Abstract
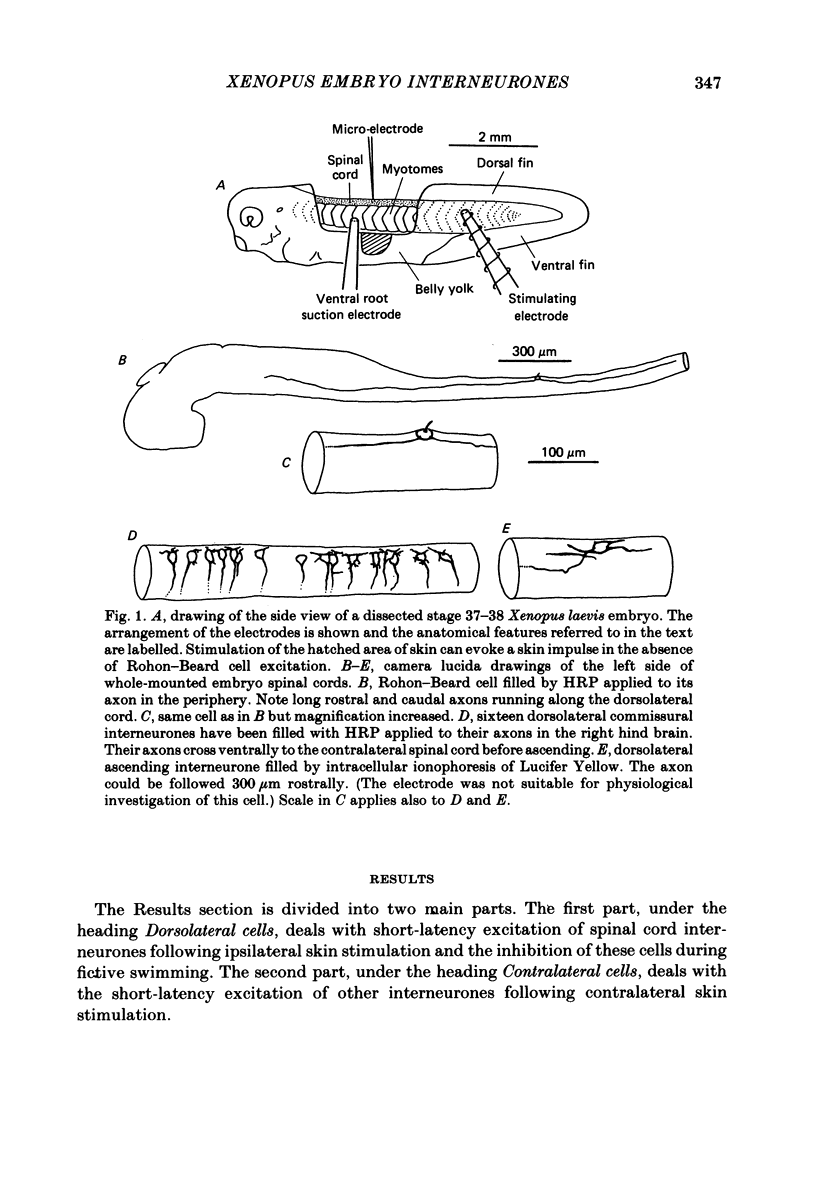
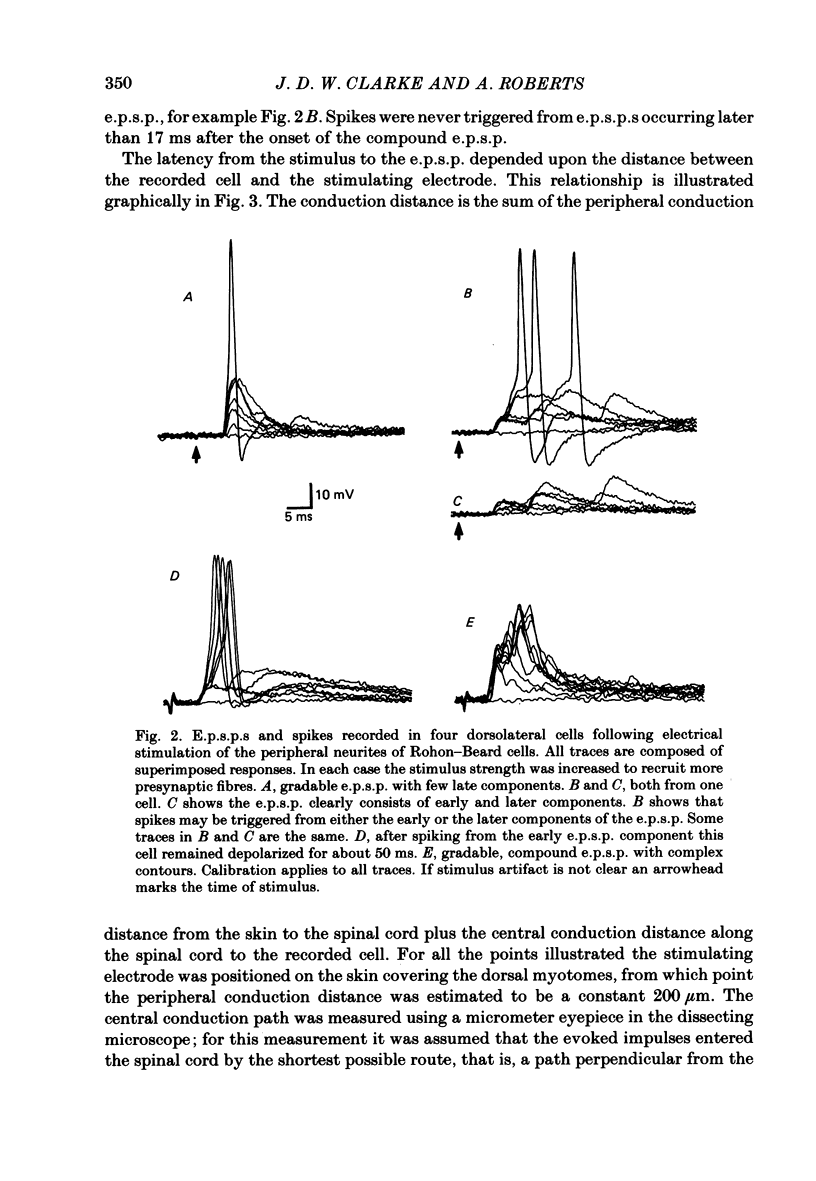
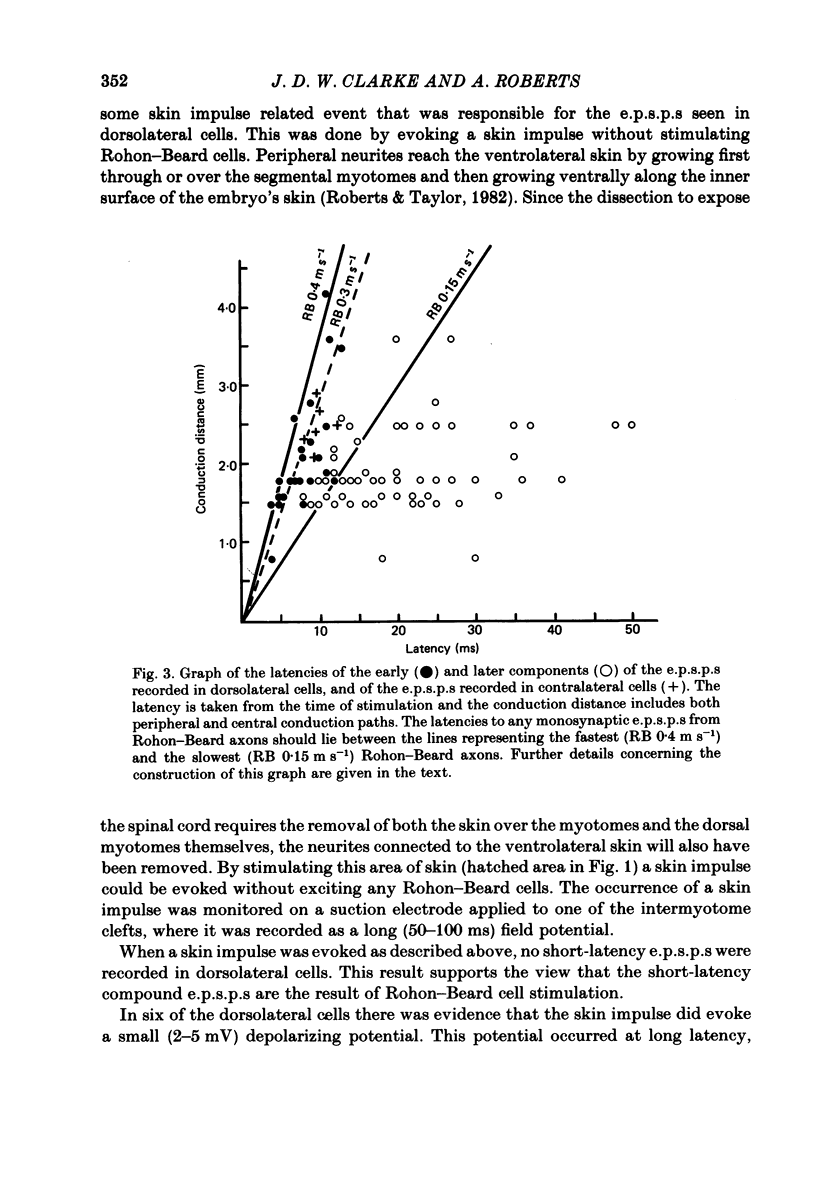
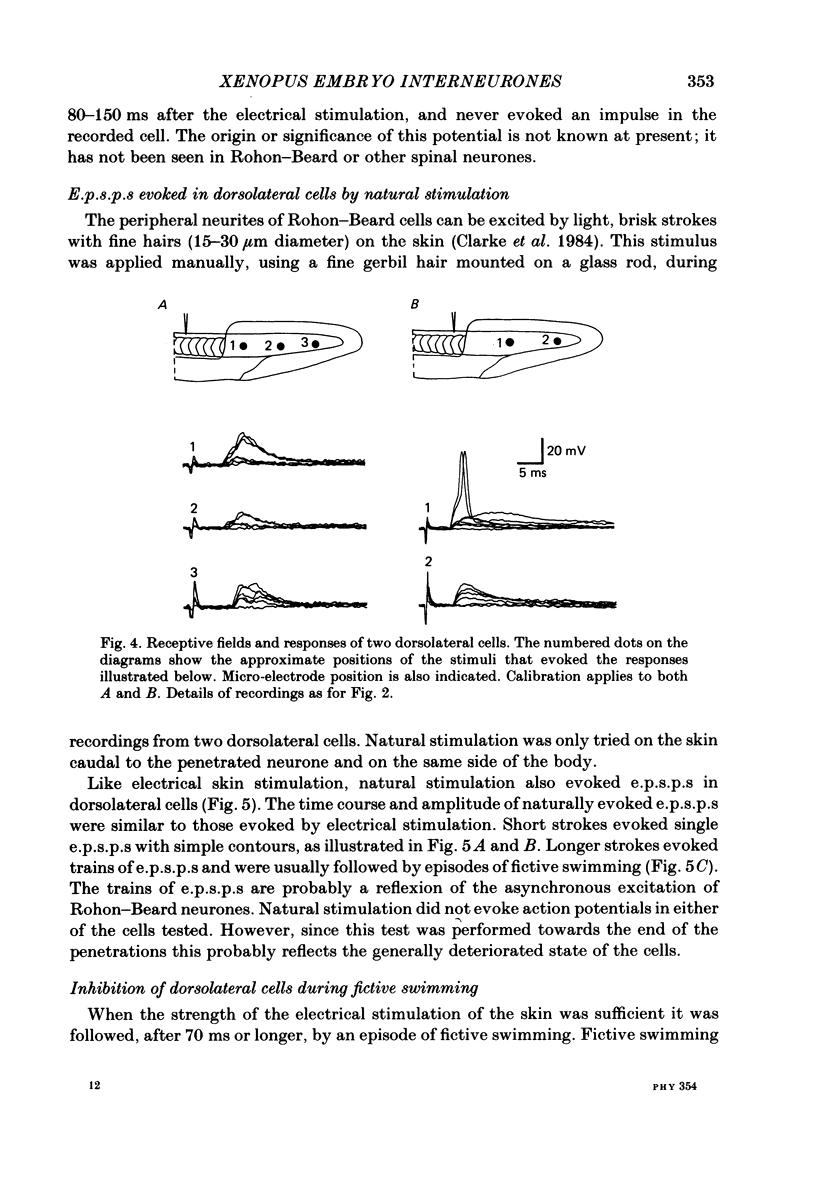
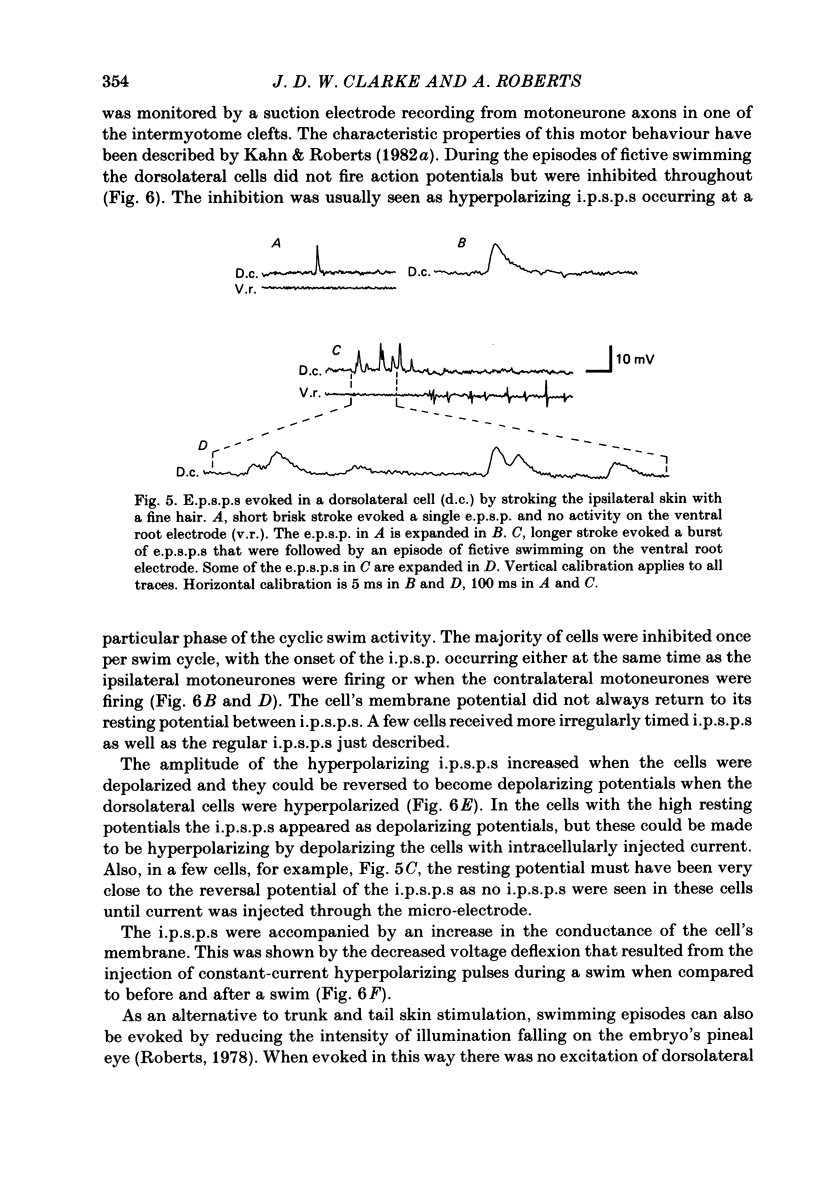
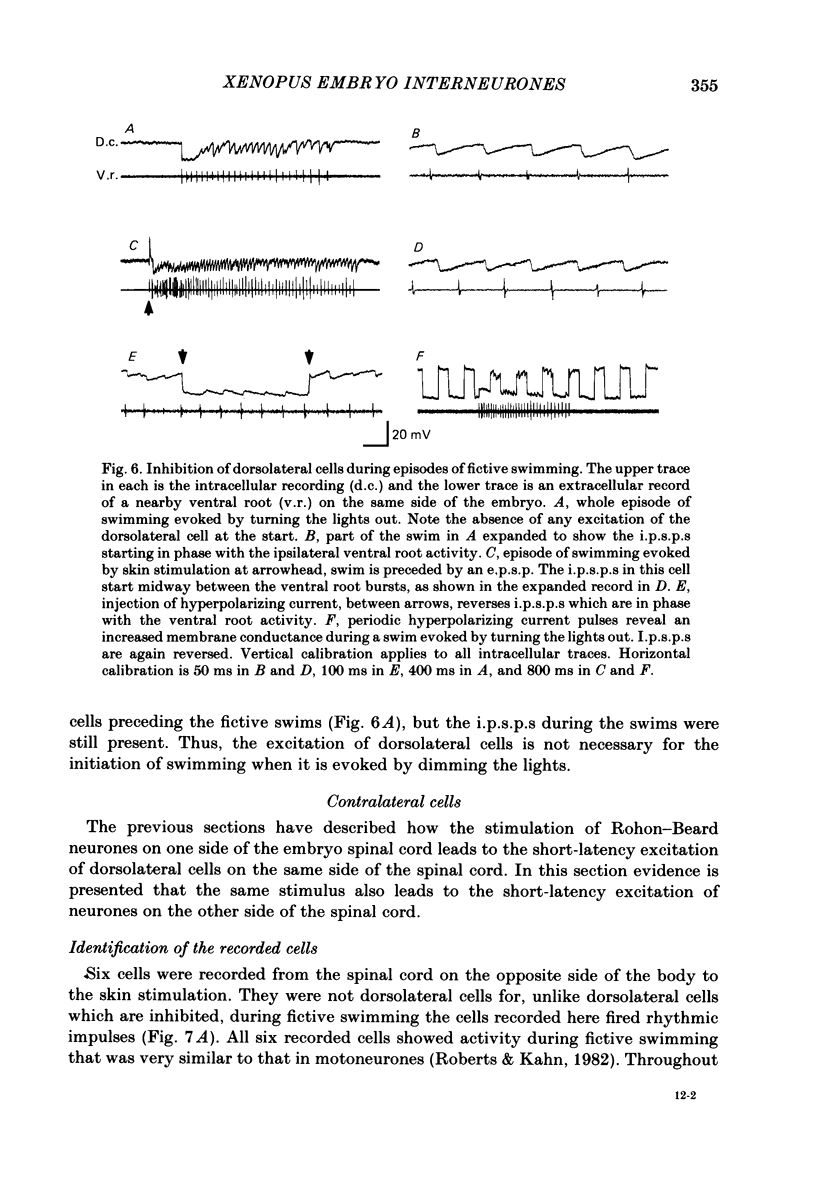
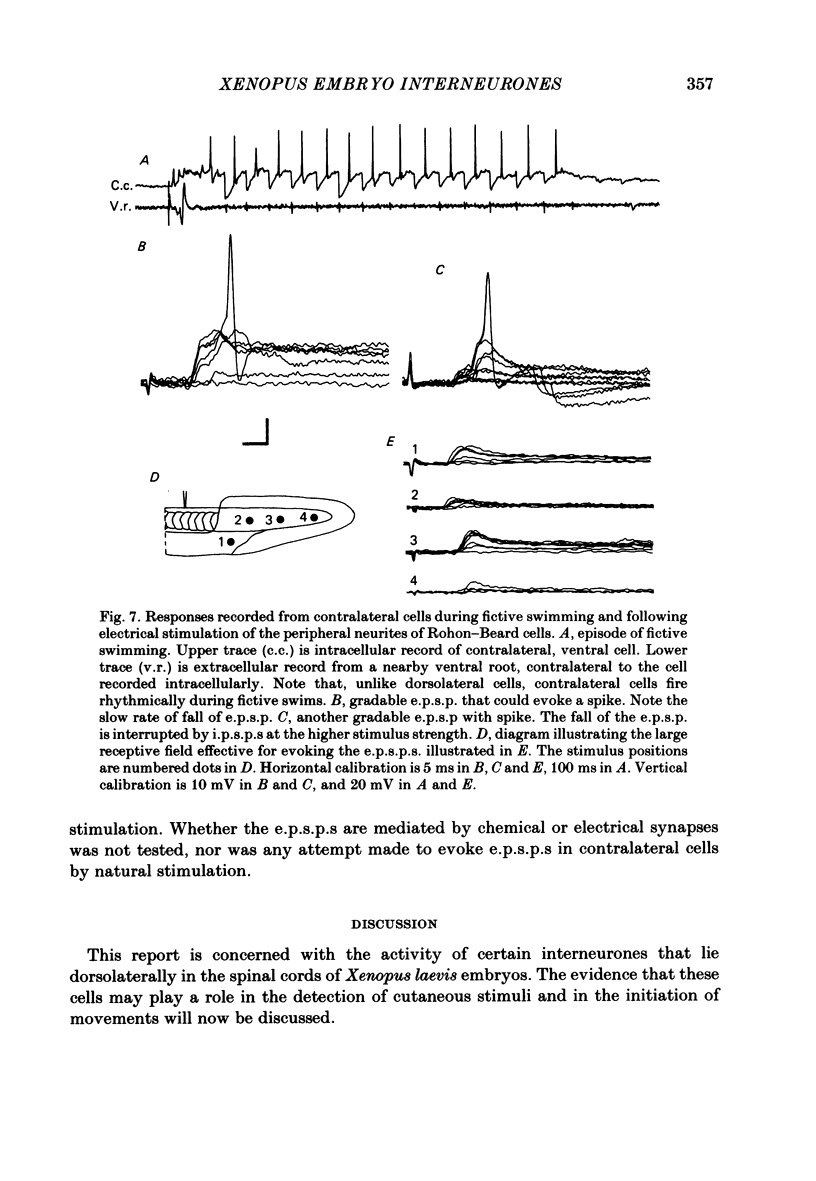
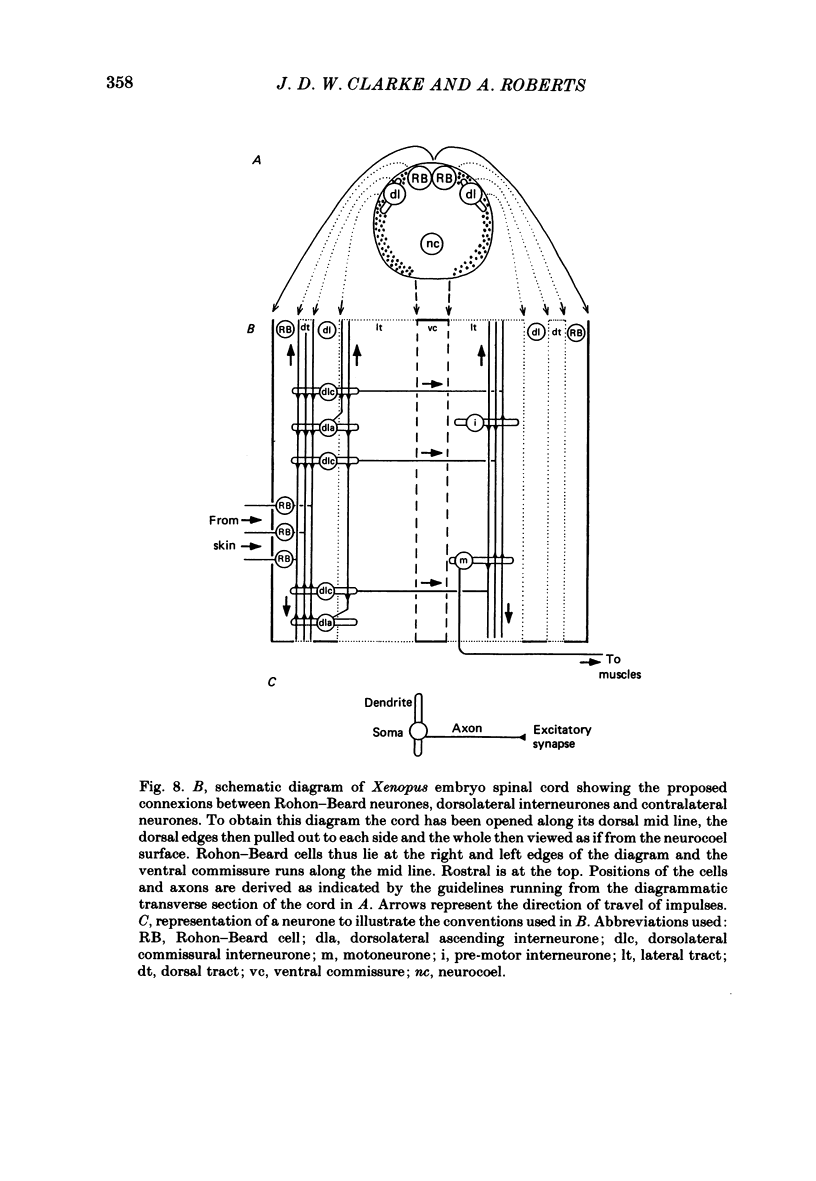
The dorsolateral spinal cord of embryonic Xenopus laevis has previously been shown to contain two anatomical classes of interneurones with dendrites in the dorsal tract where they could be contacted by the central axons of Rohon-Beard cells (Roberts & Clarke, 1982). The activity of these neurones within the dorsolateral spinal cord has been examined using intracellular micro-electrodes. Following electrical stimulation of Rohon-Beard neurites within the ipsilateral skin, dorsolateral neurones receive a short-latency, compound, excitatory post-synaptic potential (e.p.s.p.). The amplitude of the e.p.s.p. depends upon the number of Rohon-Beard cells stimulated. The e.p.s.p. consists of early and later components. The early components may result from monosynaptic connexions from Rohon-Beard cells, the later components from some unidentified interposed neurones. During episodes of fictive swimming the dorsolateral neurones are inhibited by rhythmic inhibitory post-synaptic potentials. Following Rohon-Beard neurite stimulation, neurones in the contralateral spinal cord receive e.p.s.p.s. These contralateral e.p.s.p.s are probably one of the post-synaptic effects of one of the dorsolateral neurone classes. The results suggest that the dorsolateral neurones are responsible for amplifying and distributing the primary afferent signals of Rohon-Beard cells, and may be involved in the initiation of swimming and reflex movements.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson O., Forssberg H., Grillner S., Lindquist M. Phasic gain control of the transmission in cutaneous reflex pathways to motoneurones during 'fictive' locomotion. Brain Res. 1978 Jun 30;149(2):503–507. doi: 10.1016/0006-8993(78)90493-6. [DOI] [PubMed] [Google Scholar]
- Clarke J. D., Hayes B. P., Hunt S. P., Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984 Mar;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysens J., Loeb G. E. Modulation of ipsi- and contralateral reflex responses in unrestrained walking cats. J Neurophysiol. 1980 Nov;44(5):1024–1037. doi: 10.1152/jn.1980.44.5.1024. [DOI] [PubMed] [Google Scholar]
- Duysens J., Pearson K. G. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976 Jan 26;24:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975 Feb 21;85(1):103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Res. 1977 Aug 19;132(1):121–139. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol. 1979 Jul;42(4):936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Grillner S., Rossignol S., Wallén P. The adaptation of a reflex response to the ongoing phase of locomotion in fish. Exp Brain Res. 1977 Oct 24;30(1):1–11. doi: 10.1007/BF00237854. [DOI] [PubMed] [Google Scholar]
- HUGHES A. The development of the primary sensory system in Xenopus laevis (Daudin). J Anat. 1957 Jul;91(3):323–338. [PMC free article] [PubMed] [Google Scholar]
- Kahn J. A., Roberts A. Experiments on the central pattern generator for swimming in amphibian embryos. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):229–243. doi: 10.1098/rstb.1982.0004. [DOI] [PubMed] [Google Scholar]
- Kahn J. A., Roberts A. The central nervous origin of the swimming motor pattern in embryos of Xenopus laevis. J Exp Biol. 1982 Aug;99:185–196. doi: 10.1242/jeb.99.1.185. [DOI] [PubMed] [Google Scholar]
- Lamborghini J. E. Rohon-beard cells and other large neurons in Xenopus embryos originate during gastrulation. J Comp Neurol. 1980 Jan 15;189(2):323–333. doi: 10.1002/cne.901890208. [DOI] [PubMed] [Google Scholar]
- Roberts A., Clarke J. D. The neuroanatomy of an amphibian embryo spinal cord. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):195–212. doi: 10.1098/rstb.1982.0002. [DOI] [PubMed] [Google Scholar]
- Roberts A., Hayes B. P. The anatomy and function of 'free' nerve endings in an amphibian skin sensory system. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):415–429. doi: 10.1098/rspb.1977.0048. [DOI] [PubMed] [Google Scholar]
- Roberts A., Khan J. A. Intracellular recordings from spinal neurons during 'swimming' in paralysed amphibian embryos. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):213–228. doi: 10.1098/rstb.1982.0003. [DOI] [PubMed] [Google Scholar]
- Roberts A. Pineal eye and behaviour in Xenopus tadpoles. Nature. 1978 Jun 29;273(5665):774–775. doi: 10.1038/273774a0. [DOI] [PubMed] [Google Scholar]
- Roberts A., Taylor J. S. A scanning electron microscope study of the development of a peripheral sensory neurite network. J Embryol Exp Morphol. 1982 Jun;69:237–250. [PubMed] [Google Scholar]
- Soffe S. R., Clarke J. D., Roberts A. Activity of commissural interneurons in spinal cord of Xenopus embryos. J Neurophysiol. 1984 Jun;51(6):1257–1267. doi: 10.1152/jn.1984.51.6.1257. [DOI] [PubMed] [Google Scholar]
- Soffe S. R., Roberts A. Activity of myotomal motoneurons during fictive swimming in frog embryos. J Neurophysiol. 1982 Dec;48(6):1274–1278. doi: 10.1152/jn.1982.48.6.1274. [DOI] [PubMed] [Google Scholar]
- Soffe S. R., Roberts A. Tonic and phasic synaptic input to spinal cord motoneurons during fictive locomotion in frog embryos. J Neurophysiol. 1982 Dec;48(6):1279–1288. doi: 10.1152/jn.1982.48.6.1279. [DOI] [PubMed] [Google Scholar]
- Wallén P. On the mechanisms of a phase-dependent reflex occurring during locomotion in dogfish. Exp Brain Res. 1980;39(2):193–202. doi: 10.1007/BF00237550. [DOI] [PubMed] [Google Scholar]
- Weeks J. C. Segmental specialization of a leech swim-initiating interneuron, cell 2051. J Neurosci. 1982 Jul;2(7):972–985. doi: 10.1523/JNEUROSCI.02-07-00972.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


