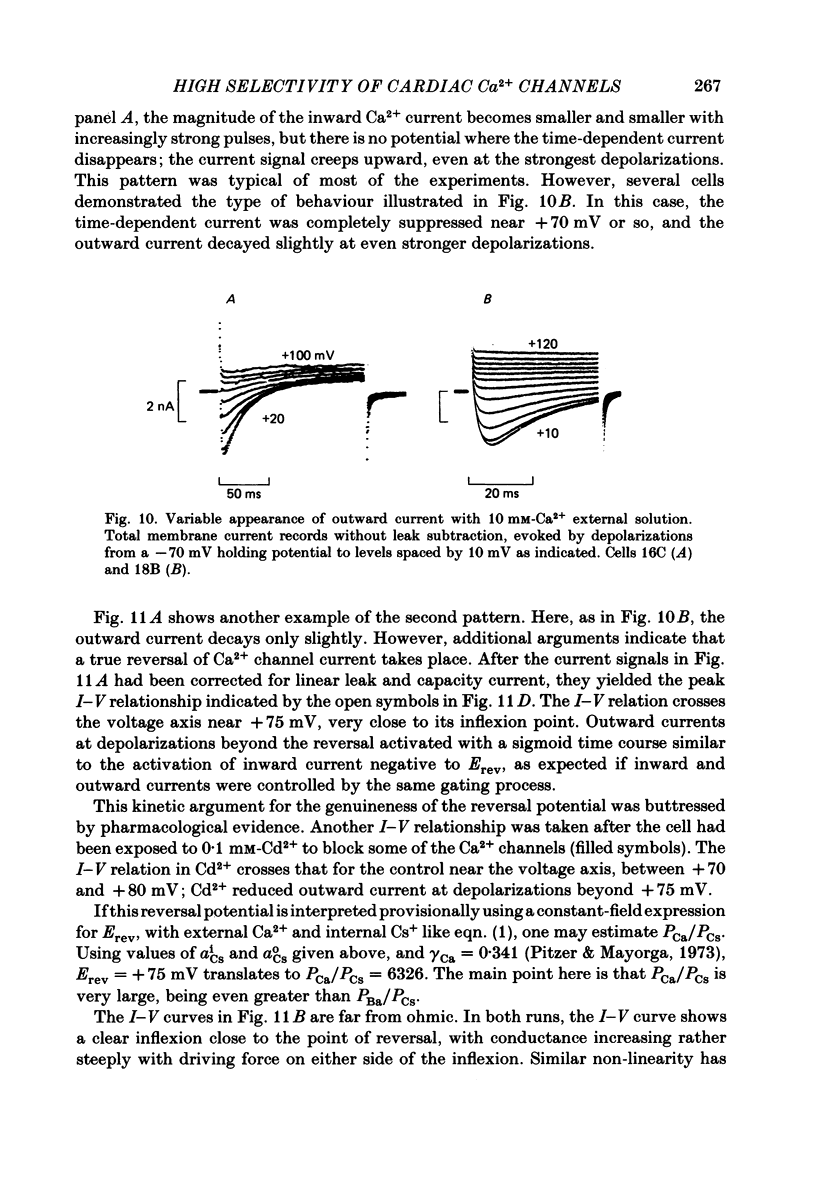
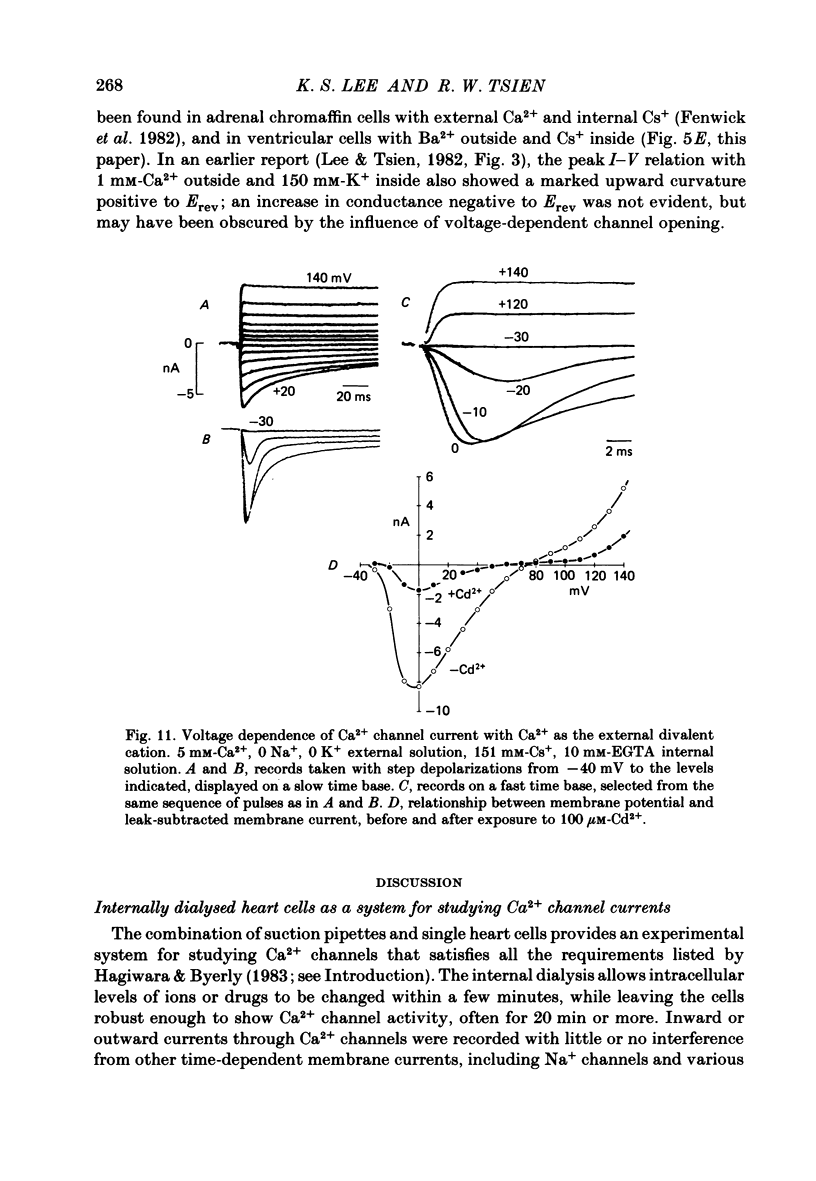
Abstract
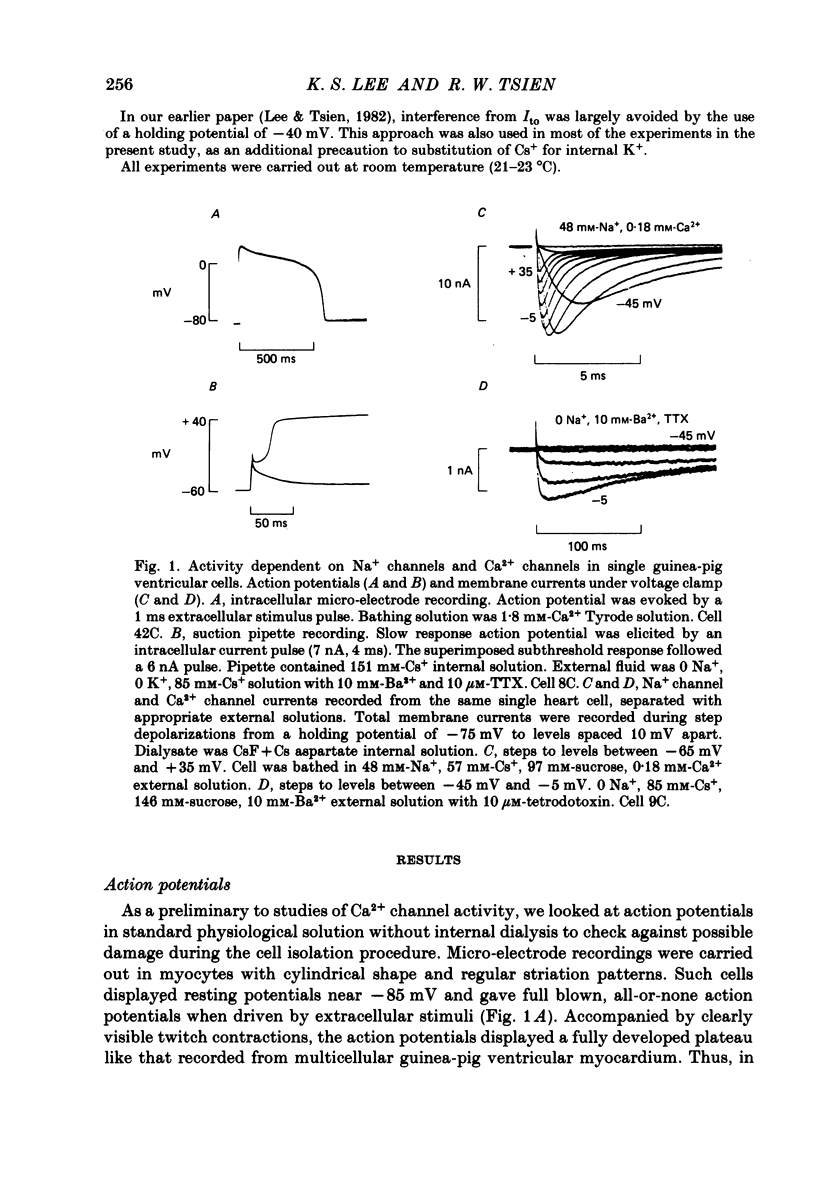
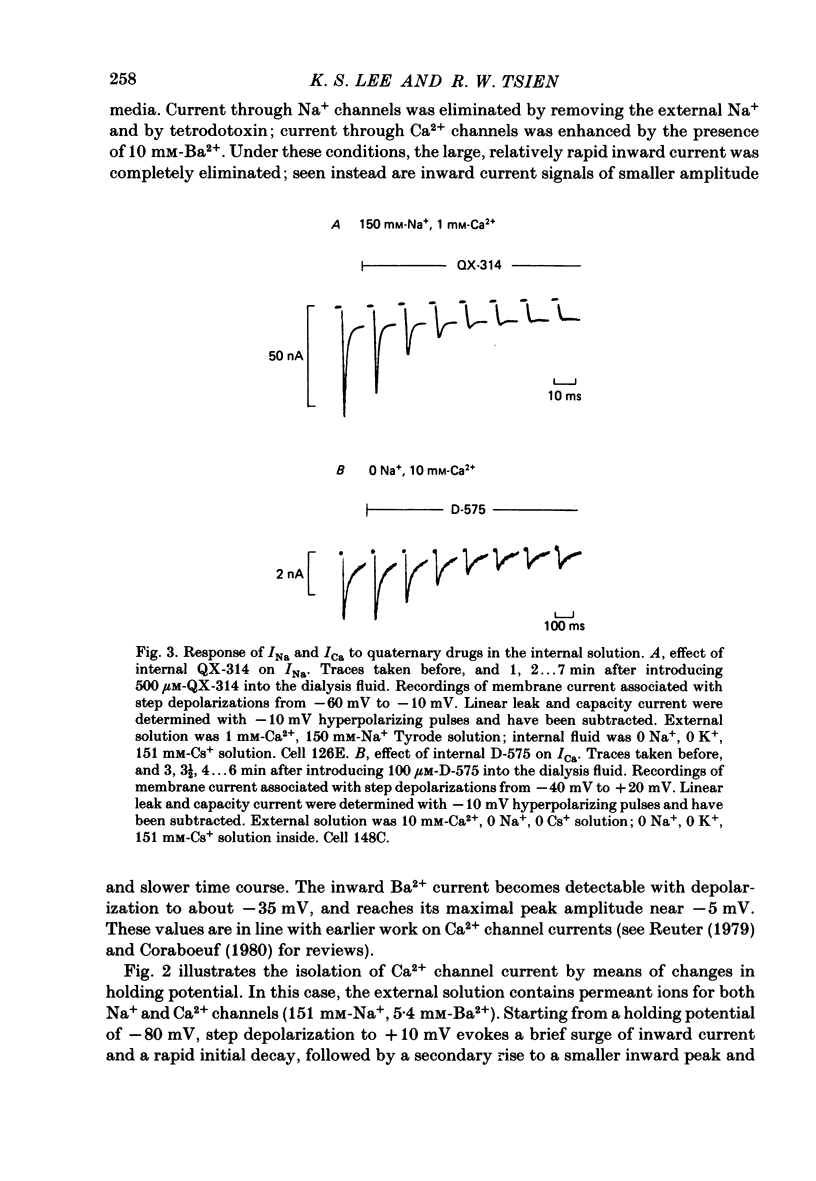
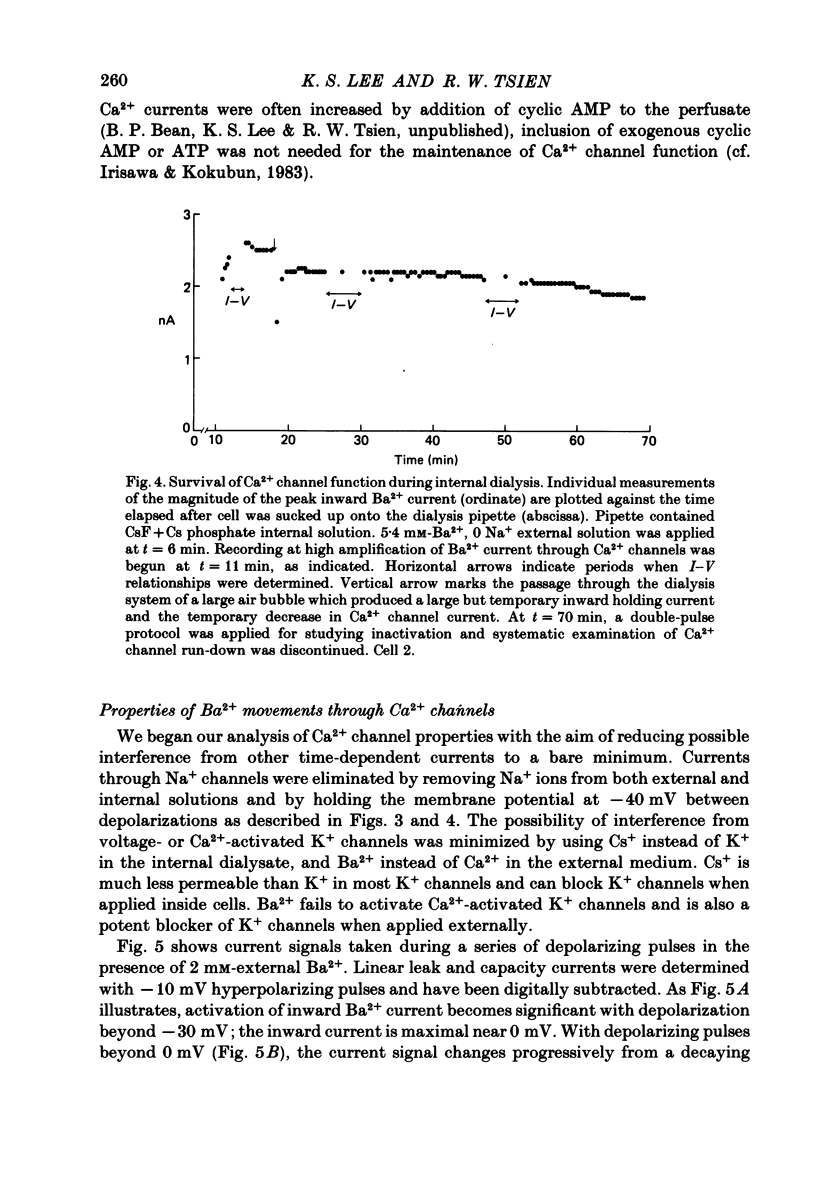
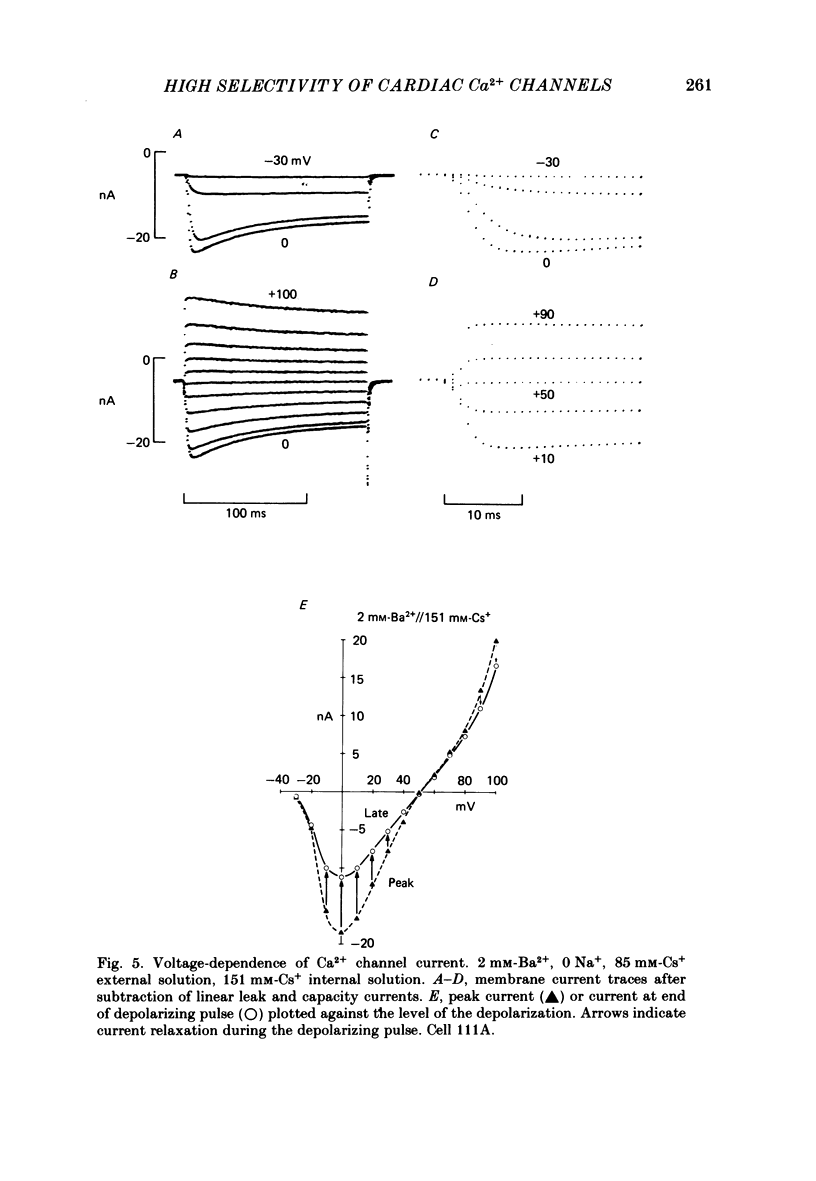
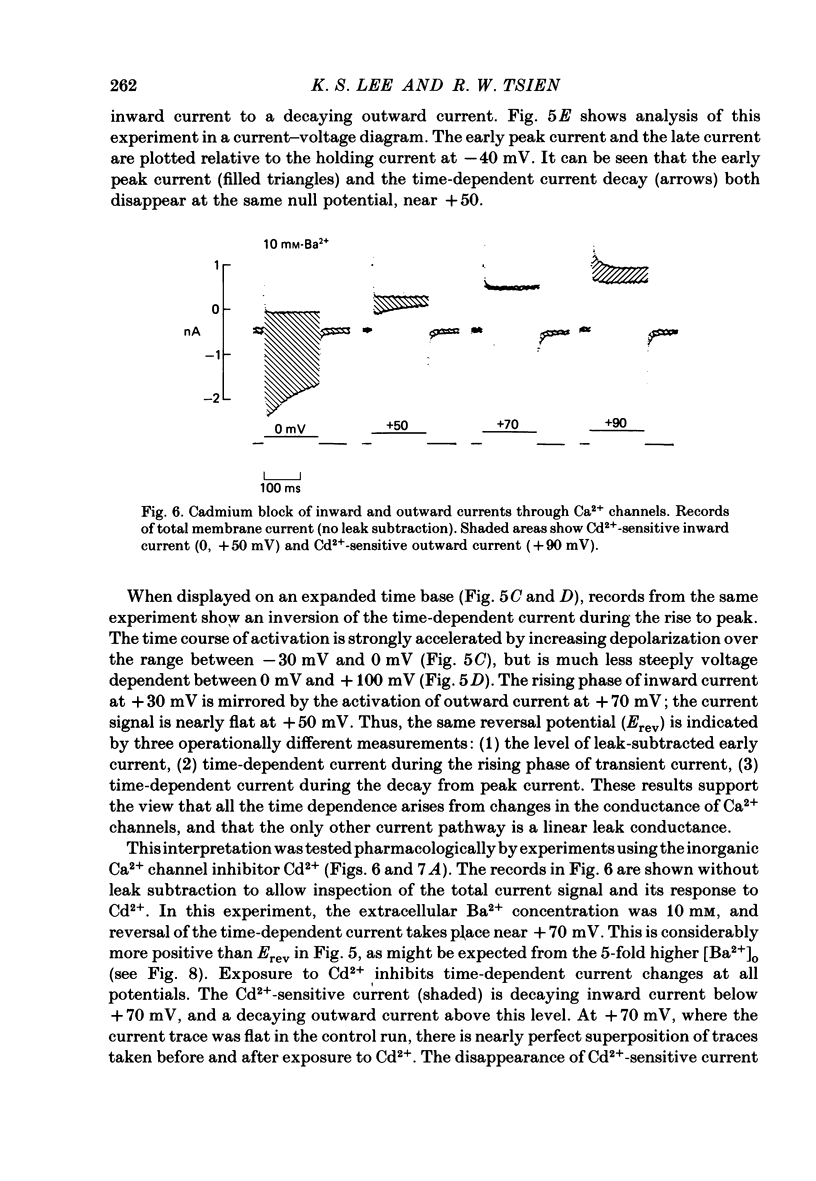
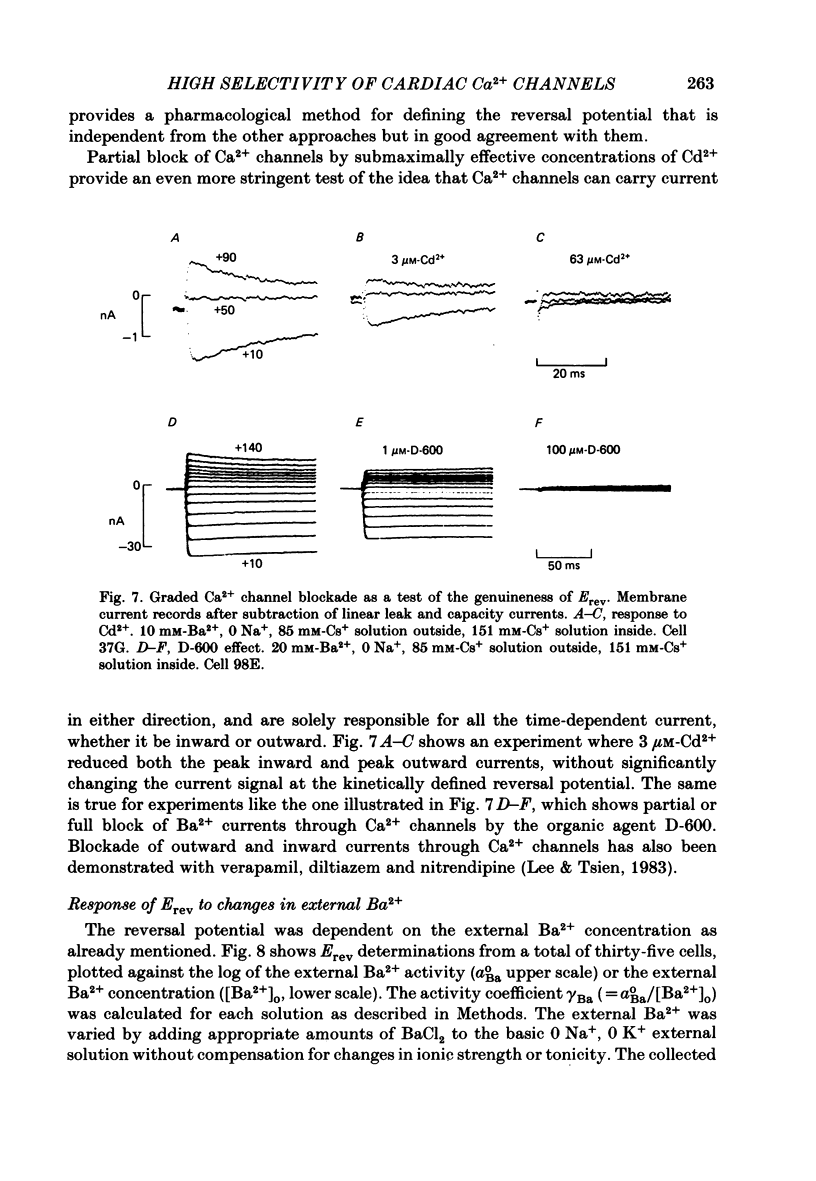
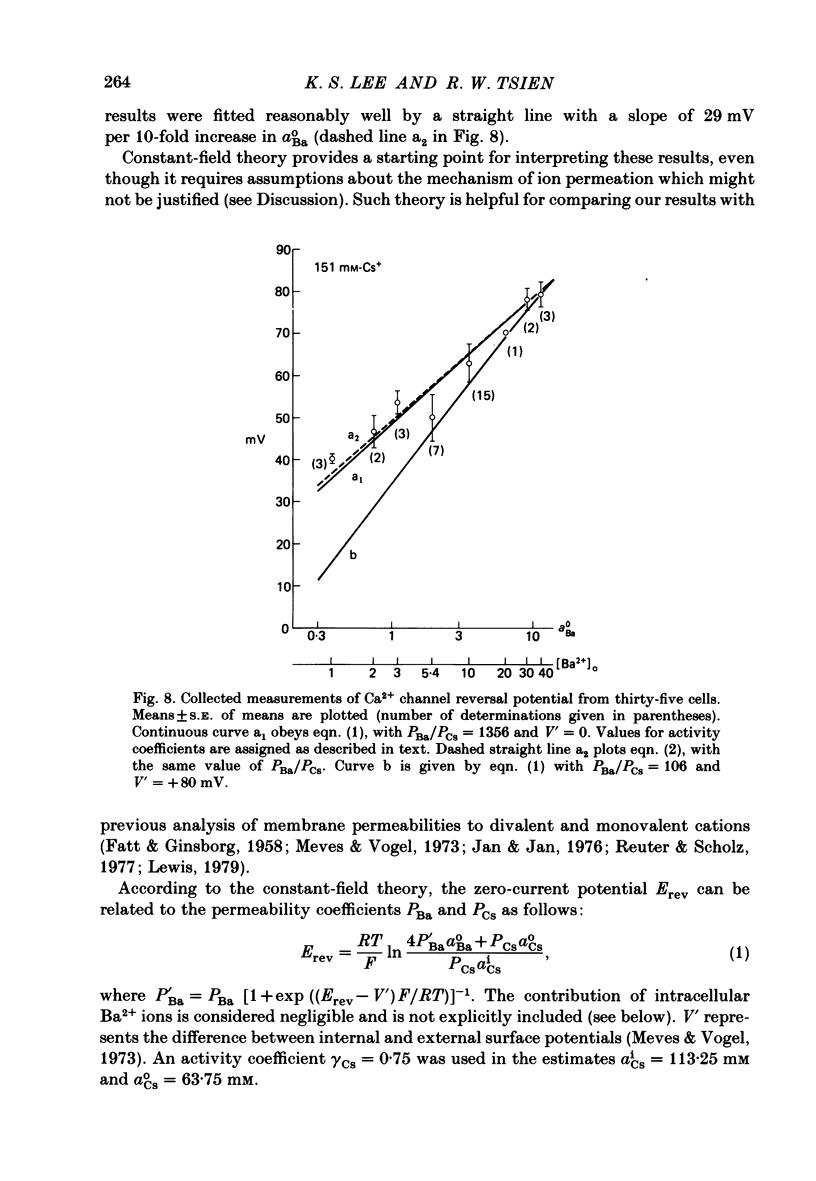
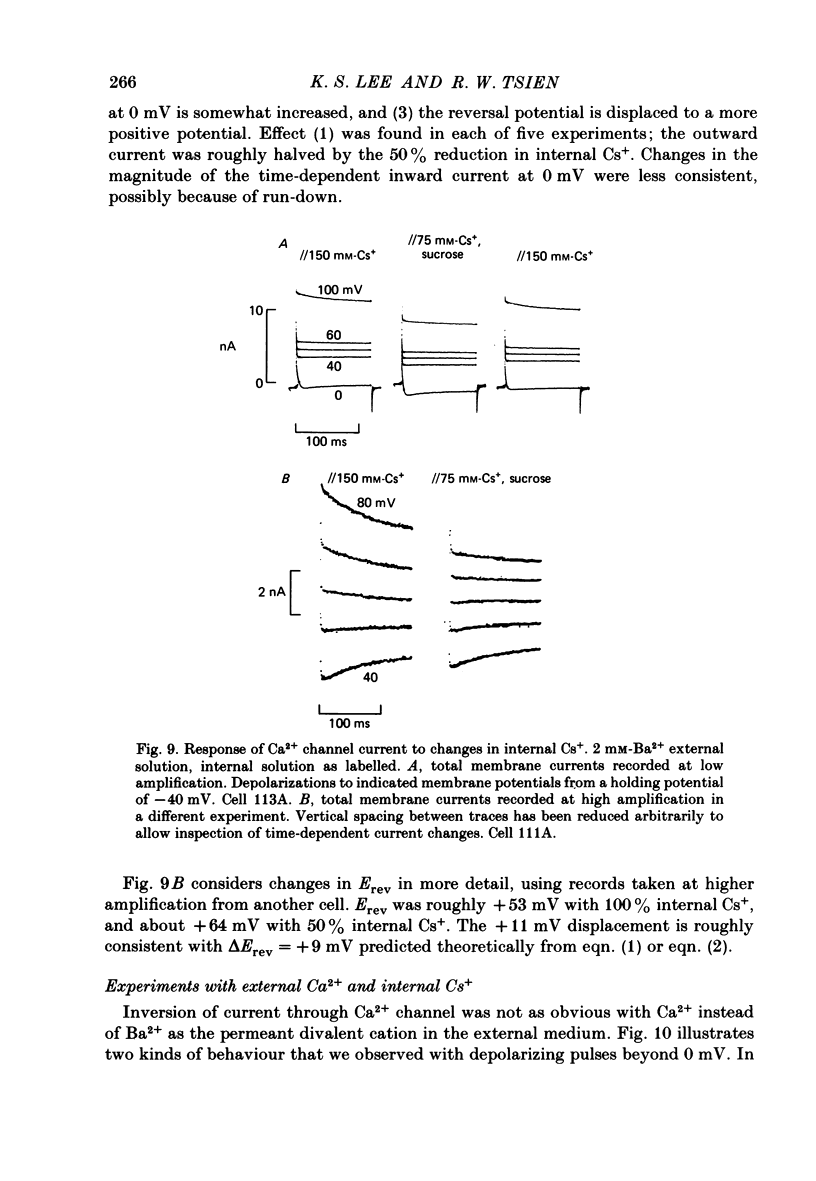
Membrane currents and action potentials were recorded in single ventricular cells obtained from guinea-pig hearts by enzymatic dissociation. Ca2+ channel currents carried by Ba2+ or Ca2+ were recorded with a suction pipette (5-10 microns diameter) for voltage clamp and internal dialysis. Currents through Na+, K+ and non-selective monovalent cation channels were suppressed by suitable holding potentials and external and internal solutions. The dialysis method allowed exchange within minutes of alkali metal cations (e.g. Cs+) and small molecules (e.g. quaternary derivatives of lidocaine and verapamil). Nevertheless, Ca2+ channels remained functional for considerable periods, typically 20 min and sometimes more than 1 h. With Ba2+ outside and Cs+ inside, current flow through Ca2+ channels changed from inward to outward at strongly positive levels beyond a clear-cut reversal potential Erev. Several methods for defining Erev were in close agreement: (1) zero-crossing of leak-subtracted peak current, (2) inversion of time-dependent current changes during channel activation or inactivation, (3) inversion of drug-sensitive current as defined by channel blockers such as Cd2+ or D-600. Erev varied with external Ba2+ or internal Cs+. Erev increased by 29 mV per 10-fold increase in Ba2+. Interpreted with constant-field theory, Erev values correspond to PBa/PCs of approximately 1360. With 5 mM-Ca2+ outside and 151 mM-Cs+ inside, Ca2+ channel current reversed near + 75 mV, corresponding to PCa/PCs approximately 6000. Earlier measurements of Erev (Lee & Tsien, 1982) suggest that PCa/PK greater than 1000. At strongly positive membrane potentials where channel activation is maximal, the Ca2+ channel current-voltage relationship is strongly non-linear, with conductance increasing on either side of an inflexion point near Erev. Activation of inward or outward currents through Ca2+ channels follows a sigmoid time course, as expected if activation were a multi-step process.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Palade P. T. Slow calcium and potassium currents across frog muscle membrane: measurements with a vaseline-gap technique. J Physiol. 1981 Mar;312:159–176. doi: 10.1113/jphysiol.1981.sp013622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Calcium channels in the somatic membrane of the rat dorsal root ganglion neurons, effect of cAMP. Brain Res. 1981 Jun 9;214(1):210–214. doi: 10.1016/0006-8993(81)90457-1. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Pelzer D., Trube G., Trautwein W. Does the organic calcium channel blocker D600 act from inside or outside on the cardiac cell membrane? Pflugers Arch. 1982 Jun;393(4):287–291. doi: 10.1007/BF00581411. [DOI] [PubMed] [Google Scholar]
- Hino N., Ochi R. Effect of acetylcholine on membrane currents in guinea-pig papillary muscle. J Physiol. 1980 Oct;307:183–197. doi: 10.1113/jphysiol.1980.sp013430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Active and passive electrical properties of single bullfrog atrial cells. J Gen Physiol. 1981 Jul;78(1):19–42. doi: 10.1085/jgp.78.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Kokubun S. Modulation by intracellular ATP and cyclic AMP of the slow inward current in isolated single ventricular cells of the guinea-pig. J Physiol. 1983 May;338:321–337. doi: 10.1113/jphysiol.1983.sp014675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Glycocalyx is not required for show inward calcium current in isolated rat heart myocytes. Nature. 1980 Mar 27;284(5754):358–360. doi: 10.1038/284358a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976 Oct;262(1):215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao R. L., Christman E. W., Luh S. L., Krauhs J. M., Tyers G. F., Williams E. H. The effects of insulin and anoxia on the metabolism of isolated mature rat cardiac myocytes. Arch Biochem Biophys. 1980 Sep;203(2):587–599. doi: 10.1016/0003-9861(80)90216-7. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Scheuer T., Malloy K. J. Block of outward current in cardiac Purkinje fibers by injection of quaternary ammonium ions. J Gen Physiol. 1982 Jun;79(6):1041–1063. doi: 10.1085/jgp.79.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G. Calcium channels in the neuronal membrane. Biochim Biophys Acta. 1981 Dec;650(2-3):128–150. doi: 10.1016/0304-4157(81)90003-4. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. The suction pipette method for internal perfusion and voltage clamp of small excitable cells. J Neurosci Methods. 1980 Feb;2(1):51–78. doi: 10.1016/0165-0270(80)90045-x. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Reversal of current through calcium channels in dialysed single heart cells. Nature. 1982 Jun 10;297(5866):498–501. doi: 10.1038/297498a0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Weeks T. A., Kao R. L., Akaike N., Brown A. M. Sodium current in single heart muscle cells. Nature. 1979 Mar 15;278(5701):269–271. doi: 10.1038/278269a0. [DOI] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Nagy K. Single channel Ca2+ currents in Helix pomatia neurons. Pflugers Arch. 1981 Sep;391(3):252–254. doi: 10.1007/BF00596179. [DOI] [PubMed] [Google Scholar]
- Marban E., Tsien R. W. Effects of nystatin-mediated intracellular ion substitution on membrane currents in calf purkinje fibres. J Physiol. 1982 Aug;329:569–587. doi: 10.1113/jphysiol.1982.sp014320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F. The slow inward calcium current in the heart. Annu Rev Physiol. 1982;44:425–434. doi: 10.1146/annurev.ph.44.030182.002233. [DOI] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F., Tsien R. W., Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982 Jun 10;297(5866):501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Schwarz W., Palade P. T., Hille B. Local anesthetics. Effect of pH on use-dependent block of sodium channels in frog muscle. Biophys J. 1977 Dec;20(3):343–368. doi: 10.1016/S0006-3495(77)85554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Nowycky M. Calcium channels: mechanisms of beta-adrenergic modulation and ion permeation. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):201–212. doi: 10.1101/sqb.1983.048.01.023. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]


