Abstract
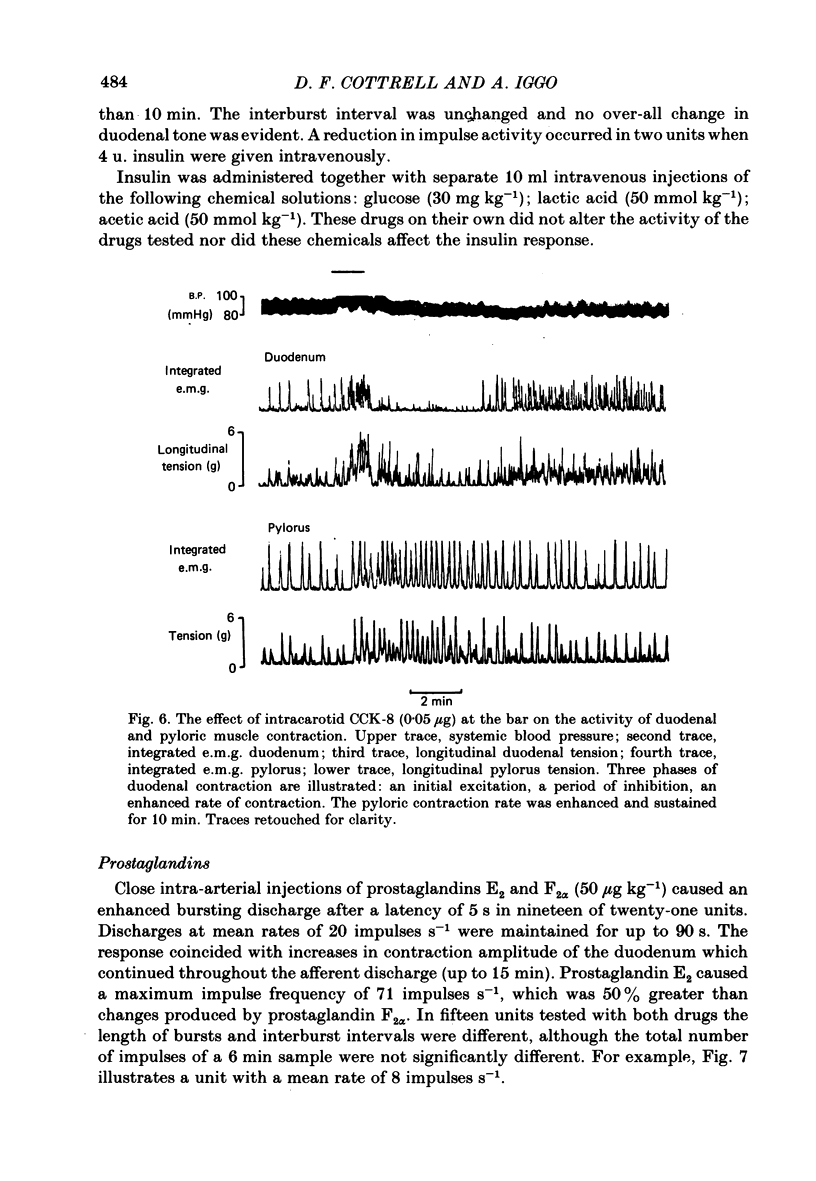
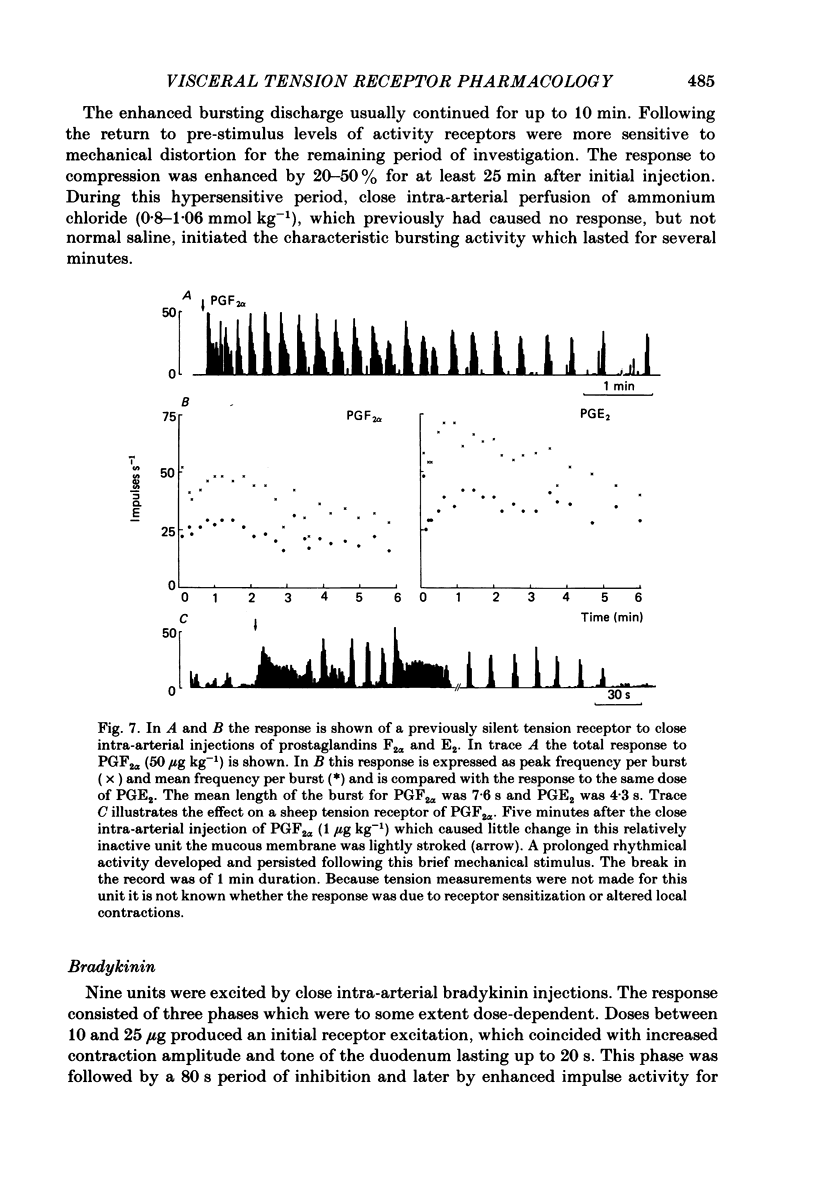
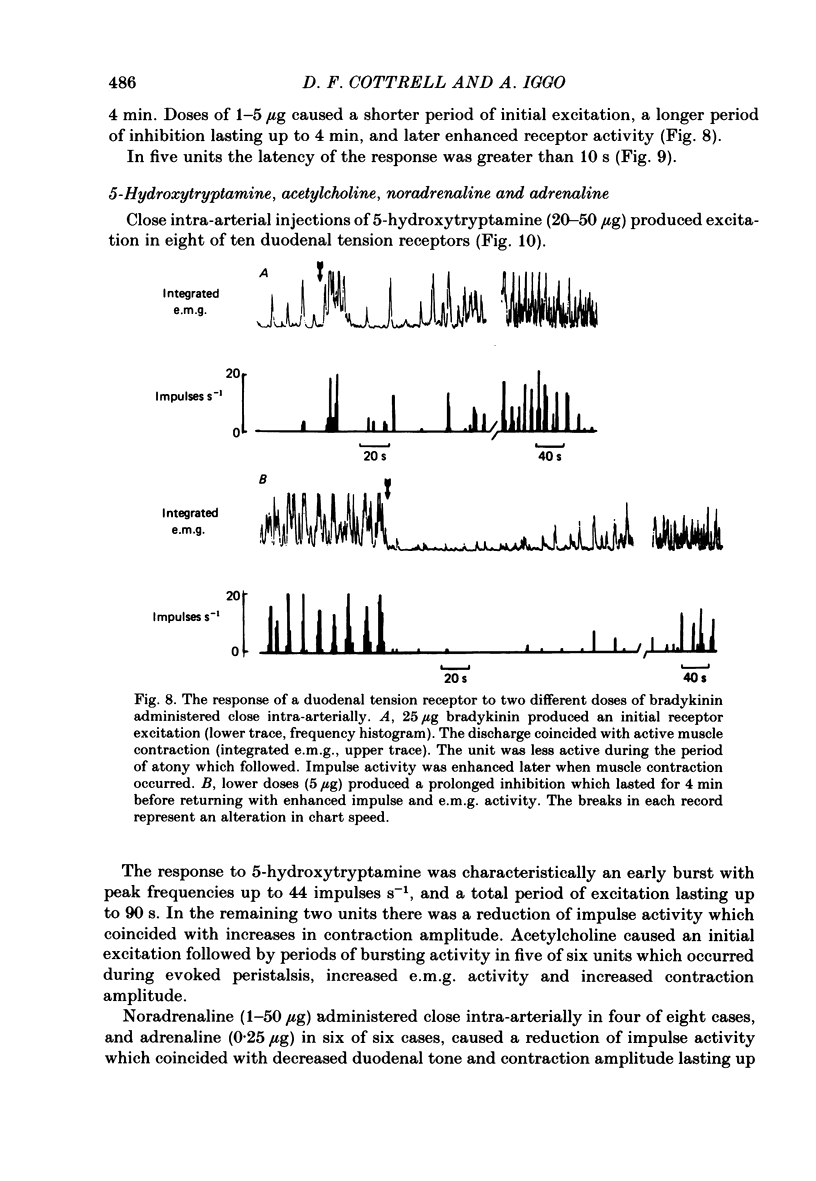
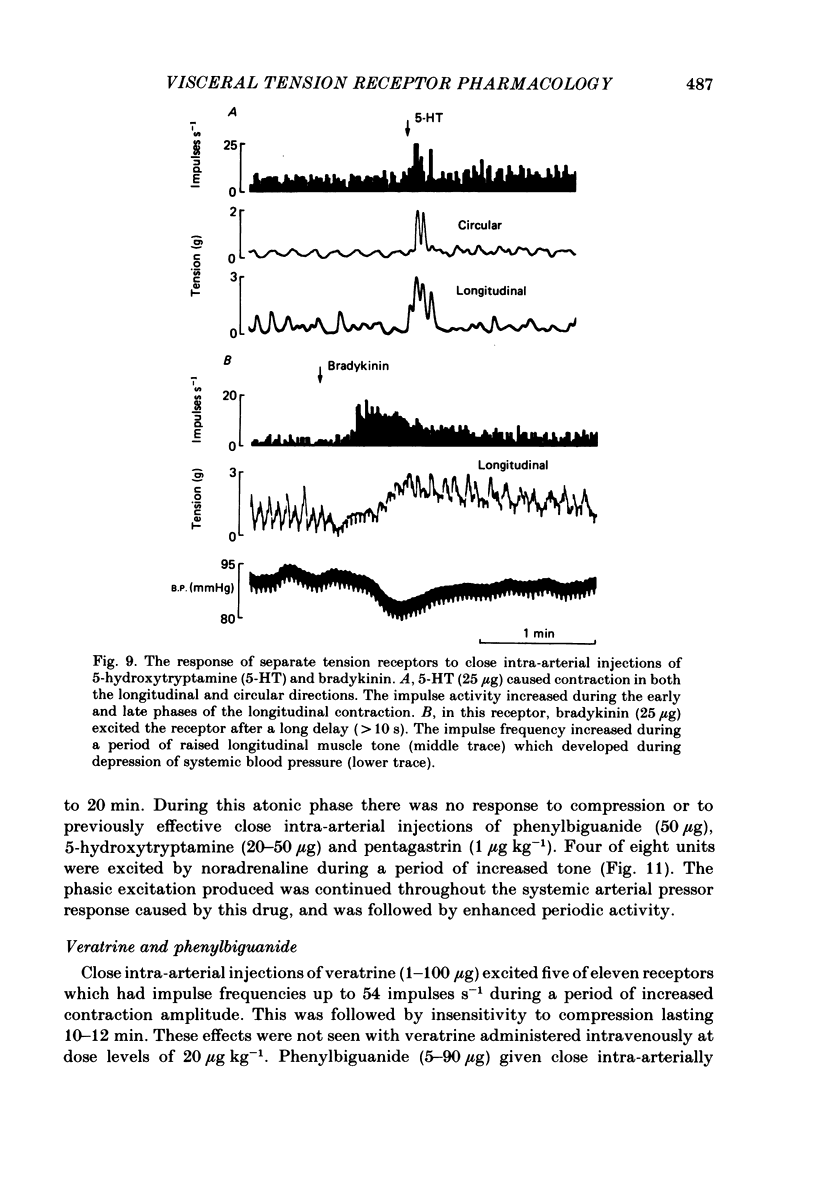
The proposal that some post-prandially released alimentary hormones modify ingestive behaviour and gastric emptying by altering impulse activity in alimentary enteroceptors has been tested using a number of gastrointestinal peptide hormone analogues. These and other drugs were applied to single-unit afferent preparations of duodenal tension receptors in chloralose-anaesthetized sheep. In separate experiments the effect of pentagastrin and cholecystokinin on duodenal motor activity was recorded without unitary afferent activity measurements. Local intra-arterial bolus injections of pentagastrin, cholecystokinin, insulin, prostaglandin, acetylcholine, phenylbiguanide, veratrine, 5-hydroxytryptamine and bradykinin aroused or enhanced activity in tension receptors. With the exception of a short-latency effect of insulin B.P., these responses occurred together with local increases in tension and electromyographic activity of the duodenum. Combinations of atropine and hexamethonium reduced duodenal motor activity and abolished most drug-evoked afferent responses. Intracarotid bolus injections of pentagastrin at first increased, then reduced duodenal tension, electromyographic activity and impulse activity of tension receptors. Cholecystokinin (CCK-8) injected by this route caused similar alterations of these parameters, and the response was characterized by periods of reduced activity followed by a prolonged excitation of duodenal motility. From the responses to bolus injections of humoral agents it is concluded that some alimentary hormones released after a meal may have a peripheral excitatory action on the tension receptor environment which causes increased afferent activity. The mechanism probably involves both an alteration in duodenal motility and a sensitization of the receptor ending. In addition, the peptide hormones gastrin and cholecystokinin may act centrally to alter duodenal motor control and thus may influence gastric emptying and post-prandial satiety mechanisms.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anika S. M., Houpt T. R., Houpt K. A. Satiety elicited by cholecystokinin in intact and vagotomized rats. Physiol Behav. 1977 Dec;19(6):761–766. doi: 10.1016/0031-9384(77)90312-2. [DOI] [PubMed] [Google Scholar]
- Bell F. R., Titchen D. A., Watson D. J. The effects of the gastrin analogue, pentagastrin, on the gastric electromyogram and abomasal emptying in the calf. Res Vet Sci. 1977 Sep;23(2):165–170. [PubMed] [Google Scholar]
- Buffa R., Solcia E., Go V. L. Immunohistochemical identification of the cholecystokinin cell in the intestinal mucosa. Gastroenterology. 1976 Apr;70(4):528–532. [PubMed] [Google Scholar]
- Bunnett N. W., Harrison F. A. Endocrine cells in the alimentary tract of the sheep. Ann Rech Vet. 1979;10(2-3):197–199. [PubMed] [Google Scholar]
- Buéno L., Ruckebusch M. Insulin and jejunal electrical activity in dogs and sheep. Am J Physiol. 1976 Jun;230(6):1538–1544. doi: 10.1152/ajplegacy.1976.230.6.1538. [DOI] [PubMed] [Google Scholar]
- Chapman H. W., Grovum W. L., Newhook J. C. The site of action of pentagastrin-induced inhibition of the reticulum. Ann Rech Vet. 1979;10(2-3):200–201. [PubMed] [Google Scholar]
- Cottrell D. F., Iggo A. Mucosal enteroceptors with vagal afferent fibres in the proximal duodenum of sheep. J Physiol. 1984 Sep;354:497–522. doi: 10.1113/jphysiol.1984.sp015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell D. F., Iggo A. Tension receptors with vagal afferent fibres in the proximal duodenum and pyloric sphincter of sheep. J Physiol. 1984 Sep;354:457–475. doi: 10.1113/jphysiol.1984.sp015388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Fera M. A., Baile C. A. Cholecystokinin octapeptide: continuous picomole injections into the cerebral ventricles of sheep suppress feeding. Science. 1979 Oct 26;206(4417):471–473. doi: 10.1126/science.504989. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Gregory R. A., Hutchison J. B., Harris J. I., Runswick M. J. Isolation, structure and biological activity of two cholecystokinin octapeptides from sheep brain. Nature. 1978 Aug 17;274(5672):711–713. doi: 10.1038/274711a0. [DOI] [PubMed] [Google Scholar]
- Dockray G. J., Gregory R. A., Tracy H. J., Zhu W. Y. Transport of cholecystokinin-octapeptide-like immunoreactivity toward the gut in afferent vagal fibres in cat and dog. J Physiol. 1981 May;314:501–511. doi: 10.1113/jphysiol.1981.sp013721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd K., Hick V. E., Koley J., Morrison J. F. Effects of bradykinin mediated by autonomic efferent nerves. Q J Exp Physiol Cogn Med Sci. 1977 Jan;62(1):11–17. doi: 10.1113/expphysiol.1977.sp002372. [DOI] [PubMed] [Google Scholar]
- Floyd K., Hick V. E., Koley J., Morrison J. F. The effects of bradykinin on afferent units in intra-abdominal sympathetic nerve trunks. Q J Exp Physiol Cogn Med Sci. 1977 Jan;62(1):19–25. doi: 10.1113/expphysiol.1977.sp002373. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Smith G. P. Cholecystokinin and satiety in rats and rhesus monkeys. Am J Clin Nutr. 1977 May;30(5):758–761. doi: 10.1093/ajcn/30.5.758. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Young R. C., Smith G. P. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973 Sep;84(3):488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Young R. C., Smith G. P. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature. 1973 Oct 12;245(5424):323–325. doi: 10.1038/245323a0. [DOI] [PubMed] [Google Scholar]
- Glick Z., Thomas D. W., Mayer J. Absence of effect of injections of the intestinal hormones secretin and choecystokinin-pancreozymin upon feeding behavior. Physiol Behav. 1971 Jan;6(1):5–8. doi: 10.1016/0031-9384(71)90004-7. [DOI] [PubMed] [Google Scholar]
- Greenway F. L., Bray G. A. Cholecystokinin and satiety. Life Sci. 1977 Sep 15;21(6):769–772. doi: 10.1016/0024-3205(77)90403-9. [DOI] [PubMed] [Google Scholar]
- Grossman M. I. Candidate hormones of the gut. I. Introduction. Gastroenterology. 1974 Oct;67(4):730–731. [PubMed] [Google Scholar]
- Grossman M. I. Letters: What is physiological? Gastroenterology. 1973 Dec;65(6):994–994. [PubMed] [Google Scholar]
- Grovum W. L., Chapman H. W. Pentagastrin in the circulation acts directly on the brain to depress motility of the stomach in sheep. Regul Pept. 1982 Dec;5(1):35–42. doi: 10.1016/0167-0115(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Grovum W. L. Factors affecting the voluntary intake of food by sheep. 3. The effect of intravenous infusions of gastrin, cholecystokinin and secretin on motility of the reticulo-rumen and intake. Br J Nutr. 1981 Jan;45(1):183–201. doi: 10.1079/bjn19810091. [DOI] [PubMed] [Google Scholar]
- Grube D., Maier V., Raptis S., Schlegel W. Immunoreactivity of the endocrine pancreas. Evidence for the presence of cholecystokinin- pancreozymin within the A-cell. Histochemistry. 1978 Jun 2;56(1):13–35. doi: 10.1007/BF00492250. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Ljungdahl A., Lundberg J. M., Schultzberg M. Peptidergic neurones. Nature. 1980 Apr 10;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- IGGO A. Gastric mucosal chemoreceptors with vagal afferent fibres in the cat. Q J Exp Physiol Cogn Med Sci. 1957 Oct;42(4):398–409. doi: 10.1113/expphysiol.1957.sp001284. [DOI] [PubMed] [Google Scholar]
- IGGO A. Gastro-intestinal tension receptors with unmyelinated afferent fibres in the vagus of the cat. Q J Exp Physiol Cogn Med Sci. 1957 Jan;42(1):130–143. doi: 10.1113/expphysiol.1957.sp001228. [DOI] [PubMed] [Google Scholar]
- Kraly F. S., Carty W. J., Resnick S., Smith G. P. Effect of cholecystokinin on meal size and intermeal interval in the sham-feeding rat. J Comp Physiol Psychol. 1978 Aug;92(4):697–707. doi: 10.1037/h0077501. [DOI] [PubMed] [Google Scholar]
- LEITNER J. M., PERL E. R. RECEPTORS SUPPLIED BY SPINAL NERVES WHICH RESPOND TO CARDIOVASCULAR CHANGES AND ADRENALINE. J Physiol. 1964 Dec;175:254–274. doi: 10.1113/jphysiol.1964.sp007516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J., Elde R., Said S. Peptide neurons in the vagus, splanchnic and sciatic nerves. Acta Physiol Scand. 1978 Dec;104(4):499–501. doi: 10.1111/j.1748-1716.1978.tb06307.x. [DOI] [PubMed] [Google Scholar]
- Maddison S. Intraperitoneal and intracranial cholecystokinin depress operant responding for food. Physiol Behav. 1977 Dec;19(6):819–824. doi: 10.1016/0031-9384(77)90322-5. [DOI] [PubMed] [Google Scholar]
- Makhlouf G. M. The neuroendocrine design of the gut. The play of chemicals in a chemical playground. Gastroenterology. 1974 Jul;67(1):159–184. [PubMed] [Google Scholar]
- Mei N. Vagal glucoreceptors in the small intestine of the cat. J Physiol. 1978 Sep;282:485–506. doi: 10.1113/jphysiol.1978.sp012477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff C. B., Osbahr A. J., 3rd, Bissette G., Jahnke G., Lipton M. A., Prange A. J. Cholecystokinin inhibits tail pinch-induced eating in rats. Science. 1978 May 19;200(4343):793–794. doi: 10.1126/science.565535. [DOI] [PubMed] [Google Scholar]
- Nicholson T. The mode of action of intravenous pentagastrin injections on forestomach motility of sheep. Q J Exp Physiol. 1982 Oct;67(4):537–542. doi: 10.1113/expphysiol.1982.sp002674. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. A method of locating the receptors of visceral afferent fibres. J Physiol. 1954 Apr 28;124(1):166–172. doi: 10.1113/jphysiol.1954.sp005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. A study of gastric stretch receptors; their role in the peripheral mechanism of satiation of hunger and thirst. J Physiol. 1954 Nov 29;126(2):255–270. doi: 10.1113/jphysiol.1954.sp005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. EFFECTS OF DRUGS ON VERTEBRATE MECHANORECEPTORS. Pharmacol Rev. 1964 Dec;16:341–380. [PubMed] [Google Scholar]
- PAINTAL A. S. Impulses in vagal afferent fibres from stretch receptors in the stomach and their role in the peripheral mechanism of hunger. Nature. 1953 Dec 26;172(4391):1194–1195. doi: 10.1038/1721194a0. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. The response of gastric stretch receptors and certain other abdominal and thoracic vagal receptors to some drugs. J Physiol. 1954 Nov 29;126(2):271–285. doi: 10.1113/jphysiol.1954.sp005208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckebusch Y. The effects of pentagastrin on the motility of the ruminant stomach. Experientia. 1971 Oct 15;27(10):1185–1186. doi: 10.1007/BF02286916. [DOI] [PubMed] [Google Scholar]
- Sarna S. K. Gastrointestinal electrical activity: terminology. Gastroenterology. 1975 Jun;68(6):1631–1635. [PubMed] [Google Scholar]
- Schneider B. S., Monahan J. W., Hirsch J. Brain cholecystokinin and nutritional status in rats and mice. J Clin Invest. 1979 Nov;64(5):1348–1356. doi: 10.1172/JCI109591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Cholecystokinin in the brains of obese and nonobese mice. Science. 1979 Jan 5;203(4375):68–69. doi: 10.1126/science.758680. [DOI] [PubMed] [Google Scholar]
- Sturdevant R. A., Goetz H. Cholecystokinin both stimulates and inhibits human food intake. Nature. 1976 Jun 24;261(5562):713–715. doi: 10.1038/261713a0. [DOI] [PubMed] [Google Scholar]
- Szurszewski J. H. Mechanism of action of pentagastrin and acetylcholine on the longitudinal muscle of the canine antrum. J Physiol. 1975 Nov;252(2):335–361. doi: 10.1113/jphysiol.1975.sp011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi S. E., Bertaccini G., Impicciatore M., Knoll J. Evidence that acetylcholine released by gastrin and related polypeptides contributes to their effect on gastrointestinal motility. Gastroenterology. 1973 Feb;64(2):268–277. [PubMed] [Google Scholar]


