Abstract
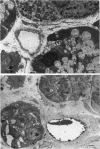
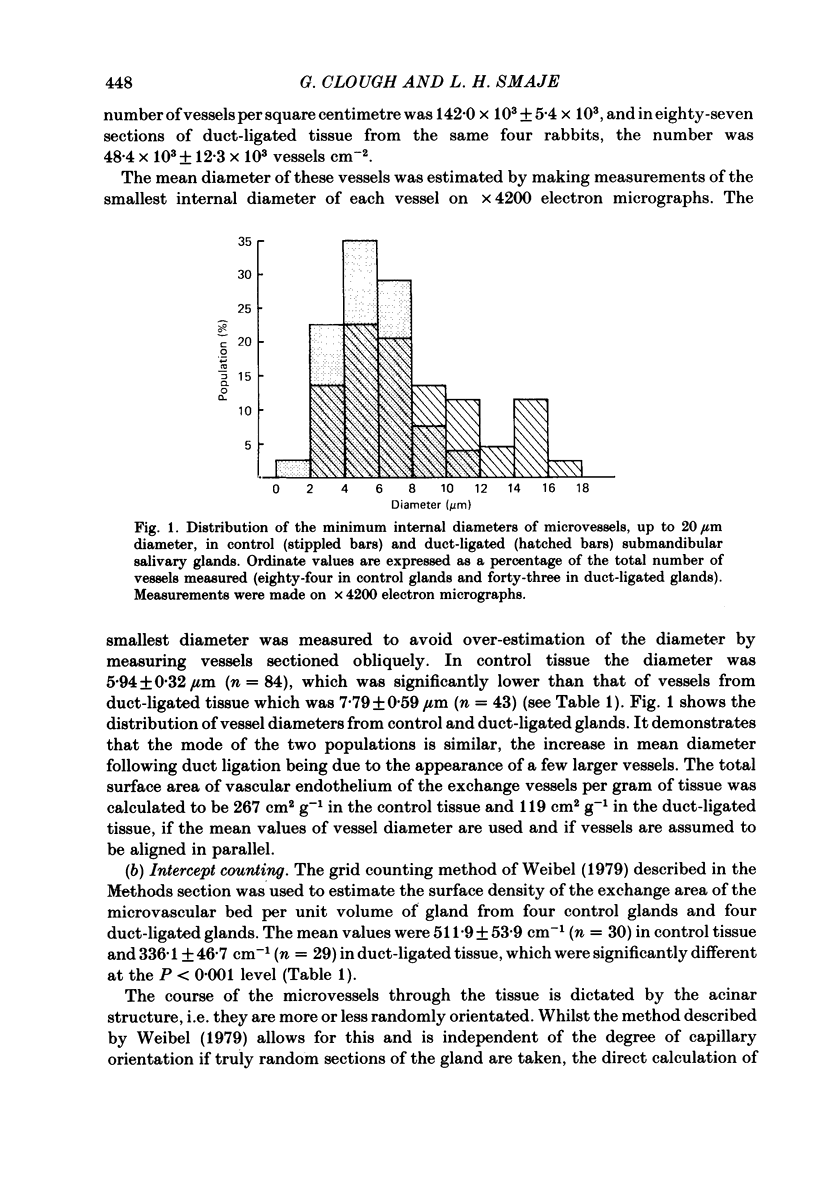
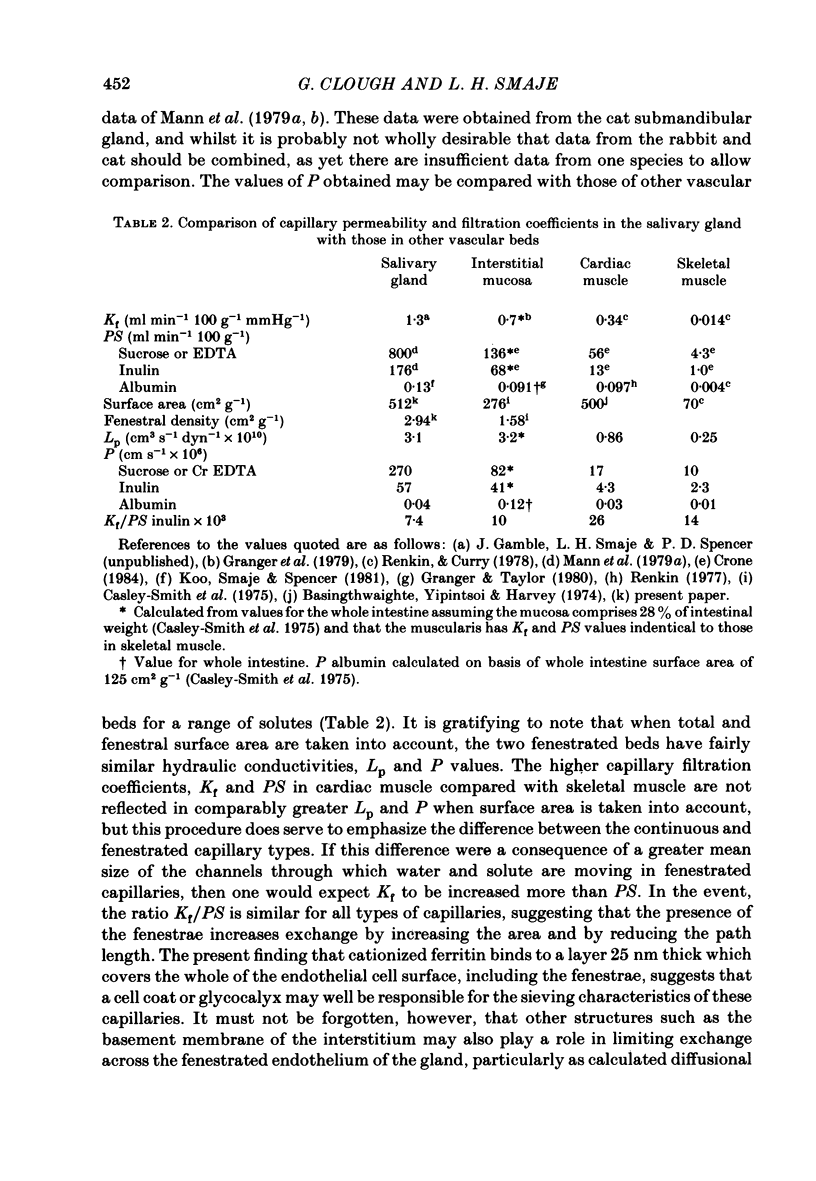
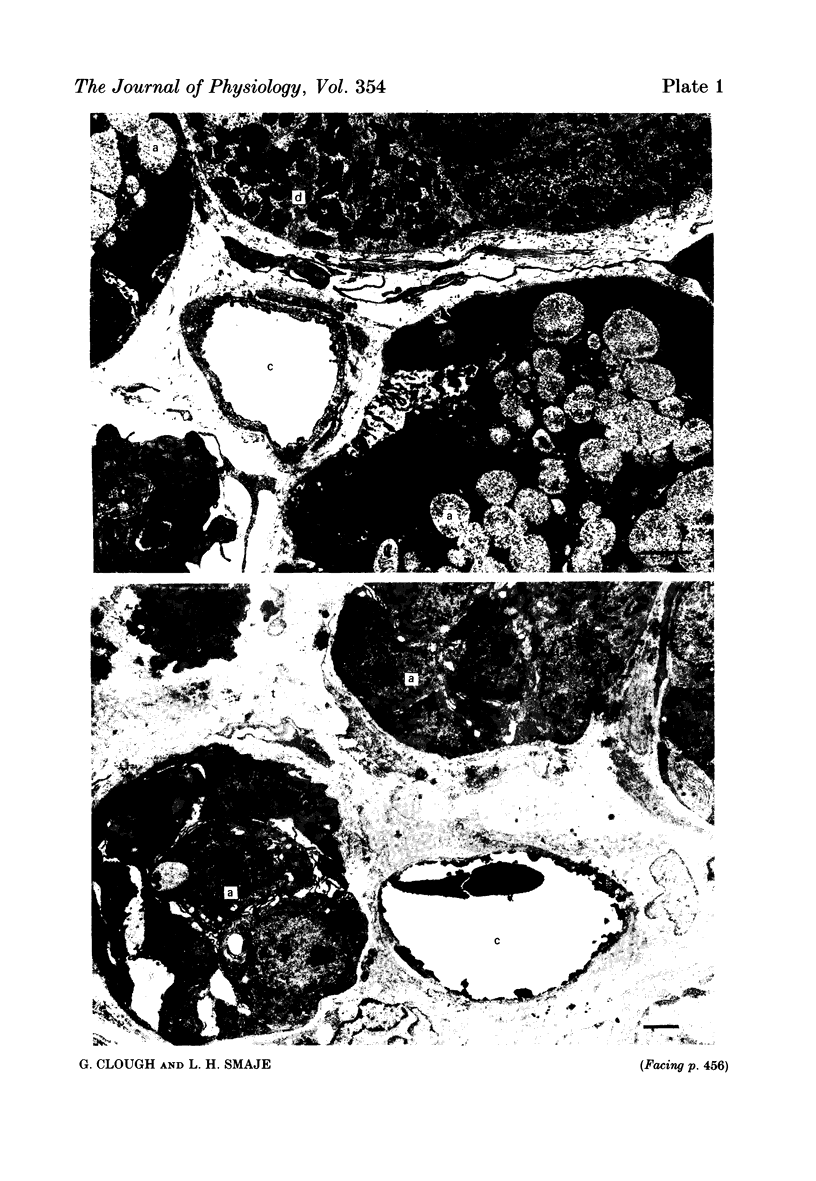
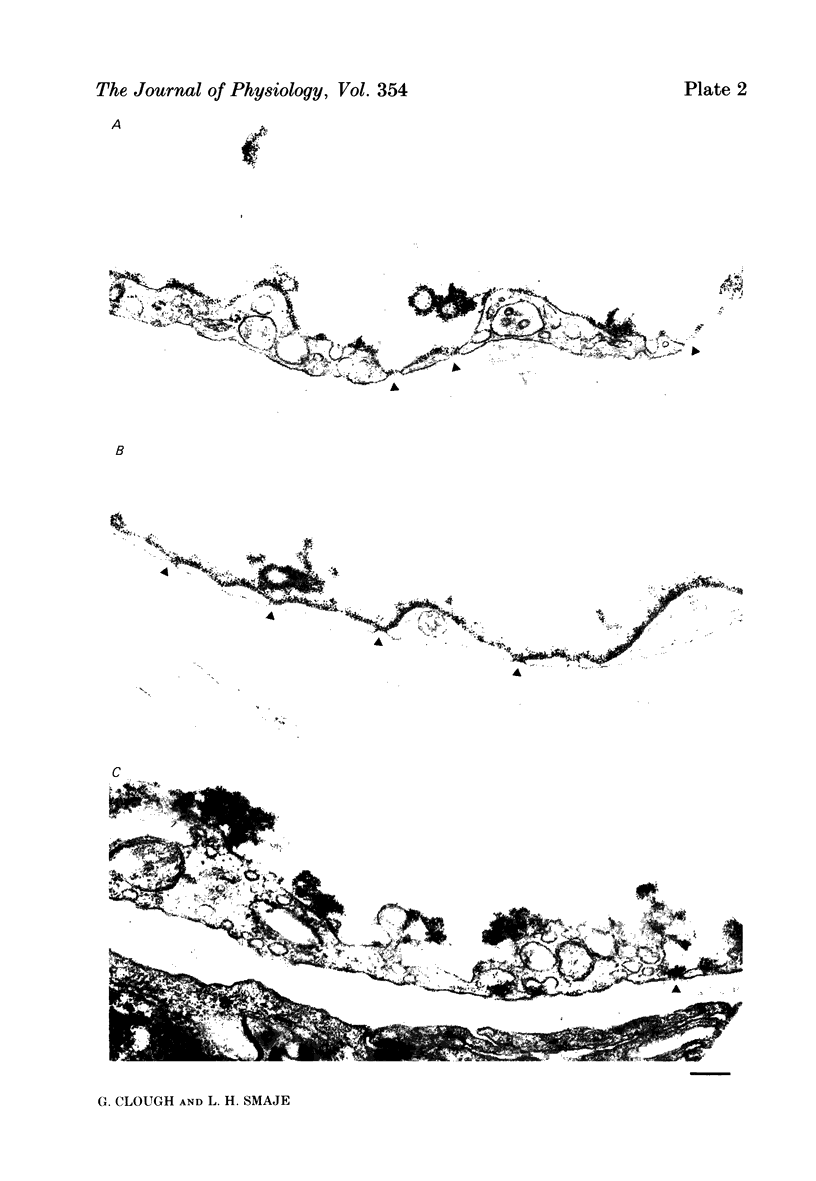
The exchange area of the submandibular salivary gland microvasculature has been measured to allow the value of microvascular permeability (P) to hydrophilic solutes to be calculated from previous measurements of permeability-surface area (PS) products. Glands whose ducts had been ligated for 2 weeks and the contralateral control glands were perfusion-fixed with a modified Karnovsky's fixative after perfusion with a solution containing cationized ferritin, and examined with transmission electron microscopy. Stereological techniques were used to estimate the surface area of the exchange vessels on random thin sections from four control and four duct-ligated glands. The mean exchange surface area in control glands was 512 cm2 g-1 and 336 cm2 g-1 in duct-ligated glands. The fenestral density was calculated to be 0.57% of the exchange surface in control glands and 0.30% in duct-ligated tissue. Molecules of cationized ferritin appeared bound to the luminal surface of the microvascular endothelium, including the surface of the fenestrae to a depth of about 25 nm in both control and ligated glands. These experiments have shown that the exchange surface area of the fenestrated endothelium of the submandibular salivary gland is comparable to that in cardiac muscle but the permeability (P) to small solutes is about 10 times greater. Following ligation of the salivary gland duct, solute permeability falls and an explanation of this, based on the reduced surface area and the nature of the permeability-flow relationship for small solutes is offered.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassingthwaighte J. B., Yipintsoi T., Harvey R. B. Microvasculature of the dog left ventricular myocardium. Microvasc Res. 1974 Mar;7(2):229–249. doi: 10.1016/0026-2862(74)90008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casley-Smith J. R., O'Donoghue P. J., Crocker K. W. The quantitative relationships between fenestrae in jejunal capillaries and connective tissue channels: proof of "tunnel-capillaries". Microvasc Res. 1975 Jan;9(1):78–100. doi: 10.1016/0026-2862(75)90053-9. [DOI] [PubMed] [Google Scholar]
- Clough G. The steady-state transport of cationized ferritin by endothelial cell vesicles. J Physiol. 1982 Jul;328:389–401. doi: 10.1113/jphysiol.1982.sp014272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon D., Goldstein L., Marikovsky Y., Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultrastruct Res. 1972 Mar;38(5):500–510. doi: 10.1016/0022-5320(72)90087-1. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Richardson P. D., Taylor A. E. Volumetric assessment of the capillary filtration coefficient in the cat small intestine. Pflugers Arch. 1979 Jul;381(1):25–33. doi: 10.1007/BF00582328. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Taylor A. E. Permeability of intestinal capillaries to endogenous macromolecules. Am J Physiol. 1980 Apr;238(4):H457–H464. doi: 10.1152/ajpheart.1980.238.4.H457. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966 Nov-Dec;25(6):1773–1783. [PubMed] [Google Scholar]
- MARTIN P., YUDILEVICH D. A THEORY FOR THE QUANTIFICATION OF TRANSCAPILLARY EXCHANGE BY TRACER-DILUTION CURVES. Am J Physiol. 1964 Jul;207:162–168. doi: 10.1152/ajplegacy.1964.207.1.162. [DOI] [PubMed] [Google Scholar]
- Mann G. E., Smaje L. H., Yudilevich D. L. Permeability of the fenestrated capillaries in the cat submandibular gland to lipid-insoluble molecules. J Physiol. 1979 Dec;297(0):335–354. doi: 10.1113/jphysiol.1979.sp013043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G. E., Smaje L. H., Yudilevich D. L. Transcapillary exchange in the cat salivary gland during secretion, bradykinin infusion and after chronic duct ligation. J Physiol. 1979 Dec;297(0):355–367. doi: 10.1113/jphysiol.1979.sp013044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkin E. M. Multiple pathways of capillary permeability. Circ Res. 1977 Dec;41(6):735–743. doi: 10.1161/01.res.41.6.735. [DOI] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. The role of mucopolysaccharides in vesicle architecture and endothelial transport. An electron microscope study of myocardial blood vessels. J Cell Biol. 1972 Jan;52(1):198–206. doi: 10.1083/jcb.52.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. R., Clough G., Michel C. C. The effects of cationised ferritin and native ferritin upon the filtration coefficient of single frog capillaries. Evidence that proteins in the endothelial cell coat influence permeability. Microvasc Res. 1983 Mar;25(2):205–222. doi: 10.1016/0026-2862(83)90016-x. [DOI] [PubMed] [Google Scholar]