Abstract
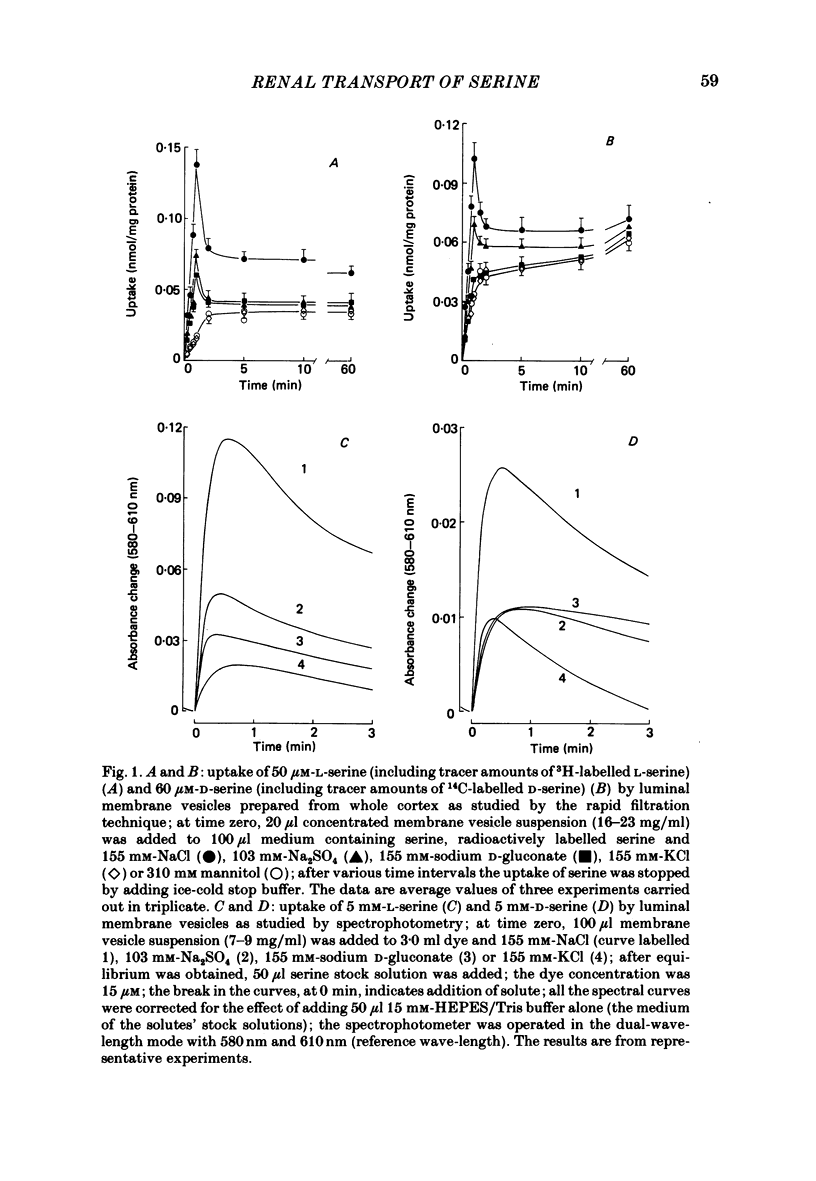
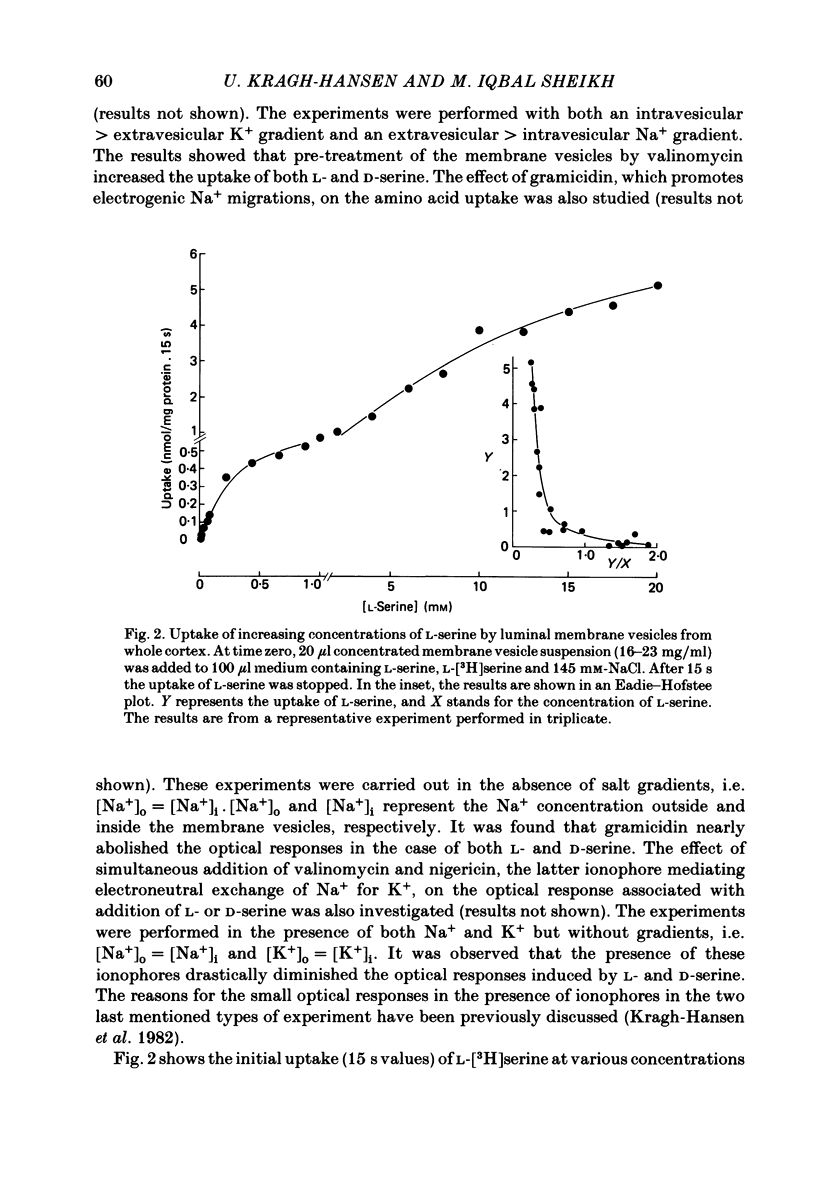
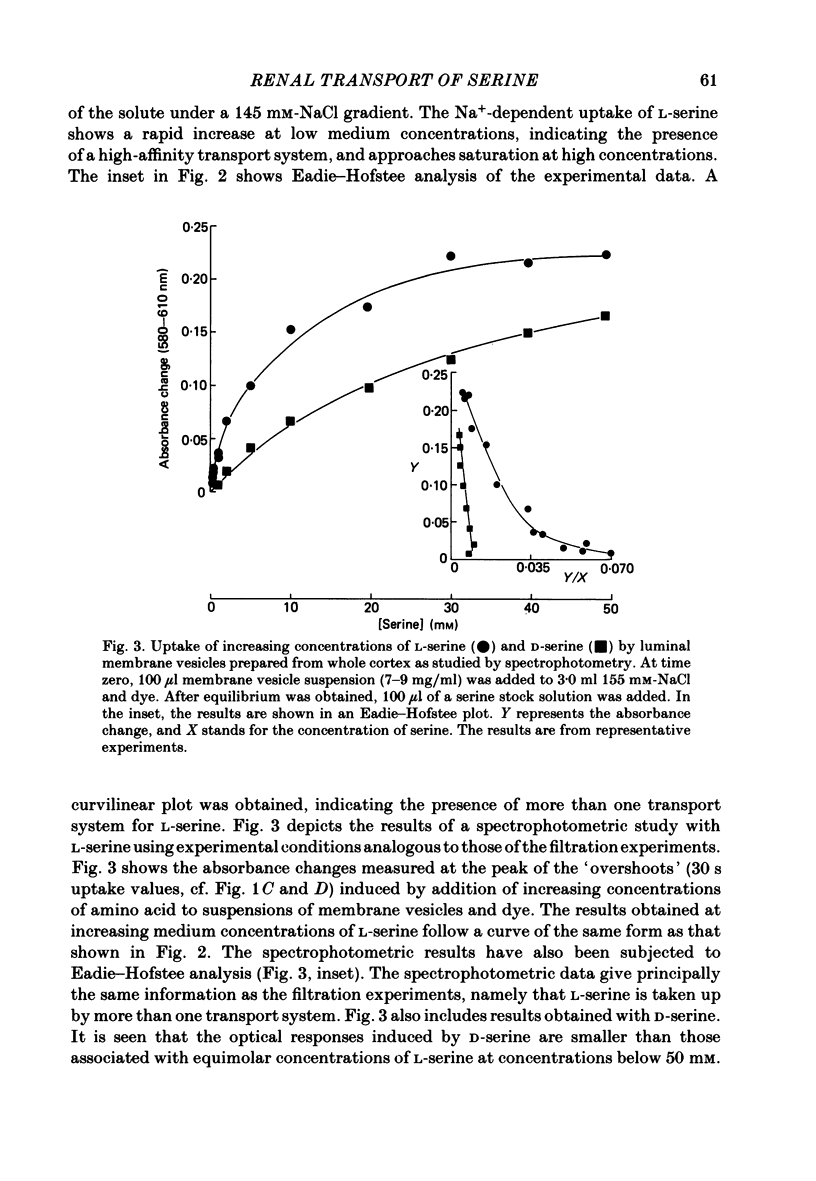
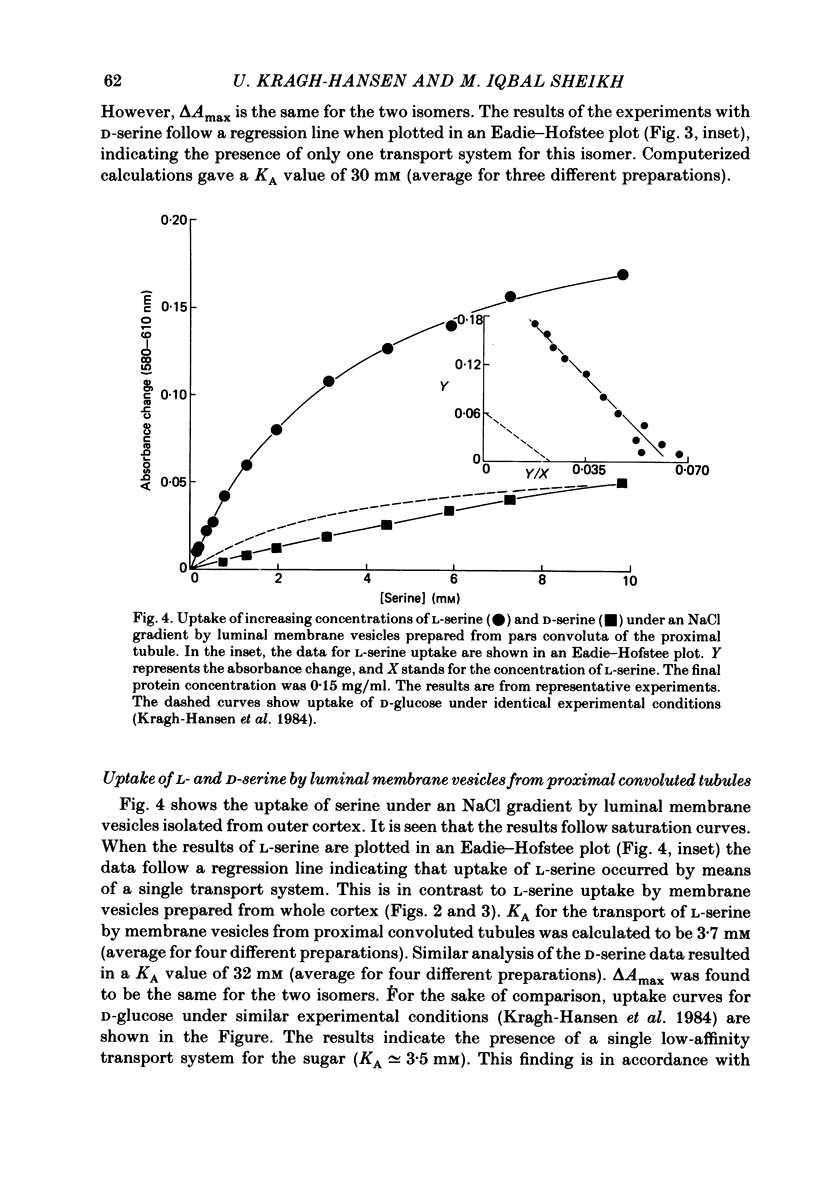
The mechanism of renal transport of L- and D-serine by membrane vesicles prepared from either whole cortex, pars convoluta or pars recta of rabbit proximal tubule was studied by a rapid filtration technique and by a spectrophotometric method using a potential-sensitive carbocyanine dye. Transport studies carried out with different salt gradients and by employing various ionophores showed that uptake of both L- and D-serine by luminal membrane vesicles from whole cortex was mediated by an Na+-dependent and electrogenic transport process. Eadie-Hofstee analysis of experimental data, obtained under extravesicular greater than intravesicular NaCl gradients, revealed the existence of multiple transport systems for L-serine but only one system for the D-isomer. The value of KA (the concentration producing a half-maximal optical response) for the D-serine transport system was calculated to be approximately 30 mM. Luminal membrane vesicles from pars convoluta take up both L- and D-serine by a single and common transport system. KA values for L- and D-serine transport were calculated to be 3.7 and 30 mM, respectively. Luminal membrane vesicles from pars recta take up L-serine by means of two transport systems, one of high affinity (KA = 0.37 mM) and the other of low affinity (KA = 10 mM). By contrast, no D-serine transport by these membrane vesicles could be detected. Uptake of L-serine by basolateral membrane vesicles is Na+ independent and electroneutral. Filtration studies showed that the transport is saturable (Km = 25-30 mM) and is inhibited by the presence of L-phenylalanine (but not by D-serine), indicating carrier-mediated uptake of L-serine.
Full text
PDF



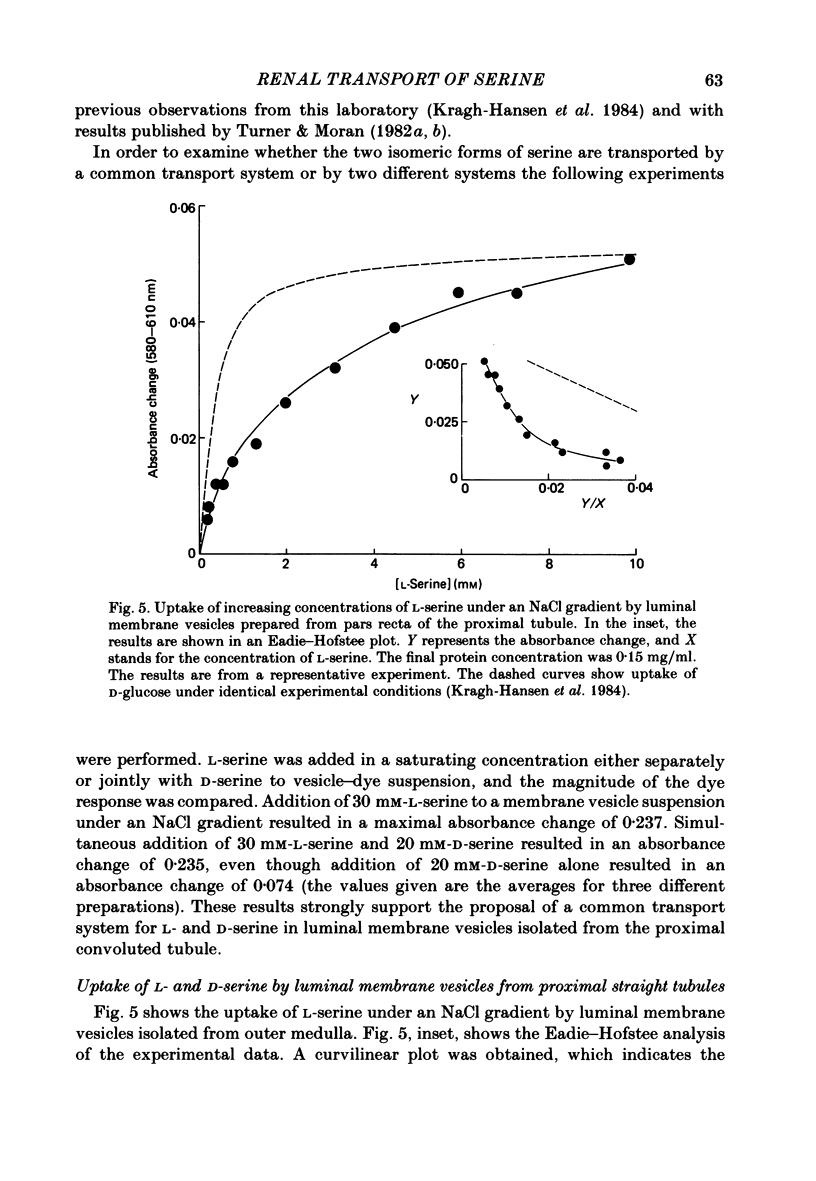
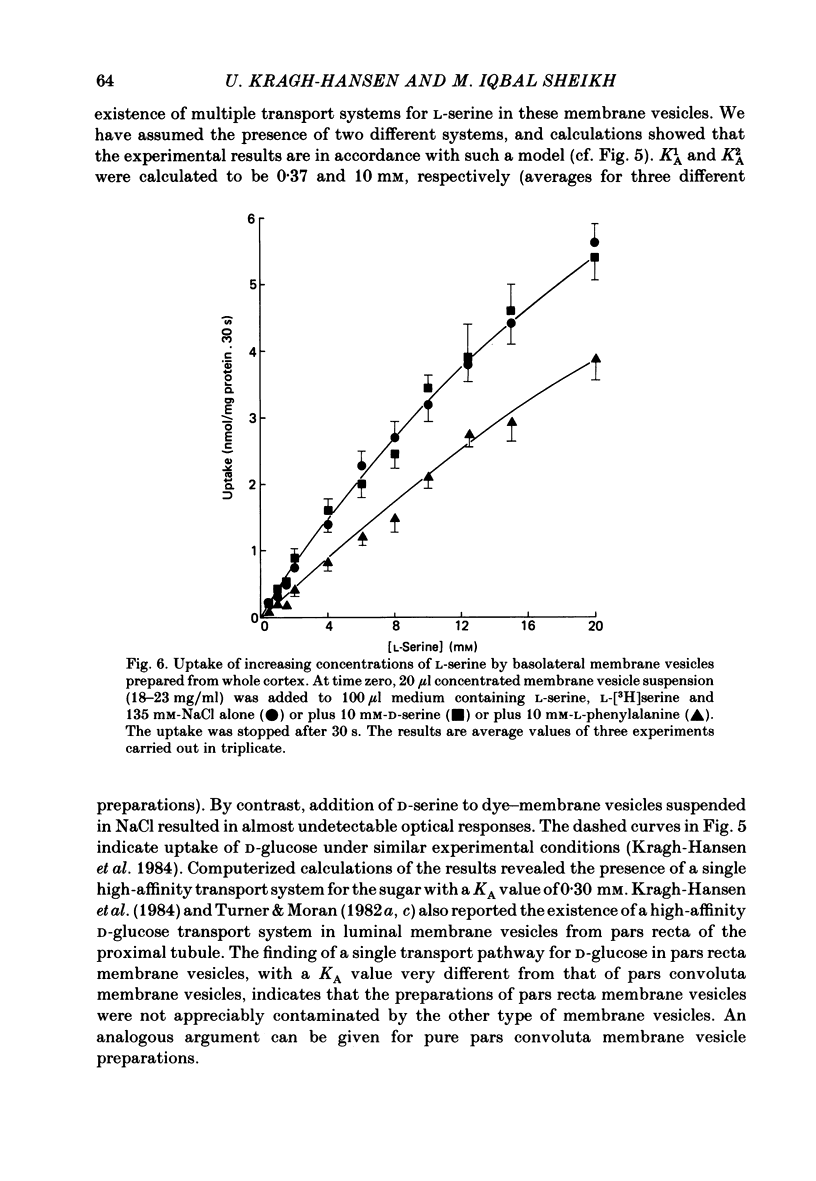



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron M., Morel F. Amino acid transport in rat renal tubules. Am J Physiol. 1969 May;216(5):1139–1149. doi: 10.1152/ajplegacy.1969.216.5.1139. [DOI] [PubMed] [Google Scholar]
- DOOLAN P. D., HARPER H. A., HUTCHIN M. E., SHREEVE W. W. Renal clearance of eighteen individual amino acids in human subjects. J Clin Invest. 1955 Aug;34(8):1247–1255. doi: 10.1172/JCI103171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVERED D. F. The excretion of amino acids by the human; a quantitative study with ion-exchange chromatography. Biochem J. 1956 Mar;62(3):416–427. doi: 10.1042/bj0620416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Jacobsen C., Frich J. R., Steensgaard J. Determination of affinity of monoclonal antibodies against human IgG. J Immunol Methods. 1982;50(1):77–88. doi: 10.1016/0022-1759(82)90305-2. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U., Jørgensen K. E., Sheikh M. I. The use of potential-sensitive cyanine dye for studying ion-dependent electrogenic renal transport of organic solutes. Spectrophotometric measurements. Biochem J. 1982 Nov 15;208(2):359–368. doi: 10.1042/bj2080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh-Hansen U., Røigaard-Petersen H., Jacobsen C., Sheikh M. I. Renal transport of neutral amino acids. Tubular localization of Na+-dependent phenylalanine- and glucose-transport systems. Biochem J. 1984 May 15;220(1):15–24. doi: 10.1042/bj2200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ottolenghi P. The reversible delipidation of a solubilized sodium-plus-potassium ion-dependent adenosine triphosphatase from the salt gland of the spiny dogfish. Biochem J. 1975 Oct;151(1):61–66. doi: 10.1042/bj1510061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Røigaard-Petersen H., Sheikh M. I. Renal transport of neutral amino acids. Demonstration of Na+-independent and Na+-dependent electrogenic uptake of L-proline, hydroxy-L-proline and 5-oxo-L-proline by luminal-membrane vesicles. Biochem J. 1984 May 15;220(1):25–33. doi: 10.1042/bj2200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh M. I., Kragh-Hansen U., Jørgensen K. E., Røigaard-Petersen H. An efficient method for the isolation and separation of basolateral-membrane and luminal-membrane vesicles from rabbit kidney cortex. Biochem J. 1982 Nov 15;208(2):377–382. doi: 10.1042/bj2080377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh M. I. Renal handling of phenol red. I. A comparative study on the accumulation of phenol red and p-aminohippurate in rabbit kidney tubules in vitro. J Physiol. 1972 Dec;227(2):565–590. doi: 10.1113/jphysiol.1972.sp010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh M. I. Renal handling of phenol red. II. The mechanism of substituted phenolsulphophthalein (PSP) dye transport in rabbit kidney tubules in vitro. J Physiol. 1976 Mar;256(1):175–195. doi: 10.1113/jphysiol.1976.sp011319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbernagl S., Foulkes E. C., Deetjen P. Renal transport of amino acids. Rev Physiol Biochem Pharmacol. 1975;74:105–167. doi: 10.1007/3-540-07483-x_20. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Further studies of proximal tubular brush border membrane D-glucose transport heterogeneity. J Membr Biol. 1982;70(1):37–45. doi: 10.1007/BF01871587. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol. 1982 Apr;242(4):F406–F414. doi: 10.1152/ajprenal.1982.242.4.F406. [DOI] [PubMed] [Google Scholar]
- Turner R. J., Moran A. Stoichiometric studies of the renal outer cortical brush border membrane D-glucose transporter. J Membr Biol. 1982;67(1):73–80. doi: 10.1007/BF01868649. [DOI] [PubMed] [Google Scholar]


