Abstract
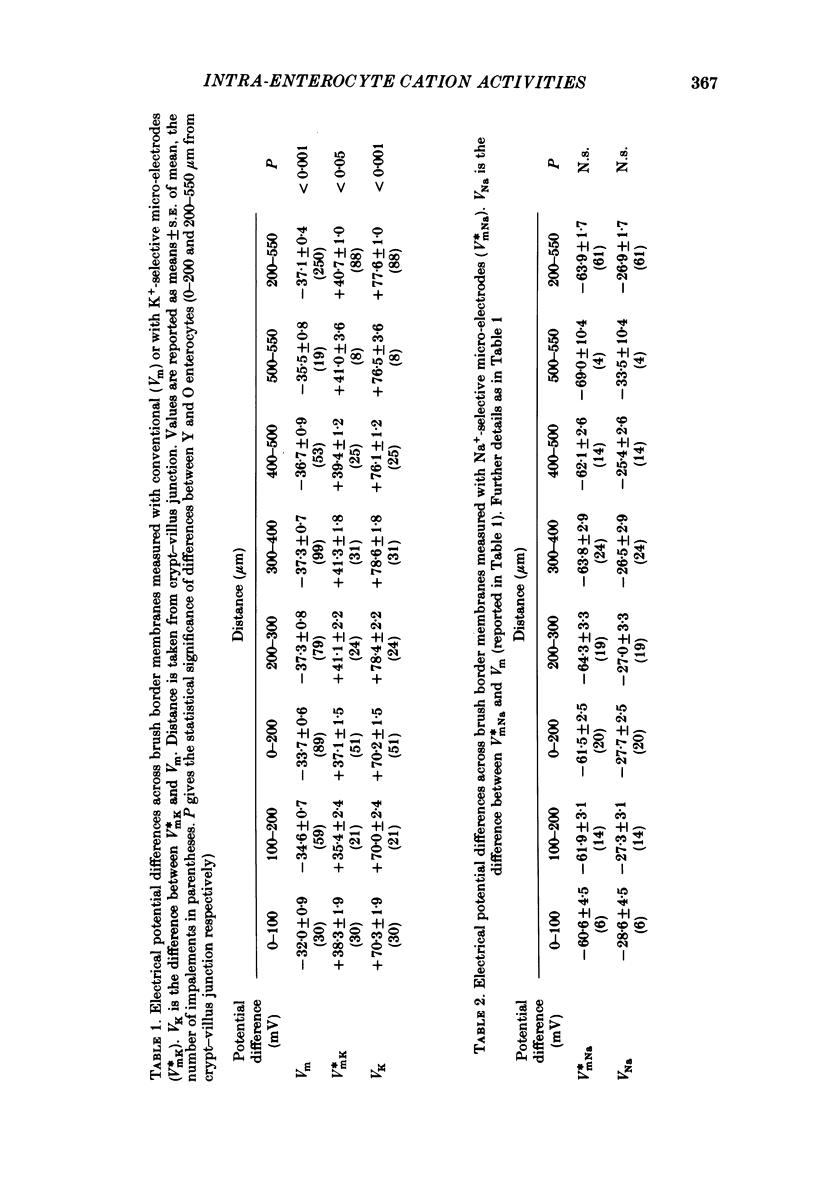
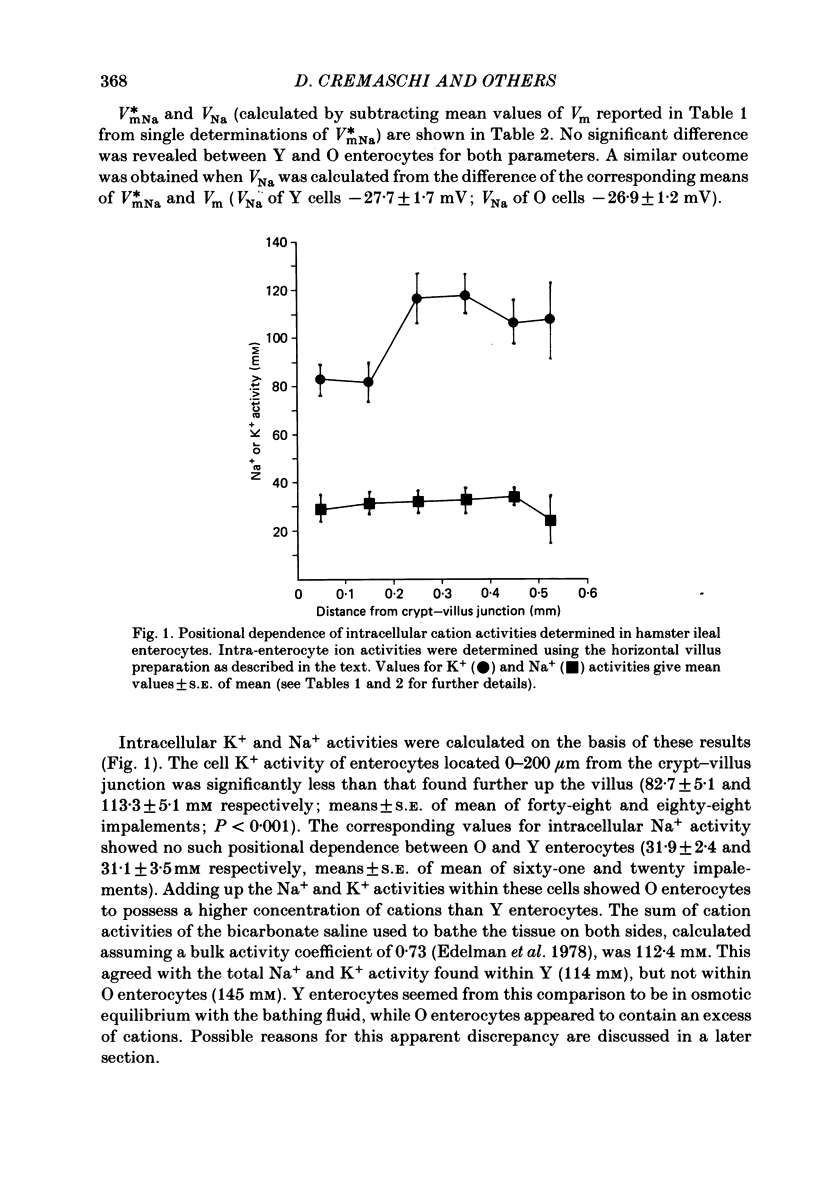
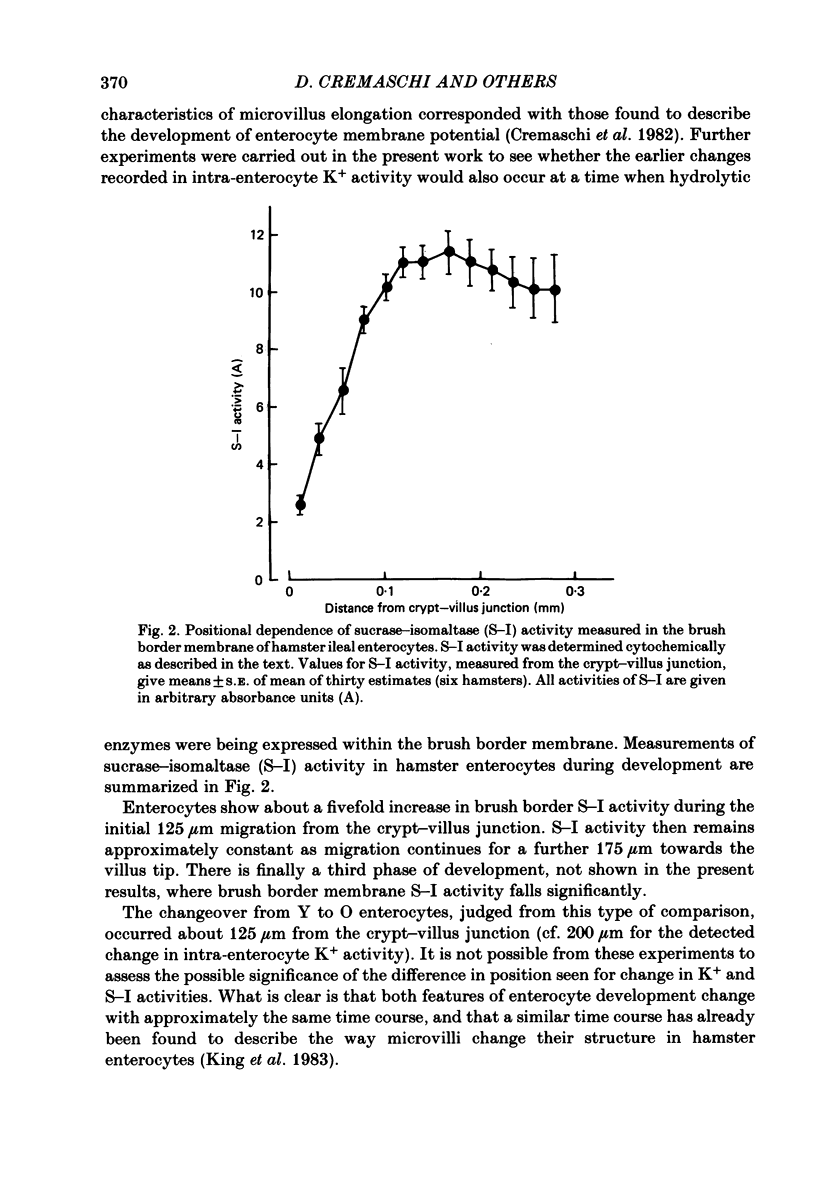
Intracellular Na+ and K+ activities have been determined under visual control in enterocytes located at known positions along hamster ileal villi in vitro. The intracellular K+ activity found in enterocytes migrating over the basal third of villi was significantly less than that found in older enterocytes (83 and 113 mM respectively). The intracellular Na+ activity remained unaffected by the state of enterocyte development (32 mM throughout). Extracellular K+ and Na+ activities measured close to the luminal surface of the intestine were always similar to those measured in the bulk of the bathing medium. There was, however, a small difference in K+ activity detected between the bulk solution and solution near the luminal surface of enterocytes (3 . 4 and 3 . 6-3 . 7 mM respectively). Na+ activity in the villus core was also similar to that of the bathing medium. The brush border membrane content of sucrase-isomaltase, determined cytochemically, was found to increase rapidly as enterocytes migrated over the basal third of the villus and to plateau at a point roughly equivalent to that where intracellular K+ activities became maximal. The numerous ways by which enterocytes might control their intracellular K+ concentrations during development are emphasized. Attention is nevertheless drawn to the close correspondence seen between different aspects of brush border membrane development and the ability of enterocytes to maintain high internal concentrations of K+.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado F., Lherminier M. Phenylalanine transport in guinea pig jejunum. A general mechanism for organic solute and sodium cotransport. J Physiol (Paris) 1982 Aug;78(2):131–145. [PubMed] [Google Scholar]
- Charney A. N., Gots R. E., Giannella R. A. Na+-K+)-stimulated adenosinetriphosphatase in isolated intestinal villus tip and crypt cells. Biochim Biophys Acta. 1974 Nov 15;367(3):265–270. doi: 10.1016/0005-2736(74)90083-2. [DOI] [PubMed] [Google Scholar]
- Cremaschi D., James P. S., Meyer G., Peacock M. A., Smith M. W. Membrane potentials of differentiating enterocytes. Biochim Biophys Acta. 1982 May 21;688(1):271–274. doi: 10.1016/0005-2736(82)90603-4. [DOI] [PubMed] [Google Scholar]
- Cremaschi D., Meyer G., Rossetti C. Bicarbonate effects, electromotive forces and potassium effluxes in rabbit and guinea-pig gall-bladder. J Physiol. 1983 Feb;335:51–64. doi: 10.1113/jphysiol.1983.sp014518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A., Curci S., Samarzija I., Frömter E. Determination of intracellular K+ activity in rat kidney proximal tubular cells. Pflugers Arch. 1978 Dec 15;378(1):37–45. doi: 10.1007/BF00581956. [DOI] [PubMed] [Google Scholar]
- Gratecos D., Knibiehler M., Benoit V., Sémériva M. Plasma membranes from rat intestinal epithelial cells at different stages of maturation. I. Preparation and characterization of plasma membrane subfractions originating from crypt cells and from villous cells. Biochim Biophys Acta. 1978 Oct 4;512(3):508–524. doi: 10.1016/0005-2736(78)90161-x. [DOI] [PubMed] [Google Scholar]
- Gutschmidt S., Kaul W., Riecken E. O. A quantitative histochemical technique for the characterisation of alpha-glucosidases in the brush-border membrane of rat jejunum. Histochemistry. 1979 Sep;63(1):81–101. doi: 10.1007/BF00508014. [DOI] [PubMed] [Google Scholar]
- Haljamäe H., Jodal M., Lundgren O. Countercurrent multiplication of sodium in intestinal villi during absorption of sodium chloride. Acta Physiol Scand. 1973 Dec;89(4):580–593. doi: 10.1111/j.1748-1716.1973.tb05552.x. [DOI] [PubMed] [Google Scholar]
- IRVINE R. O., SAUNDERS S. J., MILNE M. D., CRAWFORD M. A. Gradients of potassium and hydrogen ion in potassiumdeficient voluntary muscle. Clin Sci. 1961 Feb;20:1–18. [PubMed] [Google Scholar]
- King I. S., Paterson J. Y., Peacock M. A., Smith M. W., Syme G. Effect of diet upon enterocyte differentiation in the rat jejunum. J Physiol. 1983 Nov;344:465–481. doi: 10.1113/jphysiol.1983.sp014952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M. L., Cannon M. J. Measurement of sodium ion concentration in the unstirred layer of rat small intestine by polymer Na+-sensitive electrodes. Biochim Biophys Acta. 1983 Apr 21;730(1):41–48. doi: 10.1016/0005-2736(83)90314-0. [DOI] [PubMed] [Google Scholar]
- Lukie B. E., Westergaard H., Dietschy J. M. Validation of a chamber that allows measurement of both tissue uptake rates and unstirred layer thicknesses in the intestine under conditions of controlled stirring. Gastroenterology. 1974 Oct;67(4):652–661. [PubMed] [Google Scholar]
- Okada Y., Irimajiri A., Tsuchiya W., Inouye A. Contribution of an electrogenic sodium pump to the membrane potential in the intestinal epithelial cell. Jpn J Physiol. 1978;28(4):511–525. doi: 10.2170/jjphysiol.28.511. [DOI] [PubMed] [Google Scholar]
- Read N. W., Barber D. C., Levin R. J., Holdsworth C. D. Unstirred layer and kinetics of electrogenic glucose absorption in the human jejunum in situ. Gut. 1977 Nov;18(11):865–876. doi: 10.1136/gut.18.11.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson K. W., Millar D. B., Jacobs L. R., Gray G. M. Intestinal diffusion barrier: unstirred water layer or membrane surface mucous coat? Science. 1981 Dec 11;214(4526):1241–1244. doi: 10.1126/science.7302593. [DOI] [PubMed] [Google Scholar]
- Tsuchiya W., Okada Y., Inouye A. Membrane potential measurements in cultured intestinal villi. Membr Biochem. 1980;3(1-2):147–153. doi: 10.3109/09687688009063882. [DOI] [PubMed] [Google Scholar]
- Tsuchiya W., Okada Y. Membrane potential changes associated with differentiation of enterocytes in the rat intestinal villi in culture. Dev Biol. 1982 Dec;94(2):284–290. doi: 10.1016/0012-1606(82)90348-7. [DOI] [PubMed] [Google Scholar]
- Westergaard H., Dietschy J. M. Delineation of the dimensions and permeability characteristics of the two major diffusion barriers to passive mucosal uptake in the rabbit intestine. J Clin Invest. 1974 Sep;54(3):718–732. doi: 10.1172/JCI107810. [DOI] [PMC free article] [PubMed] [Google Scholar]


