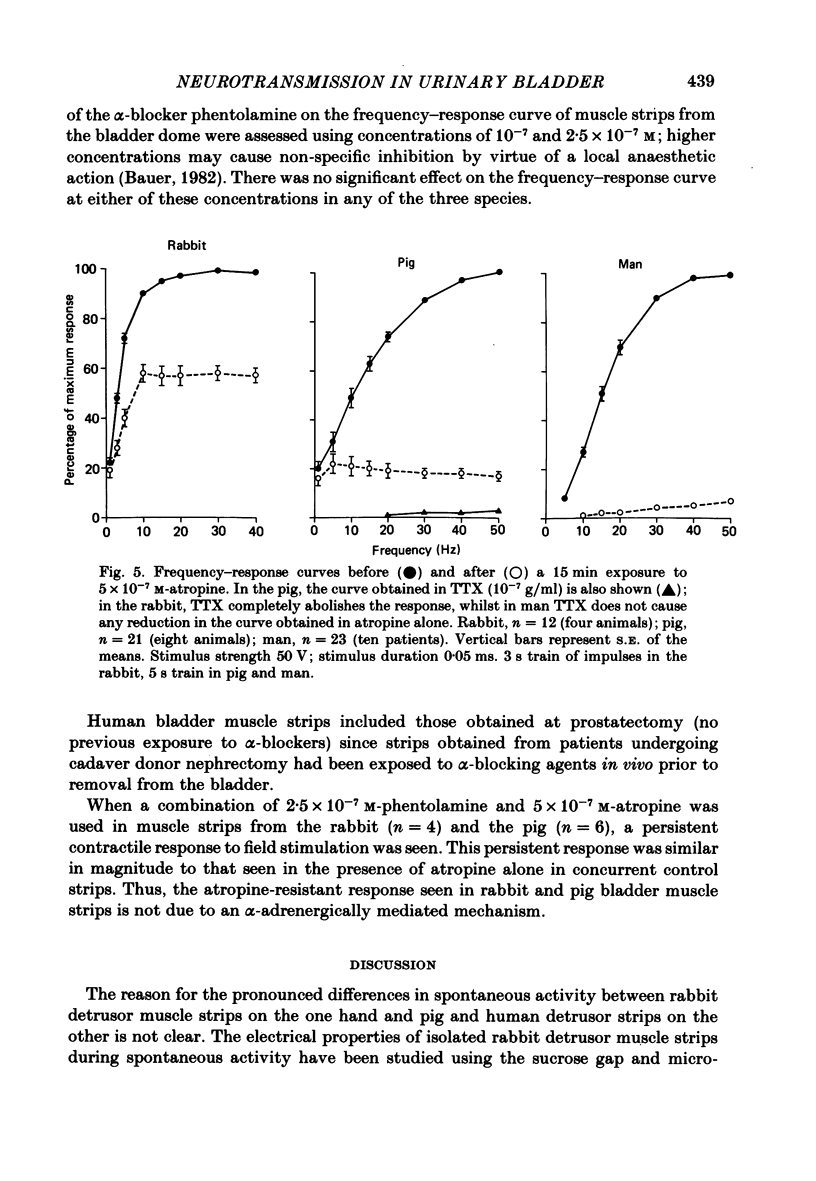
Abstract
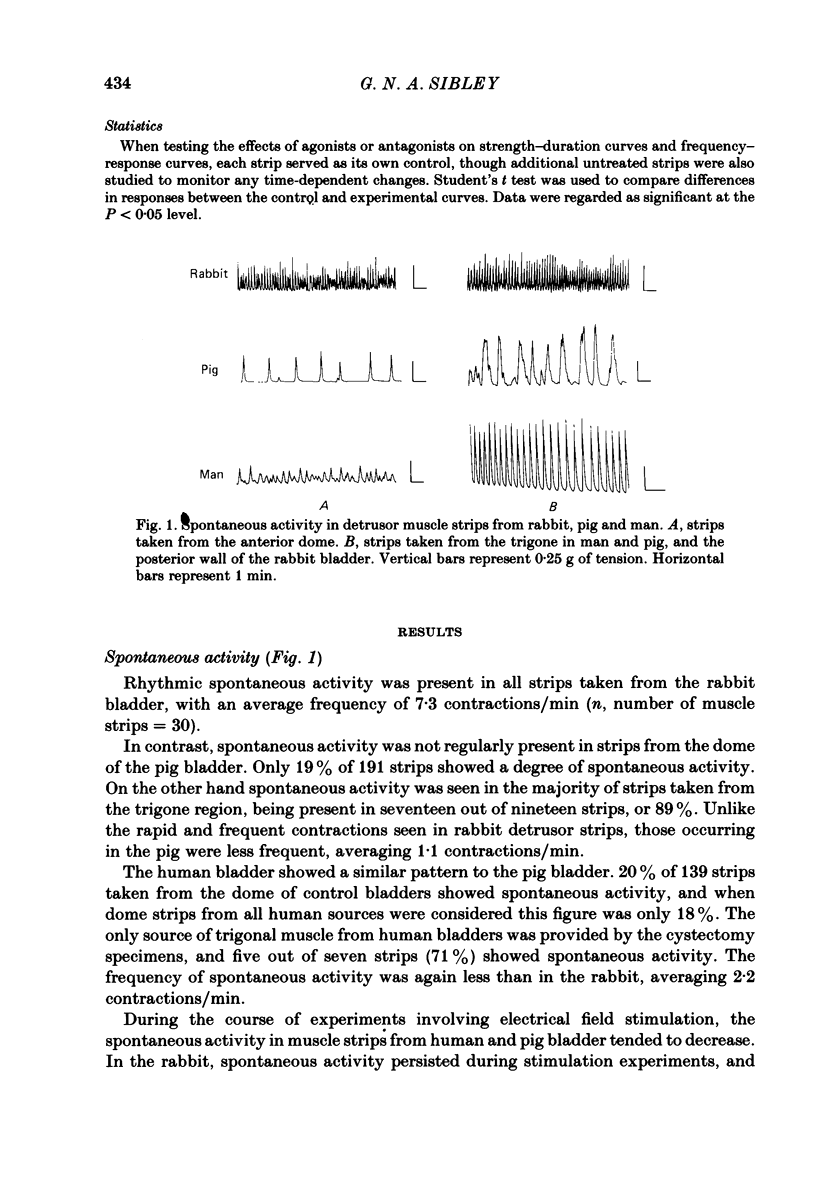
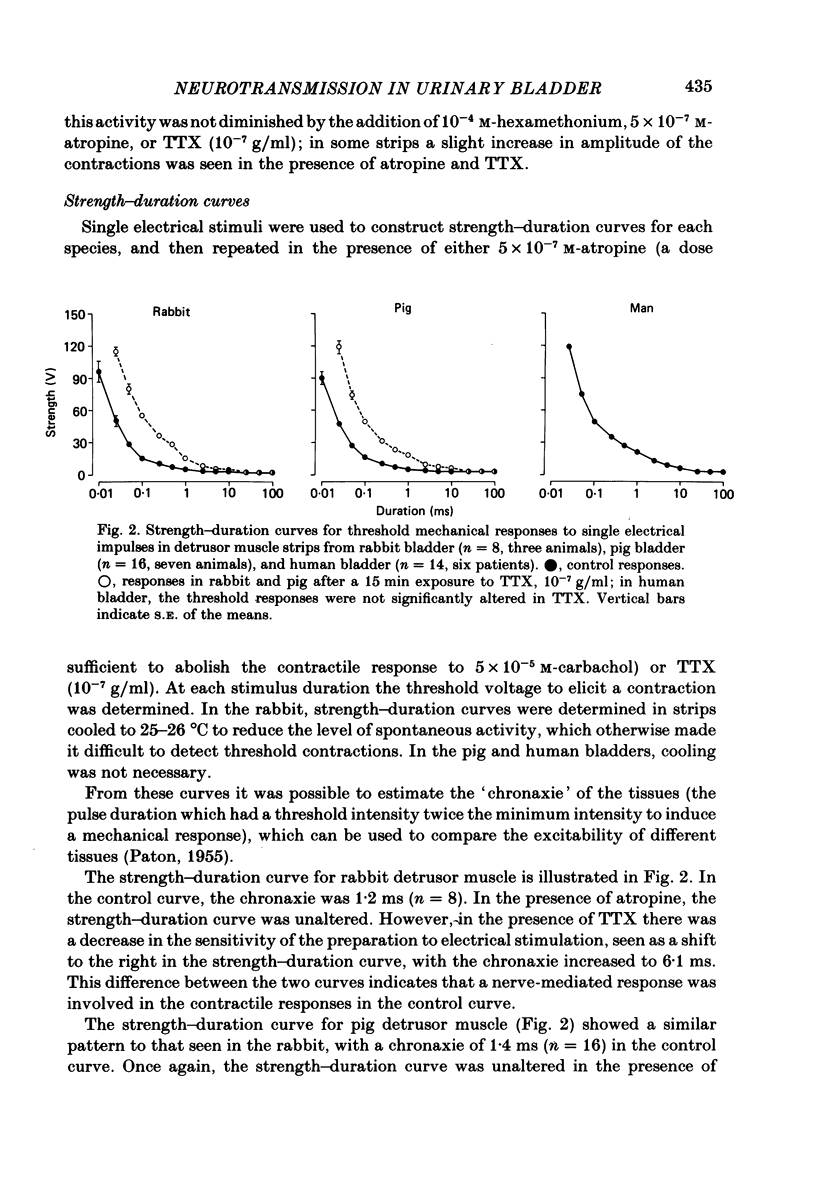
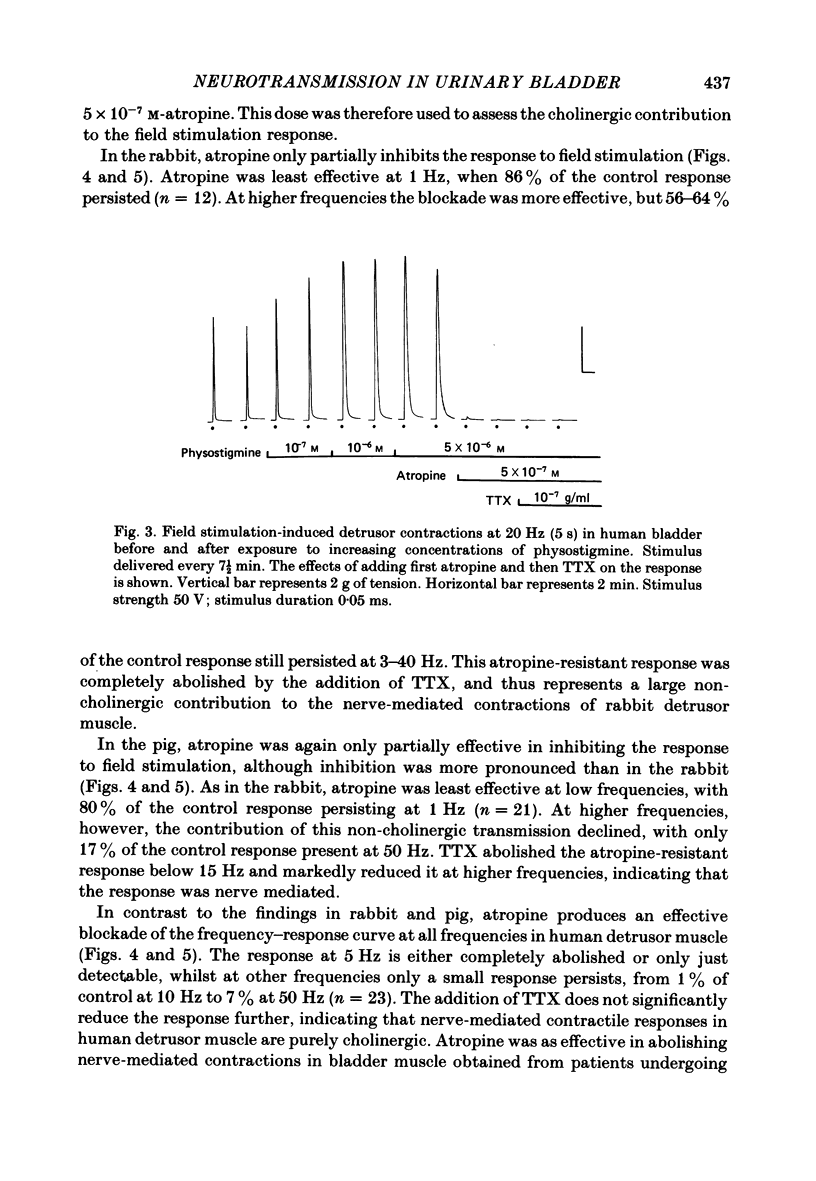
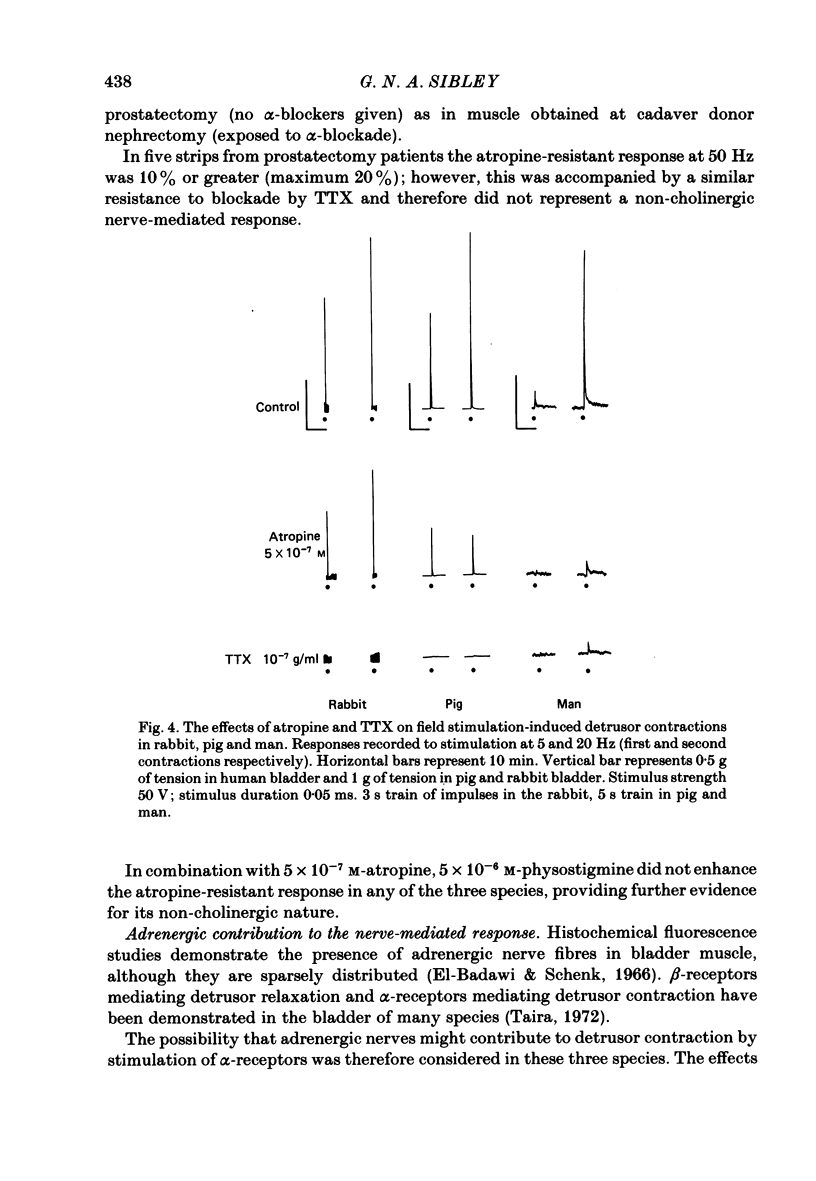
Spontaneous activity in bladder muscle strips from man, pig and rabbit has been compared using an in vitro superfusion technique. Field stimulation was used to study nerve-mediated activity. Bladder muscle strips from all areas of the rabbit bladder displayed rhythmic spontaneous activity. Spontaneous activity was regularly present in strips from the trigone region in man and pig, but was present in only 18 and 19% respectively of strips from the dome of the bladder. Strength-duration curves in the presence of tetrodotoxin (10(-7) g/ml) were constructed. The 'chronaxie' of the muscle was found to be considerably shorter than that of other smooth muscles, ranging from 6.1 ms in the rabbit to 12.9 ms in man. Frequency-response curves were constructed using trains of stimuli. The responses were not antagonized by hexamethonium (10(-4) M), but were markedly inhibited by tetrodotoxin (10(-7) g/ml), indicating that the responses were mediated by excitation of post-ganglionic nerves. Physostigmine (10(-7)-5 X 10(-6) M) produced a dose-related increase in the contractile response to field stimulation in all three species. Atropine (10(-8)-10(-6) M) produced an inhibition of the contractile response, but the maximum degree of inhibition differed considerably between the species. In the rabbit, 58% of the control response was attained, whilst in the pig this was only 22%. Atropine completely abolished nerve-mediated contractions in human bladder muscle. Phentolmaine (10(-7)-2.5 X 10(-7) M) had no significant effect on the frequency-response curve in any of the three species, and did not depress the atropine-resistant component in rabbit and pig. It is concluded that nerve-mediated activity in human bladder muscle is exclusively cholinergic, in contrast to most other mammals studied in which there is a significant non-cholinergic component. The finding of a shorter chronaxie in bladder muscle than in other smooth muscles suggests important differences in its physiological properties that merit further investigation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams P. H., Feneley R. C. The actions of prostaglandins on the smooth muscle of the human urinary tract in vitro. Br J Urol. 1975;47(7):909–915. doi: 10.1111/j.1464-410x.1975.tb04075.x. [DOI] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. J Physiol. 1970 Oct;210(3):761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K. E., Husted S., Sjögren C. Contribution of prostaglandins to the adenosine triphosphate-induced contraction of rabbit urinary bladder. Br J Pharmacol. 1980 Nov;70(3):443–452. doi: 10.1111/j.1476-5381.1980.tb08722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer V. Distribution and types of adrenoceptors in the guinea-pig ileum: the action of alpha- and beta-adrenoceptor blocking agents. Br J Pharmacol. 1982 Aug;76(4):569–578. doi: 10.1111/j.1476-5381.1982.tb09256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultitude M. I., Hills N. H., Shuttleworth K. E. Clinical and experimental studies on the action of prostaglandins and their synthesis inhibitors on detrusor muscle in vitro and in vivo. Br J Urol. 1976;48(7):631–637. doi: 10.1111/j.1464-410x.1976.tb06711.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R., Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978 May;63(1):125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Ishikawa S., Ito Y. Electrical and mechanical activity recorded from rabbit urinary bladder in response to nerve stimulation. J Physiol. 1983 May;338:149–164. doi: 10.1113/jphysiol.1983.sp014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. M., Downie J. W. Contribution of adrenergic and "purinergic" neurotransmission to contraction in rabbit detrusor. J Pharmacol Exp Ther. 1978 Nov;207(2):431–445. [PubMed] [Google Scholar]
- Downie J. W., Dean D. M. The contribution of cholinergic postganglionic neurotransmission to contractions of rabbit detrusor. J Pharmacol Exp Ther. 1977 Nov;203(2):417–425. [PubMed] [Google Scholar]
- Hindmarsh J. R., Idowu O. A., Yeates W. K., Zar M. A. Pharmacology of electrically evoked contractions of human bladder [proceedings]. Br J Pharmacol. 1977 Sep;61(1):115P–115P. [PMC free article] [PubMed] [Google Scholar]
- Hodson C. J., Maling T. M., McManamon P. J., Lewis M. G. The pathogenesis of reflux nephropathy (chronic atrophic pyelonephritis). Br J Radiol. 1975;Suppl 13:1–26. [PubMed] [Google Scholar]
- Holman M. E., Kasby C. B., Suthers M. B., Wilson J. A. Some properties of the smooth muscle of rabbit portal vein. J Physiol. 1968 May;196(1):111–132. doi: 10.1113/jphysiol.1968.sp008498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell R. D., Mccoy J. L., Ridley P. T. Pharmacological characterization of the excitatory innervation to the guinea-pig urinary bladder in vitro: evidence for both cholinergic and non-adrenergic-non-cholinergic neurotransmission. Br J Pharmacol. 1981 Sep;74(1):15–22. doi: 10.1111/j.1476-5381.1981.tb09950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Toida N. Effect of tetrodotoxin on smooth muscle cells of the guinea-pig taenia coli. Br J Pharmacol Chemother. 1966 Aug;27(2):366–376. doi: 10.1111/j.1476-5381.1966.tb01668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longrigg N. In vitro studies on the human renal calices. J Urol. 1975 Sep;114(3):325–331. doi: 10.1016/s0022-5347(17)67022-8. [DOI] [PubMed] [Google Scholar]
- MELICK W. F., NARYKA J. J., SCHMIDT J. H. Experimental studies of ureteral peristaltic patterns in the pig. I. Similarity of pig and human ureter and bladder physiology. J Urol. 1961 Feb;85:145–148. doi: 10.1016/S0022-5347(17)65296-0. [DOI] [PubMed] [Google Scholar]
- Nergårdh A., Kinn A. C. Neurotransmission in activation of the contractile response in the human urinary bladder. Scand J Urol Nephrol. 1983;17(2):153–157. doi: 10.3109/00365598309180160. [DOI] [PubMed] [Google Scholar]
- PATON W. D. The response of the guineapig ileum to electrical stimulation by coaxial electrodes. J Physiol. 1955 Feb 28;127(2):40–1P. [PubMed] [Google Scholar]
- Sjögren C., Andersson K. E., Husted S., Mattiasson A., Moller-Madsen B. Atropine resistance of transmurally stimulated isolated human bladder muscle. J Urol. 1982 Dec;128(6):1368–1371. doi: 10.1016/s0022-5347(17)53509-0. [DOI] [PubMed] [Google Scholar]
- Todd J. K., Mack A. J. A study of human bladder detrusor muscle. Br J Urol. 1969 Aug;41(4):448–454. doi: 10.1111/j.1464-410x.1969.tb09946.x. [DOI] [PubMed] [Google Scholar]
- Weiss R. M., Holcomb W., Bassett A. L. Excitability of in vitro normal and dilated human ureteral segments. Invest Urol. 1972 Sep;10(2):131–134. [PubMed] [Google Scholar]
- el-Badawi A., Schenk E. A. Dual innervation of the mammalian urinary bladder. A histochemical study of the distribution of cholinergic and adrenergic nerves. Am J Anat. 1966 Nov;119(3):405–427. doi: 10.1002/aja.1001190305. [DOI] [PubMed] [Google Scholar]


