Abstract
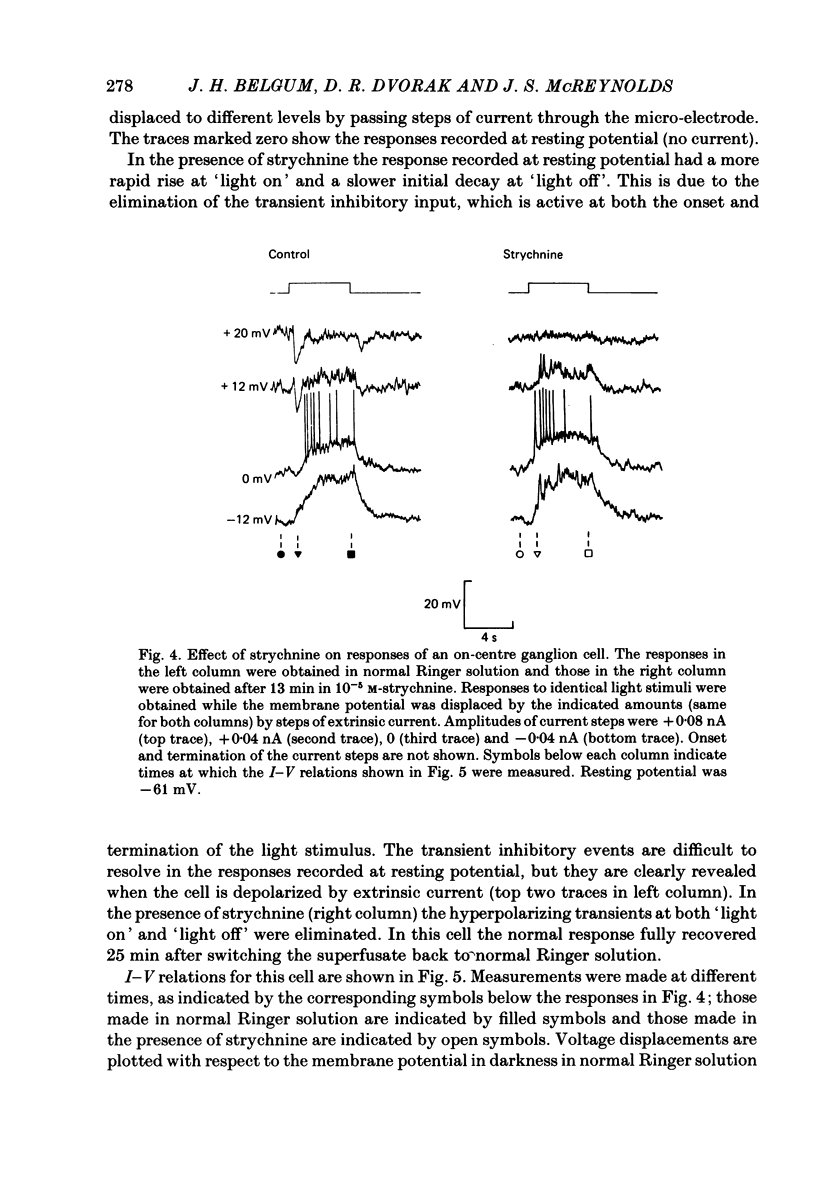
Transient and sustained inhibitory synaptic inputs to on-centre, off-centre, and on-off ganglion cells in the mudpuppy retina were studied using intracellular recording in the superfused eye-cup preparation. When chemical transmission was blocked with 4 mM-Co2+, application of either glycine or gamma-aminobutyric acid (GABA) caused a hyperpolarization and conductance increase in all ganglion cells. For both amino acids, the responses were dose dependent in the range 0.05-10 mM, with a half-maximal response at about 0.7 mM. Glycine and GABA sensitivities were very similar in all three types of ganglion cells. The response to applied glycine was selectively antagonized by 10(-5) M-strychnine and the response to applied GABA was selectively antagonized by 10(-5) M-picrotoxin. In all ganglion cells, 10(-5) M-strychnine eliminated the transient inhibitory events which occur at the onset and termination of a light stimulus. The block of transient inhibition was associated with a relative depolarization of membrane potential and decrease in conductance at these times. Strychnine had no effect on membrane potential or conductance in darkness or during sustained inhibitory responses to light. Picrotoxin (10(-5) M) did not block transient inhibitory events in any ganglion cells, but did affect other components of their responses. The results suggest that in all three classes of ganglion cells transient inhibition, but not sustained inhibition, may be mediated by glycine or a closely related substance.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belgum J. H., Dvorak D. R., McReynolds J. S. Sustained and transient synaptic inputs to on-off ganglion cells in the mudpuppy retina. J Physiol. 1983 Jul;340:599–610. doi: 10.1113/jphysiol.1983.sp014782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgum J. H., Dvorak D. R., McReynolds J. S. Sustained synaptic input to ganglion cells of mudpuppy retina. J Physiol. 1982 May;326:91–108. doi: 10.1113/jphysiol.1982.sp014179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J Physiol. 1978 Mar;276:299–310. doi: 10.1113/jphysiol.1978.sp012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W., Wyatt H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978 Mar;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Piccolino M. Synaptic transmission between photoreceptors and horizontal cells in the turtle retina. Science. 1974 Feb 1;183(4123):417–419. doi: 10.1126/science.183.4123.417. [DOI] [PubMed] [Google Scholar]
- Chan R. Y., Naka K. The amacrine cell. Vision Res. 1976;16(10):1119–1129. doi: 10.1016/0042-6989(76)90252-2. [DOI] [PubMed] [Google Scholar]
- Colburn T. R., Schwartz E. A. Linear voltage control of current passed through a micropipette with variable resistance. Med Biol Eng. 1972 Jul;10(4):504–509. doi: 10.1007/BF02474198. [DOI] [PubMed] [Google Scholar]
- Dacheux R. F., Frumkes T. E., Miller R. F. Pathways and polarities of synaptic interactions in the inner retina of the mudpuppy: I. Synaptic blocking studies. Brain Res. 1979 Jan 26;161(1):1–12. doi: 10.1016/0006-8993(79)90191-4. [DOI] [PubMed] [Google Scholar]
- Ehinger B. Cellular location of the uptake of some amino acids into the rabbit retina. Brain Res. 1972 Nov 13;46:297–311. doi: 10.1016/0006-8993(72)90021-2. [DOI] [PubMed] [Google Scholar]
- Frumkes T. E., Miller R. F., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. III. Amacrine-mediated inhibitory influences on ganglion cell receptive-field organization: a model. J Neurophysiol. 1981 Apr;45(4):783–804. doi: 10.1152/jn.1981.45.4.783. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Sheardown M. J. Transmitters mediating inhibition of ganglion cells in the cat retina: iontophoretic studies in vivo. Neuroscience. 1983 Apr;8(4):837–853. doi: 10.1016/0306-4522(83)90014-3. [DOI] [PubMed] [Google Scholar]
- Ishida A. T., Fain G. L. D-aspartate potentiates the effects of L-glutamate on horizontal cells in goldfish retina. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5890–5894. doi: 10.1073/pnas.78.9.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A. W. The effect of strychnine, bicuculline, and picrotoxin on X and Y cells in the cat retina. J Gen Physiol. 1979 Jul;74(1):71–84. doi: 10.1085/jgp.74.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R. E., Lam D. M. Glycinergic pathways in the goldfish retina. J Neurosci. 1981 Feb;1(2):152–165. doi: 10.1523/JNEUROSCI.01-02-00152.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L. The responses of retinal ganglion cells to stationary and moving visual stimuli. Vision Res. 1979;19(11):1203–1211. doi: 10.1016/0042-6989(79)90185-8. [DOI] [PubMed] [Google Scholar]
- Marchiafava P. L., Weiler R. The photoresponses of structurally identified amacrine cells in the turtle retina. Proc R Soc Lond B Biol Sci. 1982 Feb 22;214(1196):403–415. doi: 10.1098/rspb.1982.0019. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. J. Letter: Further observations on the uptake of (3H)glycine by the isolated retina of the frog. Exp Eye Res. 1976 Feb;22(2):189–191. doi: 10.1016/0014-4835(76)90045-2. [DOI] [PubMed] [Google Scholar]
- Matthews G., Wickelgren W. O. Glycine, GABA and synaptic inhibition of reticulospinal neurones of lamprey. J Physiol. 1979 Aug;293:393–415. doi: 10.1113/jphysiol.1979.sp012896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F., Frumkes T. E. Amacrine cells in Necturus retina: evidence for independent gamma-aminobutyric acid- and glycine-releasing neurons. Science. 1977 Nov 18;198(4318):748–750. doi: 10.1126/science.910159. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Frumkes T. E., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. I. Receptors, horizontal cells, bipolars, and G-cells. J Neurophysiol. 1981 Apr;45(4):743–763. doi: 10.1152/jn.1981.45.4.743. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Frumkes T. E., Slaughter M., Dacheux R. F. Physiological and pharmacological basis of GABA and glycine action on neurons of mudpuppy retina. II. Amacrine and ganglion cells. J Neurophysiol. 1981 Apr;45(4):764–782. doi: 10.1152/jn.1981.45.4.764. [DOI] [PubMed] [Google Scholar]
- Murakami M., Shimoda Y. Identification of amacrine and ganglion cells in the carp retina. J Physiol. 1977 Jan;264(3):801–818. doi: 10.1113/jphysiol.1977.sp011695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H. The effects of strychnine and bicuculline on the responses of X- and Y-cells of the isolated eye-cut preparation of the cat. Brain Res. 1981 May 11;212(1):243–248. doi: 10.1016/0006-8993(81)90061-5. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Hashimoto H., Otsu K. Bipolar-amacrine transmission in the carp retina. Vision Res. 1973 Feb;13(2):295–307. doi: 10.1016/0042-6989(73)90108-9. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S., Copenhagen D. R. Control of retinal sensitivity. 3. Lateral interactions at the inner plexiform layer. J Gen Physiol. 1974 Jan;63(1):88–110. doi: 10.1085/jgp.63.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science. 1972 Mar 3;175(4025):1008–1010. doi: 10.1126/science.175.4025.1008. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative amacrine cell depolarization and formation of on-off ganglion cell response. J Physiol. 1977 Jan;264(3):767–785. doi: 10.1113/jphysiol.1977.sp011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunk D. F., Werblin F. S. Synaptic inputs to the ganglion cells in the tiger salamander retina. J Gen Physiol. 1979 Mar;73(3):265–286. doi: 10.1085/jgp.73.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]


