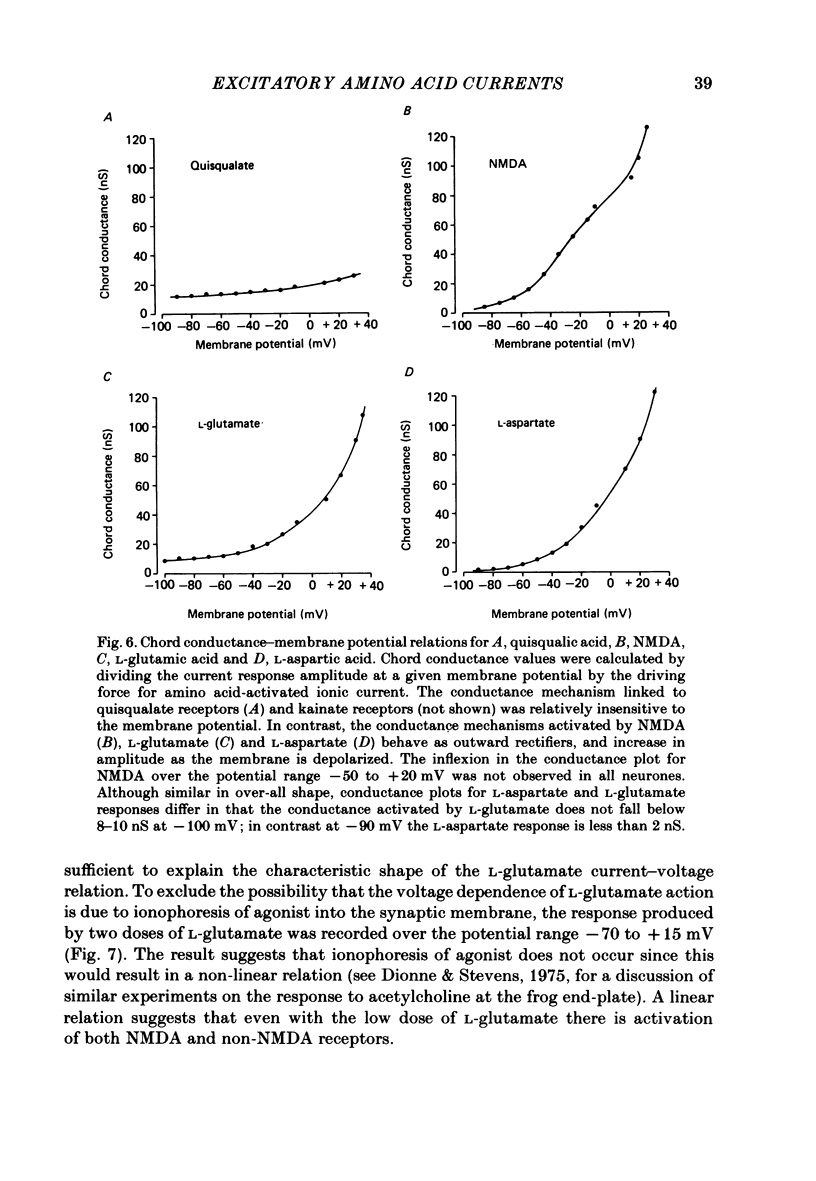
Neurones from the ventral half of mouse embryo spinal cord were grown in tissue culture and voltage clamped with two micro-electrodes. The current-voltage relation of responses evoked by brief pressure applications of excitatory amino acids was examined over a membrane potential range of -100 to +70 mV. Three types of current-voltage relation were observed. Responses to kainic and quisqualic acids were relatively linear within +/- 20 mV of the resting potential. N-methyl-D-aspartate (NMDA) and L-aspartic acid responses had a negative slope conductance at membrane potentials more negative than -30 mV. In contrast, over the same potential range the slope conductance of responses evoked by L-glutamic and L-homocysteic acids was close to zero. The membrane potential-chord conductance relation of the ionic mechanism activated by excitatory amino acids, derived using the driving force for ionic current, showed two types of behaviour. The conductance linked to NMDA receptors was highly voltage sensitive and increased on depolarization; a much weaker voltage sensitivity was observed for responses evoked by kainic and quisqualic acids. L-glutamic and L-homocysteic acid responses behaved as though due to simultaneous activation of both NMDA and either kainate or quisqualate receptors. In the presence of the NMDA receptor antagonist (+/-)-2-aminophosphonovaleric acid (2-APV) the response to L-glutamate became less voltage sensitive and resembled responses evoked by kainate or quisqualate. Simultaneous activation of both conductance mechanisms by mixtures of kainate and NMDA produced current-voltage and membrane potential-chord conductance relations similar to those of L-glutamate. The voltage sensitivity of the L-glutamate response was inversely related to the dose; for low doses of L-glutamate the slope conductance of responses recorded near the resting potential was close to zero. However, larger doses of L-glutamate evoked responses with a voltage sensitivity similar to that of kainate. We suggest that L-glutamate acts as a mixed agonist at both NMDA and non-NMDA receptors. This can explain the results of previous experiments that failed to demonstrate a membrane resistance change during L-glutamate-induced depolarizations.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Dwyer T. M., Hille B. The permeability of endplate channels to monovalent and divalent metal cations. J Gen Physiol. 1980 May;75(5):493–510. doi: 10.1085/jgp.75.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. A comparison of current-voltage relations for full and partial agonists. J Physiol. 1978 Oct;283:621–644. doi: 10.1113/jphysiol.1978.sp012523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Inactivation of delayed outward current in molluscan neurone somata. J Physiol. 1979 Jun;291:507–530. doi: 10.1113/jphysiol.1979.sp012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann H., ten Bruggencate G., Pickelmann P., Steinberg R. Effects of glutamate, aspartate, and two-presumed antagonists on feline rubrospinal neurones. Pflugers Arch. 1976 Aug 24;364(3):249–255. doi: 10.1007/BF00581763. [DOI] [PubMed] [Google Scholar]
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi G., Zieglgansberger W., Herz A., Puil E. A. Intracellular studies on the action of L-glutamic acid on spinal neurones of the cat. Brain Res. 1972 Apr 28;39(2):523–525. doi: 10.1016/0006-8993(72)90457-x. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Muller K. J., Murray G. Reversal potential for an electrophysiological event generated by conductance changes: mathematical analysis. Science. 1971 Oct 15;174(4006):318–318. doi: 10.1126/science.174.4006.318. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Miledi R., Parker I. Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J Physiol. 1981 Dec;321:195–210. doi: 10.1113/jphysiol.1981.sp013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Evans R. H., Jones A. W., Smith D. A., Watkins J. C. Differential activation and blockade of excitatory amino acid receptors in the mammalian and amphibian central nervous systems. Comp Biochem Physiol C. 1982;72(2):211–224. doi: 10.1016/0306-4492(82)90086-7. [DOI] [PubMed] [Google Scholar]
- Davies J., Francis A. A., Jones A. W., Watkins J. C. 2-Amino-5-phosphonovalerate (2APV), a potent and selective antagonist of amino acid-induced and synaptic excitation. Neurosci Lett. 1981 Jan 1;21(1):77–81. doi: 10.1016/0304-3940(81)90061-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Differentiation of kainate and quisqualate receptors in the cat spinal cord by selective antagonism with gamma-D(and L)-glutamylglycine. Brain Res. 1981 Feb 9;206(1):172–177. doi: 10.1016/0006-8993(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Effect of magnesium ions on the responses of spinal neurones to excitatory amino acids and acetylcholine. Brain Res. 1977 Jul 15;130(2):364–368. doi: 10.1016/0006-8993(77)90284-0. [DOI] [PubMed] [Google Scholar]
- Davies J., Watkins J. C. Selective antagonism of amino acid-induced and synaptic excitation in the cat spinal cord. J Physiol. 1979 Dec;297(0):621–635. doi: 10.1113/jphysiol.1979.sp013060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R. N-methyl aspartate activates voltage-dependent calcium conductance in rat hippocampal pyramidal cells. J Physiol. 1983 Oct;343:385–405. doi: 10.1113/jphysiol.1983.sp014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The action of N-methyl-D-aspartic and kainic acids on motoneurones with emphasis on conductance changes [proceedings]. Br J Pharmacol. 1978 Nov;64(3):384P–385P. [PMC free article] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The actions of excitatory amino acids on motoneurones in the feline spinal cord. J Physiol. 1979 Mar;288:227–261. [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Finkel A. S., Redman S. J. The synaptic current evoked in cat spinal motoneurones by impulses in single group 1a axons. J Physiol. 1983 Sep;342:615–632. doi: 10.1113/jphysiol.1983.sp014872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman J. A., Schwindt P. C., Crill W. E., Stafstrom C. E. Multiple actions of N-methyl-D-aspartate on cat neocortical neurons in vitro. Brain Res. 1983 Apr 25;266(1):169–173. doi: 10.1016/0006-8993(83)91323-9. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Gration K. A., Lambert J. J., Ramsey R., Usherwood P. N. Non-random openings and concentration-dependent lifetimes of glutamate-gated channels in muscle membrane. Nature. 1981 Jun 4;291(5814):423–425. doi: 10.1038/291423a0. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J. Conductance changes induced by DL-homocysteic acid and N-methyl-DL-aspartic acid in hippocampal neurons. Brain Res. 1982 Sep 9;247(1):149–153. doi: 10.1016/0006-8993(82)91040-x. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J., Langmoen I. A. Excitation of hippocampal pyramidal cells by glutamate in the guinea-pig and rat. J Physiol. 1982 Apr;325:317–331. doi: 10.1113/jphysiol.1982.sp014152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. S. Electrogenic effects of neutral amino acids on neurons of Aplysia californica. Cold Spring Harb Symp Quant Biol. 1976;40:145–155. doi: 10.1101/sqb.1976.040.01.016. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Porietis A. V. DL-quisqualic and L-aspartic acids activate separate excitatory conductances in cultured spinal cord neurons. Brain Res. 1982 Aug 5;245(1):175–178. doi: 10.1016/0006-8993(82)90356-0. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Porietis A. V., Wojtowicz J. M. L-Aspartic acid induces a region of negative slope conductance in the current-voltage relationship of cultured spinal cord neurons. Brain Res. 1982 Apr 8;237(1):248–253. doi: 10.1016/0006-8993(82)90575-3. [DOI] [PubMed] [Google Scholar]
- MacDonald J. F., Wojtowicz J. M. The effects of L-glutamate and its analogues upon the membrane conductance of central murine neurones in culture. Can J Physiol Pharmacol. 1982 Mar;60(3):282–296. doi: 10.1139/y82-039. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurones. J Physiol. 1983 Jul;340:19–45. doi: 10.1113/jphysiol.1983.sp014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H. On the nature of the receptors for various excitatory amino acids in the mammalian central nervous system. Adv Biochem Psychopharmacol. 1981;27:253–262. [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Puil E. S-Glutamate: its interactions with spinal neurons. Brain Res. 1981 Dec;228(3):229–322. doi: 10.1016/0165-0173(81)90007-2. [DOI] [PubMed] [Google Scholar]
- Ransom B. R., Neale E., Henkart M., Bullock P. N., Nelson P. G. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977 Sep;40(5):1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Effects of barium on cat spinal motoneurons studied by voltage clamp. J Neurophysiol. 1980 Oct;44(4):827–846. doi: 10.1152/jn.1980.44.4.827. [DOI] [PubMed] [Google Scholar]
- Segal M. The actions of glutamic acid on neurons in the rat hippocampal slice. Adv Biochem Psychopharmacol. 1981;27:217–225. [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982 Mar 18;236(1):221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Amino acids as neurotransmitters of corticofugal neurones in the rat: a comparison of glutamate and aspartate. Br J Pharmacol. 1979 Dec;67(4):545–551. doi: 10.1111/j.1476-5381.1979.tb08700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C. Pharmacology of excitatory amino acid transmitters. Adv Biochem Psychopharmacol. 1981;29:205–212. [PubMed] [Google Scholar]
- Wojtowicz J. M., Gysen M., MacDonald J. F. Multiple reversal potentials for responses to L-glutamic acid. Brain Res. 1981 May 25;213(1):195–200. doi: 10.1016/0006-8993(81)91261-0. [DOI] [PubMed] [Google Scholar]


