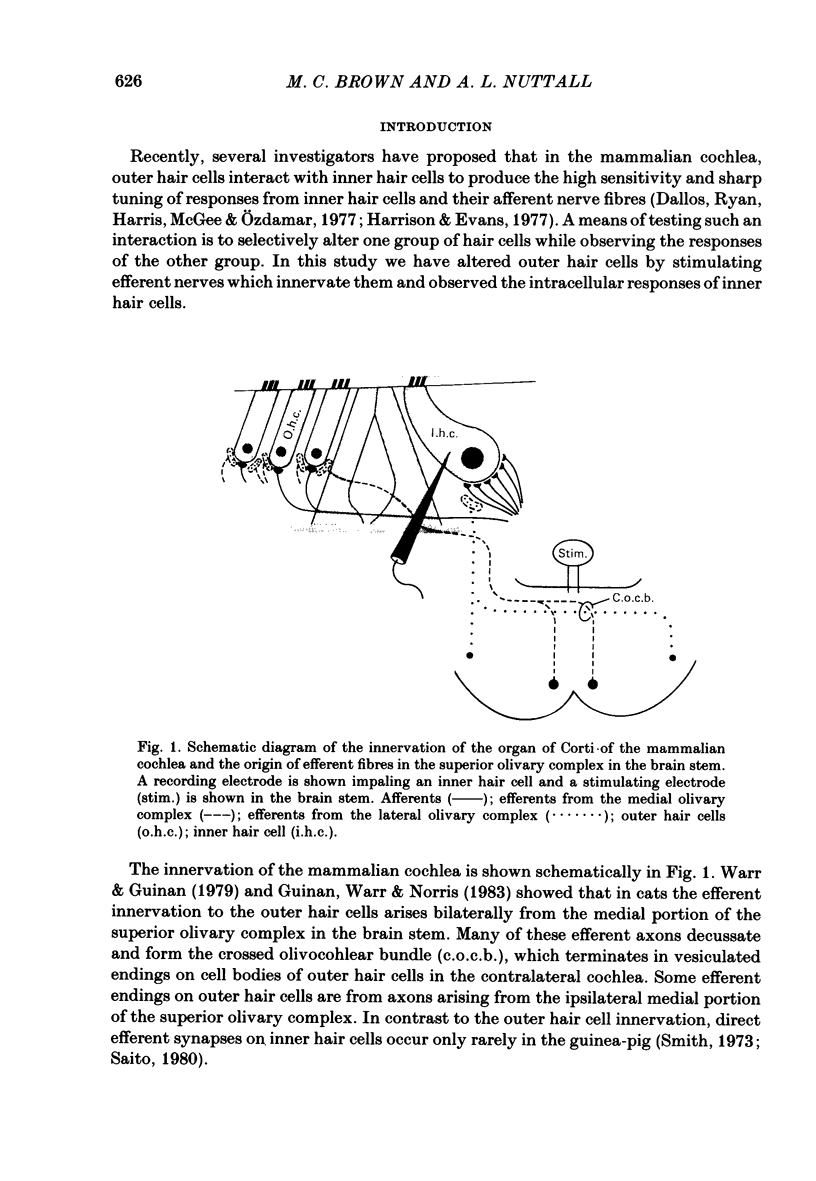
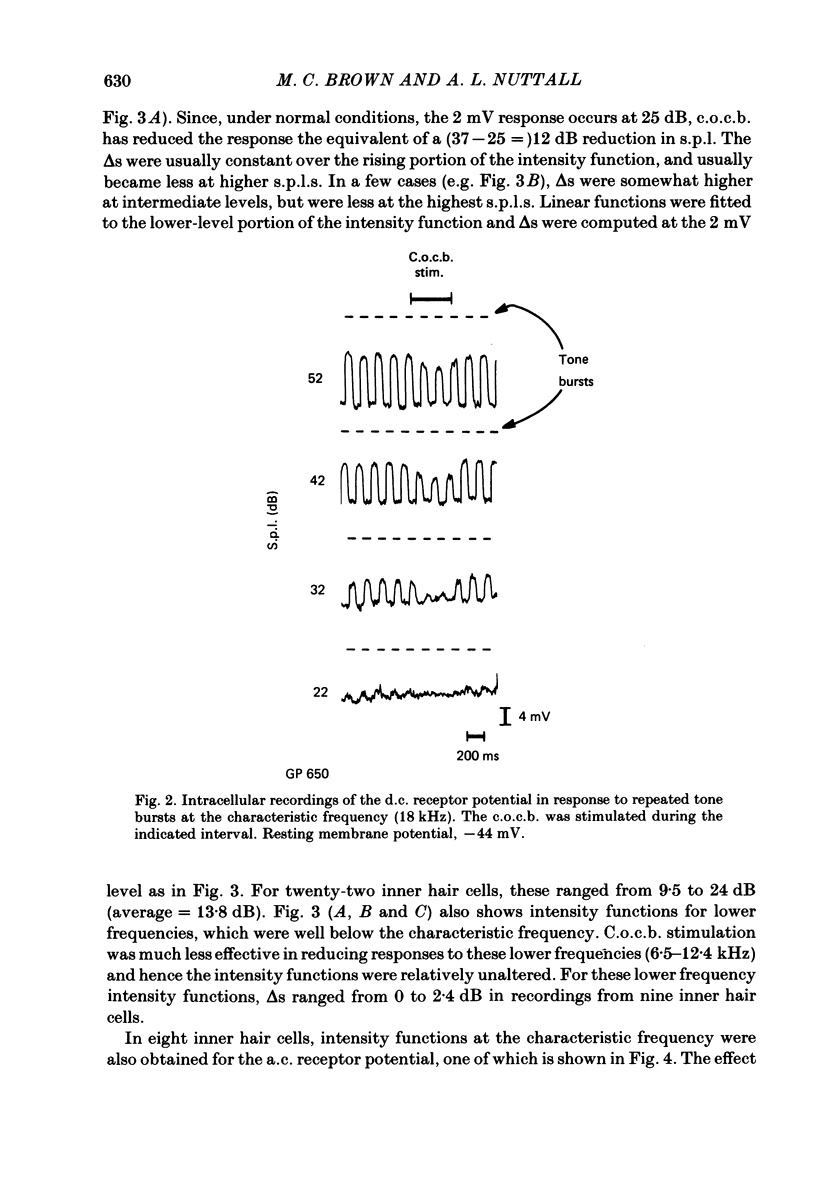
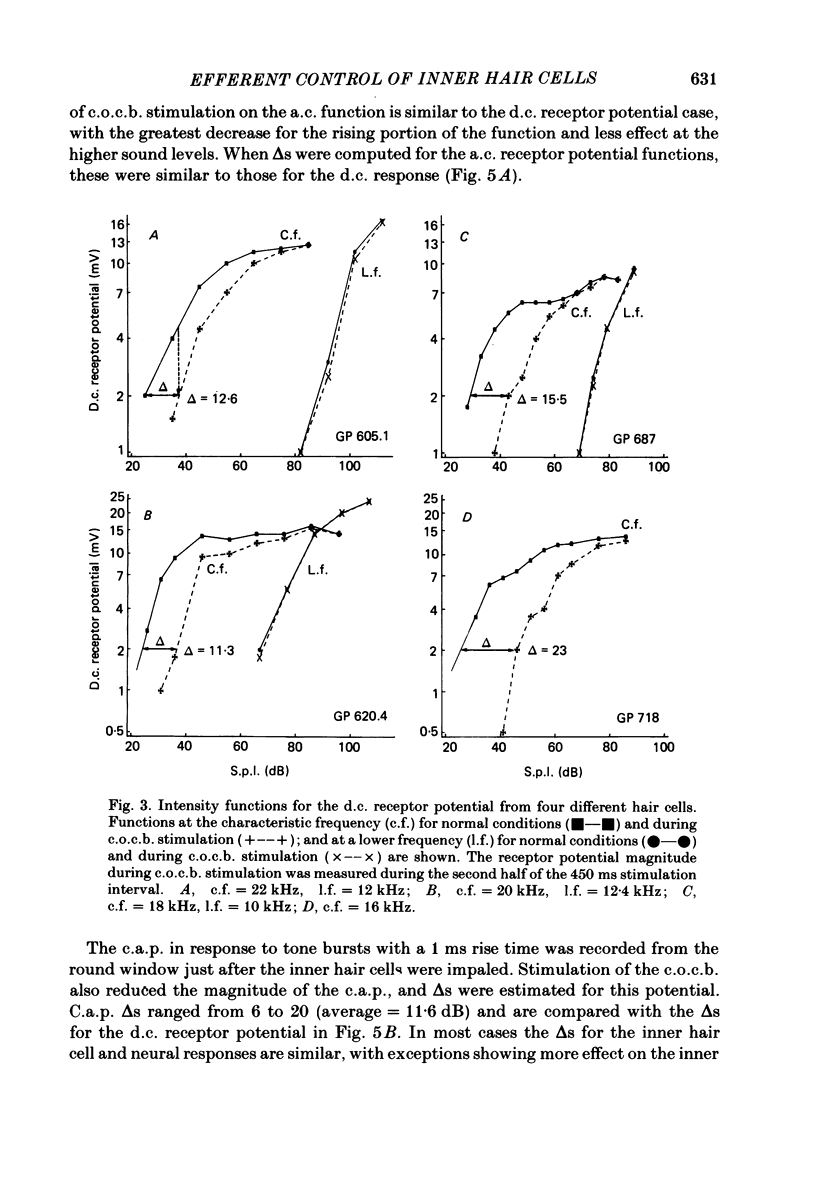
Abstract
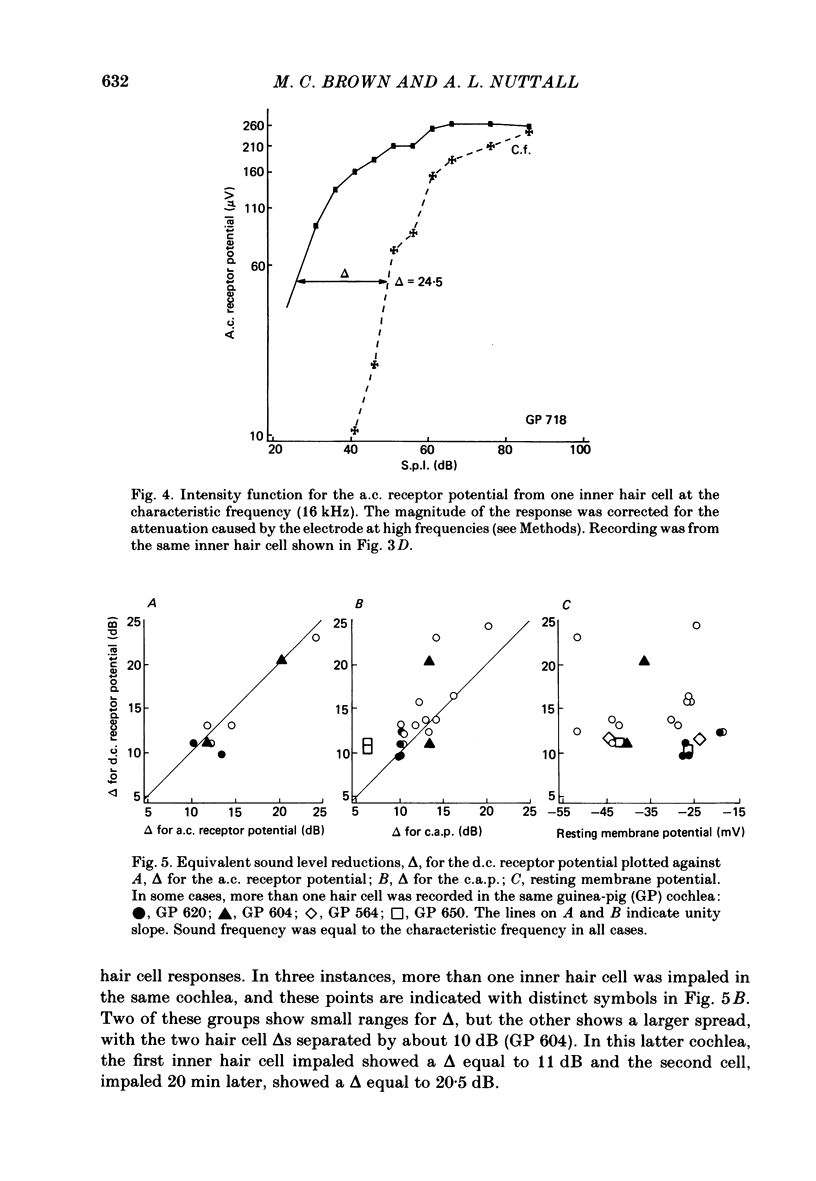
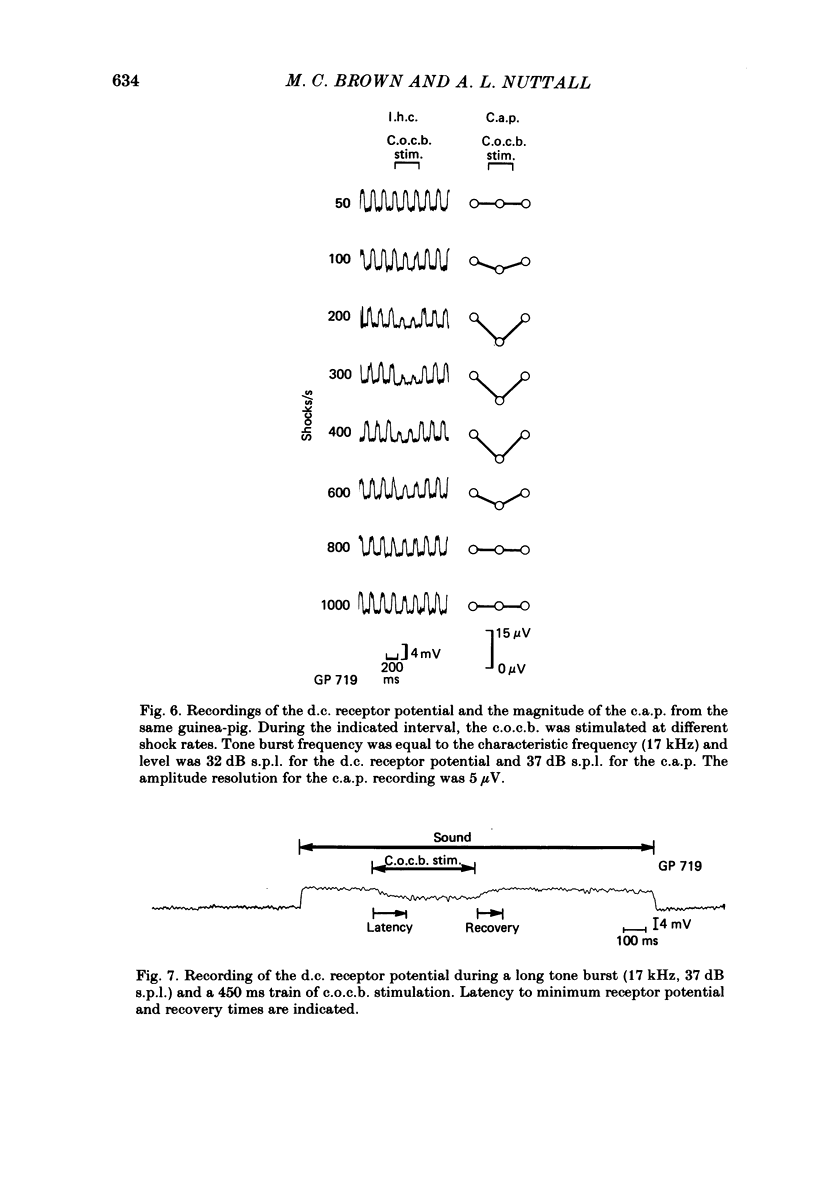
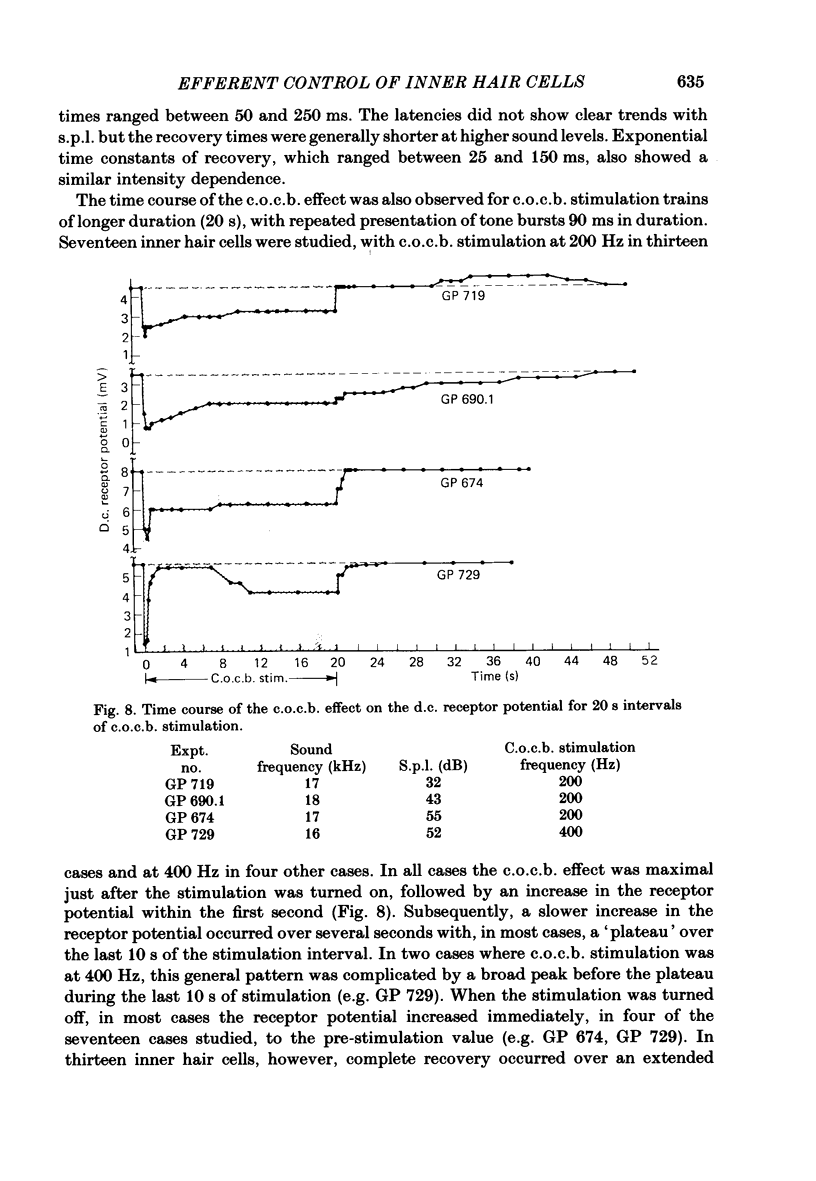
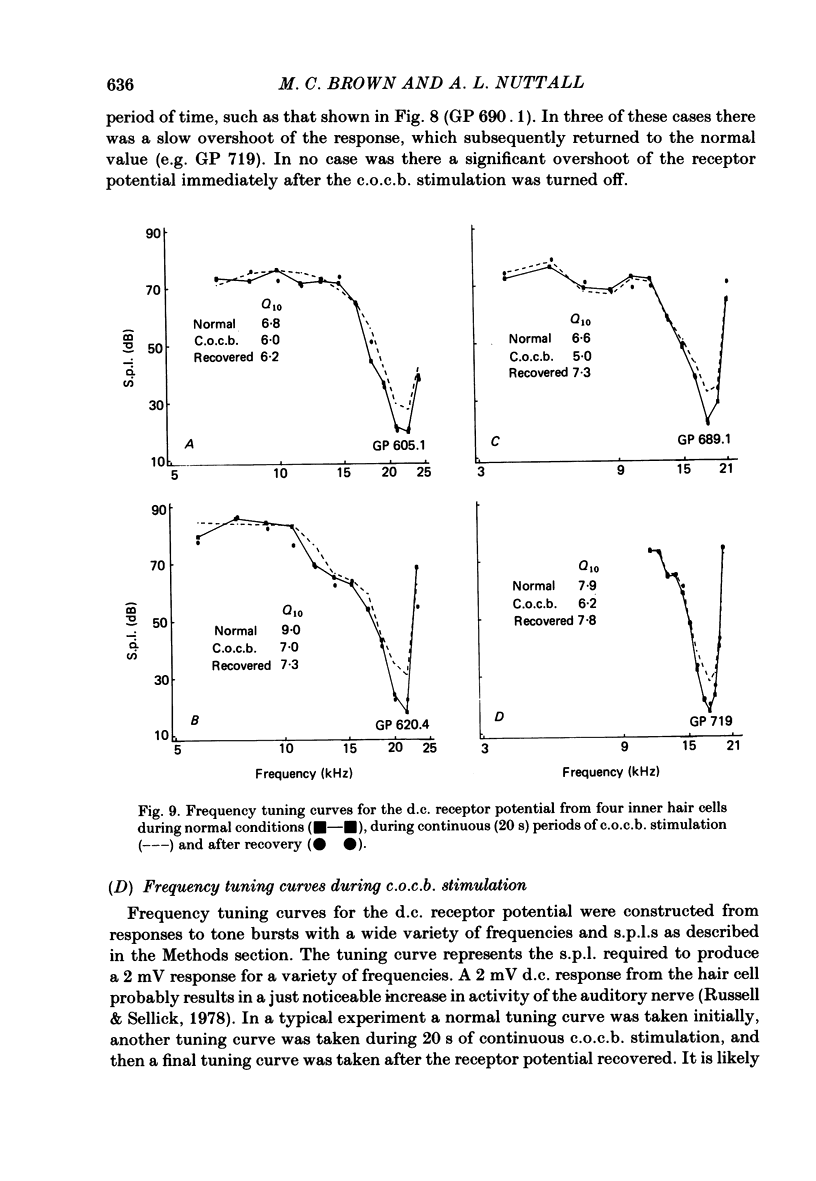
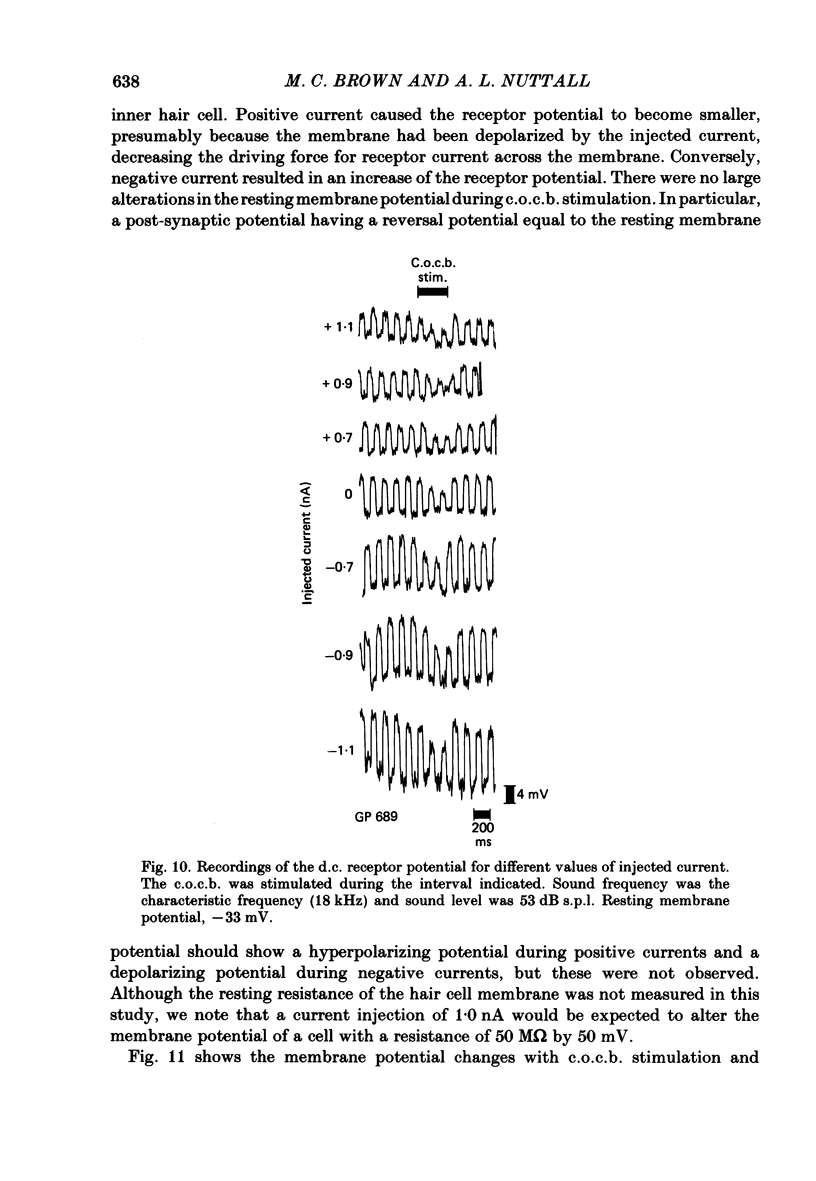
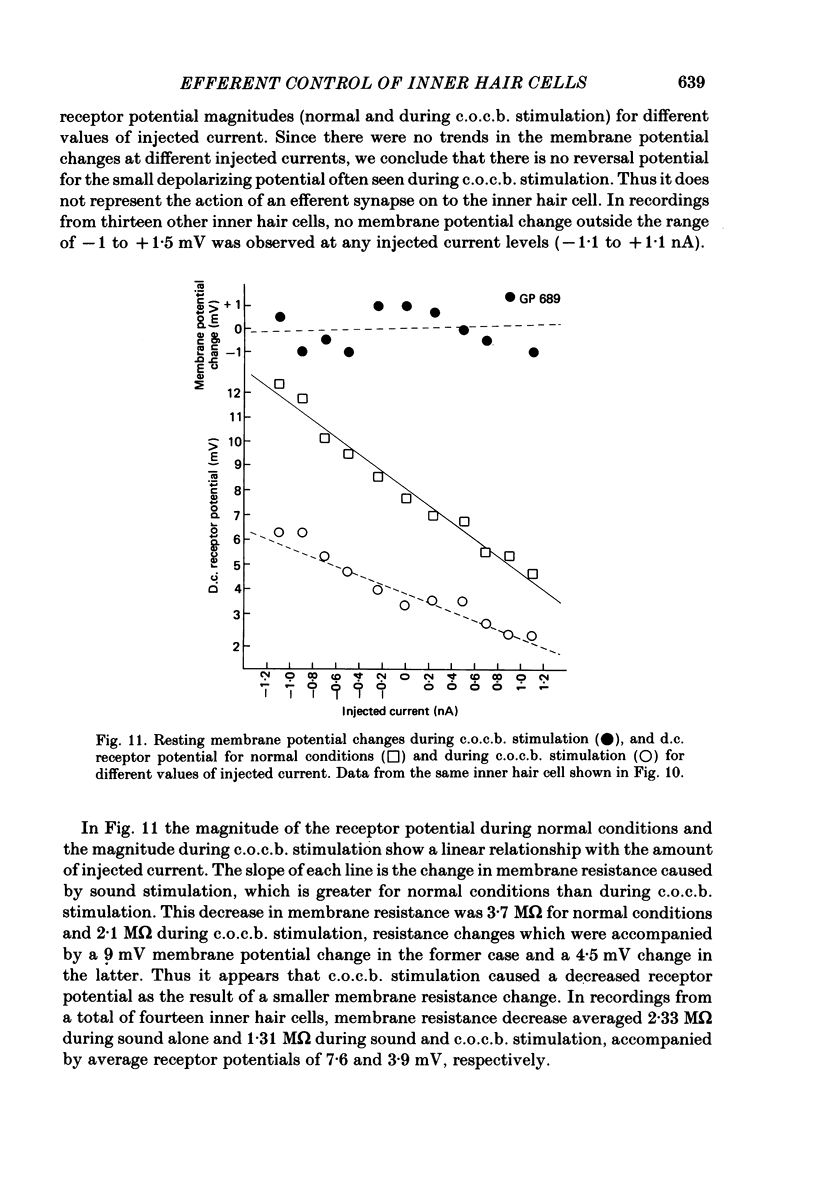
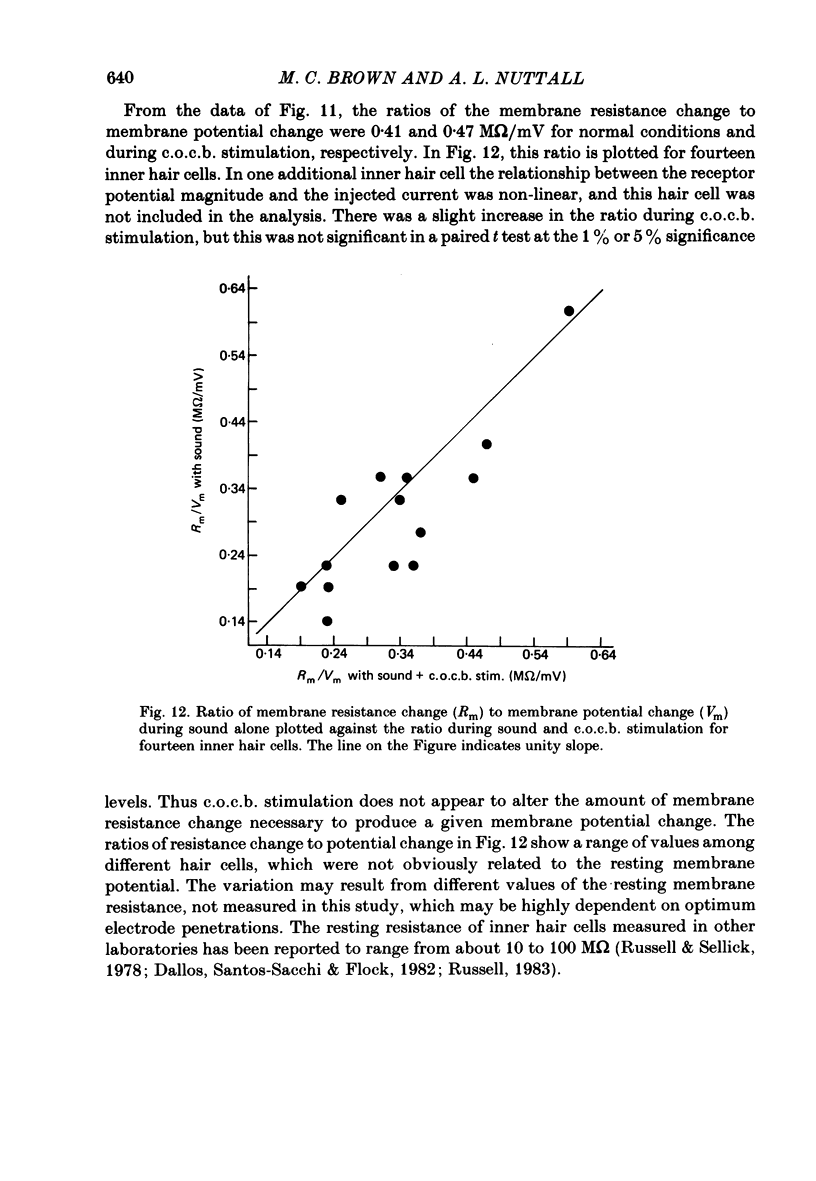
The efferent crossed olivocochlear bundle (c.o.c.b.) was electrically stimulated during intracellular recordings from cochlear inner hair cells in anaesthetized guinea-pigs. The effect of c.o.c.b. stimulation was to decrease the magnitude of the inner hair cell depolarizing component (d.c.) and alternating component (a.c.) receptor potentials evoked by tone bursts at the characteristic frequency. At low sound pressure levels, the decrease in receptor potentials caused by c.o.c.b. stimulation was equivalent to decreasing the sound intensity by 9-24 dB. C.o.c.b. stimulation usually had a similar effect on the compound action potential of the auditory nerve. The change in inner hair cell membrane resistance during moderate-level sound was measured for sound alone and when sound was accompanied by c.o.c.b. stimulation. Sound alone produced a greater membrane resistance change than sound with c.o.c.b. stimulation, in proportion to the d.c. receptor potential during the same conditions. The time course of the c.o.c.b. effect was slow, with 50-250 ms required for a full effect and for recovery. The effects of varying the frequency and voltage of electrical stimulation were similar for the d.c. receptor potential and for the compound action potential. For sounds of high level and for frequencies well below the characteristic frequency, c.o.c.b. stimulation was less effective in reducing receptor potentials. Frequency tuning curves for the d.c. receptor potential taken during intervals of continuous c.o.c.b. stimulation showed decreases in sensitivity primarily in the tip segment of the tuning curve. When no sound stimulus was present, the resting membrane potential was relatively unaltered during c.o.c.b. stimulation. The resting membrane resistance did not change during c.o.c.b. stimulation. Since the c.o.c.b. innervates mainly the outer hair cells, these results strongly suggest that changes in outer hair cell activity can influence the receptor potentials of inner hair cells and thus alter the transmission of acoustic responses to the central nervous system.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Art J. J., Crawford A. C., Fettiplace R., Fuchs P. A. Efferent regulation of hair cells in the turtle cochlea. Proc R Soc Lond B Biol Sci. 1982 Oct 22;216(1204):377–384. doi: 10.1098/rspb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Nuttall A. L., Masta R. I. Intracellular recordings from cochlear inner hair cells: effects of stimulation of the crossed olivocochlear efferents. Science. 1983 Oct 7;222(4619):69–72. doi: 10.1126/science.6623058. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Nuttall A. L., Masta R. I., Lawrence M. Cochlear inner hair cells: effects of transient asphyxia on intracellular potentials. Hear Res. 1983 Feb;9(2):131–144. doi: 10.1016/0378-5955(83)90023-0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Smith D. I., Nuttall A. L. Anesthesia and surgical trauma: their influence on the guinea pig compound action potential. Hear Res. 1983 Jun;10(3):345–358. doi: 10.1016/0378-5955(83)90097-7. [DOI] [PubMed] [Google Scholar]
- Dallos P., Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978 Mar;41(2):365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Dallos P., Santos-Sacchi J., Flock A. Intracellular recordings from cochlear outer hair cells. Science. 1982 Nov 5;218(4572):582–584. doi: 10.1126/science.7123260. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Kellner J., Mannherz H. G., Gröschel-Stewart U., Kendrick-Jones J., Scholey J. Absence of myosin-like immunoreactivity in stereocilia of cochlear hair cells. Nature. 1982 Dec 9;300(5892):531–532. doi: 10.1038/300531a0. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D., Kellner J., Mannherz H. G., Gröschel-Stewart U., Kendrick-Jones J., Scholey J. Absence of myosin-like immunoreactivity in stereocilia of cochlear hair cells. Nature. 1982 Dec 9;300(5892):531–532. doi: 10.1038/300531a0. [DOI] [PubMed] [Google Scholar]
- FEX J. Augmentation of cochlear microphonic by stimulation of efferent fibres to the cochlea; preliminary report. Acta Otolaryngol. 1959 Nov-Dec;50:540–541. doi: 10.3109/00016485909129230. [DOI] [PubMed] [Google Scholar]
- Fex J. Efferent inhibition in the cochlea related to hair-cell dc activity: study of postsynaptic activity of the crossed olivocochlear fibres in the cat. J Acoust Soc Am. 1967 Mar;41(3):666–675. doi: 10.1121/1.1910395. [DOI] [PubMed] [Google Scholar]
- Flock A., Cheung H. C., Flock B., Utter G. Three sets of actin filaments in sensory cells of the inner ear. Identification and functional orientation determined by gel electrophoresis, immunofluorescence and electron microscopy. J Neurocytol. 1981 Feb;10(1):133–147. doi: 10.1007/BF01181749. [DOI] [PubMed] [Google Scholar]
- Flock A., Russell I. Inhibition by efferent nerve fibres: action on hair cells and afferent synaptic transmission in the lateral line canal organ of the burbot Lota lota. J Physiol. 1976 May;257(1):45–62. doi: 10.1113/jphysiol.1976.sp011355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALAMBOS R. Suppression of auditory nerve activity by stimulation of efferent fibers to cochlea. J Neurophysiol. 1956 Sep;19(5):424–437. doi: 10.1152/jn.1956.19.5.424. [DOI] [PubMed] [Google Scholar]
- Goodman D. A., Smith R. L., Chamberlain S. C. Intracellular and extracellular responses in the organ of Corti of the gerbil. Hear Res. 1982 Jul;7(2):161–169. doi: 10.1016/0378-5955(82)90012-0. [DOI] [PubMed] [Google Scholar]
- Guinan J. J., Jr, Warr W. B., Norris B. E. Differential olivocochlear projections from lateral versus medial zones of the superior olivary complex. J Comp Neurol. 1983 Dec 10;221(3):358–370. doi: 10.1002/cne.902210310. [DOI] [PubMed] [Google Scholar]
- Kiang N. Y., Rho J. M., Northrop C. C., Liberman M. C., Ryugo D. K. Hair-cell innervation by spiral ganglion cells in adult cats. Science. 1982 Jul 9;217(4555):175–177. doi: 10.1126/science.7089553. [DOI] [PubMed] [Google Scholar]
- Liberman M. C. Single-neuron labeling in the cat auditory nerve. Science. 1982 Jun 11;216(4551):1239–1241. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- Lim D. J. Fine morphology of the tectorial membrane. Its relationship to the organ of Corti. Arch Otolaryngol. 1972 Sep;96(3):199–215. doi: 10.1001/archotol.1972.00770090321001. [DOI] [PubMed] [Google Scholar]
- Macartney J. C., Comis S. D., Pickles J. O. Is myosin in the cochlea a basis for active motility? Nature. 1980 Dec 4;288(5790):491–492. doi: 10.1038/288491a0. [DOI] [PubMed] [Google Scholar]
- Mountain D. C. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science. 1980 Oct 3;210(4465):71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Robertson D., Johnstone B. M. Aberrant tonotopic organization in the inner ear damaged by kanamycin. J Acoust Soc Am. 1979 Aug;66(2):466–469. doi: 10.1121/1.383097. [DOI] [PubMed] [Google Scholar]
- Russell I. J. Origin of the receptor potential in inner hair cells of the mammalian cochlea--evidence for Davis' theory. Nature. 1983 Jan 27;301(5898):334–336. doi: 10.1038/301334a0. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol. 1983 May;338:179–206. doi: 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K. Fine structure of the sensory epithelium of the guinea pig organ of Corti: afferent and efferent synapses of hair cells. J Ultrastruct Res. 1980 May;71(2):222–232. doi: 10.1016/s0022-5320(80)90108-2. [DOI] [PubMed] [Google Scholar]
- Siegel J. H., Kim D. O. Efferent neural control of cochlear mechanics? Olivocochlear bundle stimulation affects cochlear biomechanical nonlinearity. Hear Res. 1982 Feb;6(2):171–182. doi: 10.1016/0378-5955(82)90052-1. [DOI] [PubMed] [Google Scholar]
- Teas D. C., Konishi T., Nielsen D. W. Electrophysiological studies on the spatial distribution of the crossed olivocochlear bundle along the guinea pig cochlea. J Acoust Soc Am. 1972 Apr;51(4):1256–1264. doi: 10.1121/1.1912969. [DOI] [PubMed] [Google Scholar]
- Warr W. B., Guinan J. J., Jr Efferent innervation of the organ of corti: two separate systems. Brain Res. 1979 Sep 7;173(1):152–155. doi: 10.1016/0006-8993(79)91104-1. [DOI] [PubMed] [Google Scholar]
- Wiederhold M. L., Kiang N. Y. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am. 1970 Oct;48(4):950–965. doi: 10.1121/1.1912234. [DOI] [PubMed] [Google Scholar]
- Wiederhold M. L., Peake W. T. Efferent inhibition of auditory-nerve responses: dependence on acoustic-stimulus parameters. J Acoust Soc Am. 1966 Dec;40(6):1427–1430. doi: 10.1121/1.1910243. [DOI] [PubMed] [Google Scholar]
- Wiederhold M. L. Variations in the effects of electric stimulation of the crossed olivocochlear bundle on cat single auditory-nerve-fiber responses to tone bursts. J Acoust Soc Am. 1970 Oct;48(4):966–977. doi: 10.1121/1.1912235. [DOI] [PubMed] [Google Scholar]