Abstract
Background
The COVID-19 pandemic challenged healthcare systems, particularly in settings with high infectious disease burden. We examined the postpandemic long-term impacts of COVID-19 on tuberculosis (TB) services at anti-retroviral therapy (ART) clinics in lower-income countries.
Methods
Using standardised online questionnaires, we conducted a cross-sectional site survey among ART clinics providing TB services in Africa and Asia from July to September 2023 (site-level information and number of TB diagnoses and tests).
Results
Of 45 participating ART clinics, 32 (71%) were in Africa and 13 (29%) in Asia. During the COVID-19 pandemic (2020–2022), 43 (96%) clinics reported implementing social distancing or separation measures, 39 (87%) personal protections for staff members and 32 (71%) protections for patients. Infection control measures were in place in 45% of the clinics before the pandemic (until 2019), 23% introduced measures during the pandemic and 15% maintained them after the pandemic (after 2022). Service provision was affected during the pandemic in 33 (73%) clinics, including TB services in 22 (49%) clinics. TB service restrictions were addressed by introducing changes in directly observed therapy provision in 8 (18%) clinics, multimonth TB drug dispensing in 23 (51%), telehealth services in 25 (56%) and differentiated service delivery in 19 (42%). These changes were sustained after the pandemic at 4 (9%), 11 (24%), 17 (38%) and 12 (27%) clinics, respectively. Compared with 2018–2019, the number of TB diagnoses decreased sharply in 2020–2021 and improved after the pandemic.
Conclusions
COVID-19 affected TB care services in ART clinics in Africa and Asia. This was paralleled by a reduction in TB diagnoses, which partly resumed after the pandemic. Infection control measures and alternative modes of service delivery were adopted during the pandemic and only partially maintained. Efforts should be made to sustain the lessons learnt during the COVID-19 pandemic, particularly approaches that reduce the risk of transmission of infectious diseases, including TB, in ART clinics.
Keywords: Tuberculosis, COVID-19, HIV
WHAT IS ALREADY KNOWN ON THIS TOPIC
The COVID-19 pandemic challenged health systems worldwide, stretching capacities to their limits particularly in settings with a high burden of infectious diseases, including HIV and tuberculosis (TB).
WHAT THIS STUDY ADDS
We examined the long-term impact of COVID-19, beyond the pandemic, focusing on the use of infection and prevention control measures in ART clinics, virtual and decentralised TB care delivery.
During the pandemic, COVID-19 affected service provision in almost three quarters of the surveyed clinics, and in about half of them, TB services were impacted.
ART clinics adopted infection control measures and alternative modes of service delivery that were only partially continued after the pandemic.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Long-term efforts should be made beyond the pandemic to improve the sustainability of approaches that have been shown to be feasible and to reduce the risk of transmission of infectious agents, including TB, in ART clinics.
Introduction
Globally, tuberculosis (TB) has long been the leading cause of death from a single infectious pathogen, apart from the years 2020–2022, when deaths due to the COVID-19 surpassed TB-related deaths.1 TB is also among the most frequent causes of death among people living with HIV, particularly in low- and middle-income settings where both diseases are highly prevalent. The COVID-19 pandemic has been a major challenge for health systems and caused severe disruptions of essential health services, including HIV and TB services.2,4 The impact was particularly strong in low- and middle-income settings where resources were already scarce and stretched before the pandemic. The COVID-19 pandemic disrupted access to TB diagnostic and care services, leaving many people undiagnosed or without TB treatment, thereby reversing years of progress in TB control, with an increased risk of onward transmission. Indeed, the WHO estimated an 18% reduction in TB notification in 2020 compared with 2019, and for the first time in a decade, TB mortality increased globally in 2020 and 2021, before returning to the pre-COVID-19 levels in 2022.1 5
Infection prevention and control (IPC) during the COVID-19 pandemic included measures that can prevent the airborne transmission of pathogens, and thus prevent diseases such as TB and COVID-19. Given the higher risk of TB infection among people living with HIV, preventing TB transmission is especially important in anti-retroviral therapy (ART) clinics, which often provide TB care services.6 In 2021, we conducted a cross-sectional multiregional site survey on the impact of COVID-19 on TB services in Africa and the Asia-Pacific region during the pandemic.7 Since then, it remained unclear what the lingering effects of the pandemic had on the healthcare systems and TB care delivery. Thus, we conducted a follow-up survey to examine the postpandemic long-term impact of COVID-19 on TB services at HIV care clinics in low- and middle-income settings, specifically in sub-Saharan Africa and in Asia, where both HIV and TB are highly prevalent and care for both diseases is often provided under one roof. We also examined the lessons learnt from the pandemic, focusing on the prolonged use of IPC measures at the clinics and on the sustained availability of virtual and decentralised TB care delivery.
Methods
Study setting
This cross-sectional site survey was conducted in the African and Asia-Pacific regions of the International epidemiology Databases to Evaluate AIDS (IeDEA, www.iedea.org). We included ART clinics treating adults living with HIV and offering TB diagnosis and treatment on site in five IeDEA regions with a high TB burden: West Africa, East Africa, Central Africa, Southern Africa and the Asia-Pacific.
Data collection and definitions
We collected data from July to September 2023. The survey was available in English and French and administered on paper or electronically using the REDCap data collection software.8 9 Questionnaires were codeveloped and revised in collaborations with two IeDEA-wide working groups, specialised in site-level surveys and TB and lung health, respectively. The survey material was pilot-tested in English and French. A healthcare worker familiar with TB and HIV service delivery at each facility filled in the questionnaire. Data included site-level information about the clinics, IPC measures, changes in service provision associated with COVID-19 and data on whether those changes were maintained after the pandemic. We also collected aggregated quarterly numbers of TB diagnoses, Xpert MTB/RIF TB diagnostic tests performed and ART initiations between 2018 and 2023 from TB registers across sites.
We defined the degree of TB/HIV service integration as ‘full integration’ (TB diagnostics and treatment available at the same facility as HIV care services), ‘partial integration’ (either TB diagnostics or treatment available) or ‘not integrated’ (TB diagnostics and treatment not available at the participating HIV care facility).10 TB diagnosis was defined as either being bacteriologically confirmed by the methods routinely used at the ART clinics and the associated TB services, or clinically diagnosed.
Statistical analyses
We describe survey responses as the number (%) of sites per item. We further compare the proportion of measures already implemented before, only in place during, and maintained after the pandemic by site-level characteristics using χ2 contingency table tests. The quarterly numbers of TB diagnoses, new patients on ART and Xpert MTB/RIF tests performed were estimated to impute missing observations by modelling temporal trends at the level of individual sites, countries and regions. Bayesian hierarchical Gaussian processes11 were used to account for short- and long-term site-level trends before and after missing periods, as well as broader country- and region-level trends during the gaps. All analyses were performed in R V.4.3.2 and modelling in the probabilistic programming language Stan V.2.26.1.
Patient and public involvement
We discussed the objectives and the study design with the relevant working groups of the global IeDEA consortium and developed the data collection procedures in collaboration with the local sites. The site survey was pilot tested with a small number of sites. Patients were not involved in the design, recruitment or conduct of this study. We will disseminate the results of this study to the participating clinics and our broader research networks.
Results
Clinic characteristics
We surveyed 32 clinics in African countries and 13 clinics in Asian-Pacific countries. Most clinics were in urban settings (37, 82%), offered tertiary care (22, 49%) and provided both in- and outpatient care (26, 58%). TB services were fully integrated with HIV services in 30 (67%) clinics (table 1).
Table 1. Characteristics of the 45 participating HIV clinics, overall and by region.
| Variable | All(n=45) | Africa(n=32) | Asia-Pacific(n=13) |
| Clinic setting | |||
| Urban | 37 (82%) | 24 (75%) | 13 (100%) |
| Peri-urban | 5 (11%) | 5 (16%) | 0 (0%) |
| Rural | 3 (7%) | 3 (9%) | 0 (0%) |
| Clinic owner | |||
| Public | 36 (80%) | 26 (81%) | 10 (77%) |
| Other | 9 (20%) | 6 (19%) | 3 (23%) |
| Level of care | |||
| Primary | 12 (27%) | 12 (38%) | 0 (0%) |
| Secondary | 7 (16%) | 6 (19%) | 1 (8%) |
| Tertiary | 22 (49%) | 12 (38%) | 10 (77%) |
| Other | 4 (9%) | 2 (6%) | 2 (15%) |
| Age of treated patients | |||
| Adults and children | 35 (78%) | 28 (88%) | 7 (54%) |
| Only adults | 10 (22%) | 4 (12%) | 6 (46%) |
| Type of care provision | |||
| In- and outpatients | 26 (58%) | 15 (47%) | 11 (85%) |
| Only outpatients | 19 (42%) | 17 (53%) | 2 (15%) |
| TB/HIV service integration | |||
| Full | 30 (67%) | 20 (62%) | 10 (77%) |
| Partial | 13 (29%) | 10 (31%) | 3 (23%) |
| Not integrated | 2 (4%) | 2 (6%) | 0 (0%) |
TBtuberculosis
Infection prevention and control measures
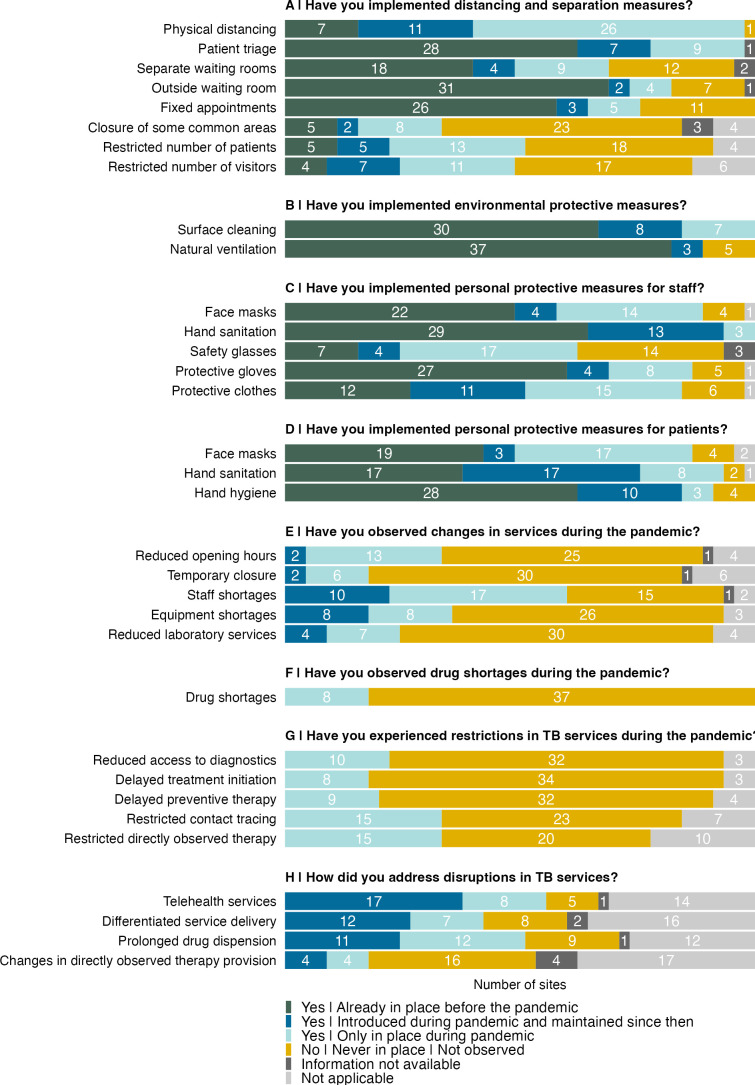
At least one distancing and separation measure was already in place before the pandemic (<2020) in most participating clinics (38, 84%), and 43 (96%) implemented at least one additional measure during the pandemic (2020–2021). Some clinics reported having maintained measures after the pandemic (≥2022), such as physical distancing at 11 (24%) clinics and patient triage at 7 (16%). Other clinics discontinued the newly introduced measures after the pandemic. All clinics had at least one distancing or separation implemented during the pandemic (figure 1A). Most of the clinics (27, 60%) reported practising regular surface cleaning and room ventilation already before the pandemic, while 15 (33%) introduced at least one of these measures during the COVID-19 pandemic and often maintained them afterward (figure 1B). During the pandemic, 39 (87%) clinics implemented personal protective measures for staff members and 32 (71%) for patients. Face masks were newly introduced in 18 (40%) and hand sanitation in 16 (36%) clinics for staff and in 20 (44%) and 25 (56%) clinics for patients, respectively (figure 1C,D).
Figure 1. Implementation of infection control and prevention measures during and after the COVID-19 pandemic in participating anti-retroviral therapy (ART) clinics. TB, tuberculosis.
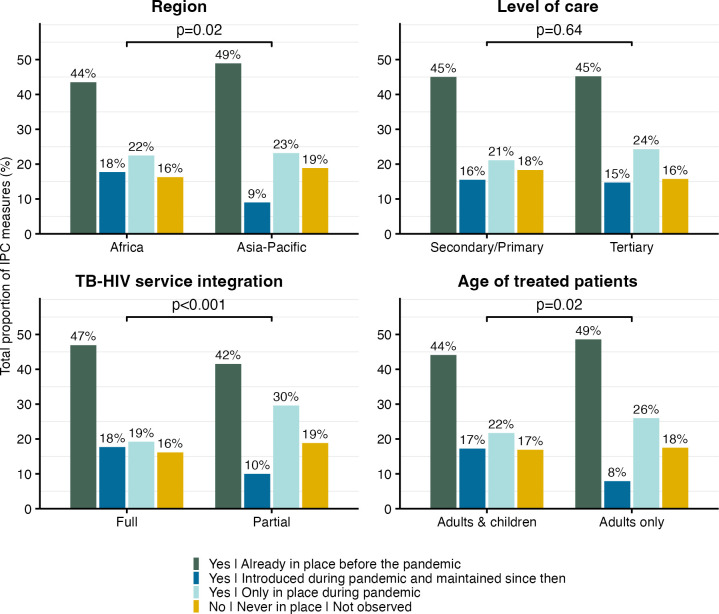
We explored differences in the total proportion of implemented IPC measures in clinics during the COVID-19 pandemic and beyond. Across clinics, 45% of all IPC measures were already implemented before the pandemic, 23% were only in place during the pandemic and 15% were maintained after the pandemic. Implementation differed by degree of TB/HIV service integration (full vs partial integration, p<0.001), with 47% versus 42% of measures already implemented before, 19% versus 30% only in place during and 18% versus 10% maintained after the pandemic. Differences were also found between regions (Africa vs Asia-Pacific, p=0.02) and age of treated patients (adults/children vs adults only, p=0.02). Level of care (secondary/primary vs tertiary, p=0.64) was not associated with IPC implementation (figure 2).
Figure 2. Total proportion of implemented infection prevention and control (IPC) measures across participating HIV clinics by subgroups of region, level of care, TB/HIV service integration and age of treated patients. P values are based on the χ2 contingency table test with the null hypothesis that the distribution of counts does not differ by subgroup. TB, tuberculosis.
Service restrictions
Fourteen (31%) clinics reported reduced opening hours and eight (18%) temporary closures during the pandemic (figure 1E). Staff shortages affected 26 (58%) clinics and drug shortages eight (18%) clinics (figure 1F). TB services were particularly affected in some clinics: 10 (22%) reported reduced access to TB diagnostic services, eight (18%) reported delayed treatment initiation and 15 (33%) restricted directly observed therapy services (figure 1G). Restrictions in TB services were addressed by providing telehealth, differentiated service delivery and multimonth TB drug dispensing. Telehealth was introduced at 25 (56%) clinics during the pandemic and has been maintained since then in 17 clinics. Differentiated service delivery was offered at 19 (42%) clinics during the pandemic, at 12 of them beyond. Multimonth TB drug dispensing was available in 23 (51%) of the clinics, 11 of which maintained this option beyond the COVID-19 pandemic (figure 1H).
Tuberculosis (TB) diagnoses and Xpert MTB/RIF tests performed over time
We observed a marked decrease in the number of new TB diagnoses in 2020–2021, compared with 2018–2019 in the African regions of IeDEA (figure 3), and a smaller decrease in the Asia-Pacific sites. The number of TB diagnoses has since almost returned to prepandemic levels. The same trends were observed for the number of Xpert MTB/RIF diagnostic tests performed. This decrease in TB diagnoses was accompanied by a decrease in ART initiation, which declined in both regions in 2020 and remained slightly below prepandemic levels since then (figure 3).
Figure 3. Number of people newly diagnosed with tuberculosis (TB), number of Xpert tests performed, and the number of people newly started on anti-retroviral therapy (ART) in 39 sites contributing aggregated data. Median estimate as black lines with 95% credibility intervals as ribbons, globally (across all sites) and by the International epidemiology Databases to Evaluate AIDS region (Africa and the Asia-Pacific).
Discussion
We surveyed HIV care clinics in Africa and the Asia-Pacific to understand the impact of COVID-19 on care provision beyond the pandemic, with a focus on TB services. During the pandemic, COVID-19 affected service provision in almost three quarters of the surveyed clinics, and in about half of them, TB services were impacted. These restrictions in TB services were paralleled by a reduction in quarterly TB diagnoses, which partly resumed after the pandemic. During the pandemic, most clinics introduced distancing or separation measures and used personal protective measures. Some of these measures were sustained beyond the pandemic. About 40% of the clinics adopted or strengthened measures to adapt care delivery during the pandemic, including with longer TB medication refills and telehealth, or with differentiated service delivery. Most facilities maintained these client-centred services beyond the pandemic.
We had previously described the impact of COVID-19 on service provision in ART clinics in 2021, while the pandemic was still ongoing.7 In this follow-up survey, we observed that some of the measures used during the pandemic were temporary, while others were sustained. IPC implementation was associated with the degree of TB/HIV service integration, likely reflecting transmission reduction measures commonly in place at clinics offering both services under one roof. Access to diagnostic tools and to drugs was often impaired during the pandemic due to disrupted supply chains. Furthermore, access to care facilities was generally restricted during lockdown periods to dedicate resources to COVID-19 care.12 A global study in 16 countries showed that lockdowns severely impeded TB care provision because of reassigned resources at clinics, fear of exposure and reduced public transportation.13 Mathematical modelling showed that interruptions in ART supply could lead to a marked increase in HIV transmission and death.14 The impact of COVID-19 on health systems was especially severe in sub-Saharan Africa where the dual TB and HIV epidemics were already overstretching care provision before COVID-19.15 16 The observed differences in the impact of COVID-19 on TB services between ART clinics in Africa and Asia-Pacific may reflect several underlying factors, such as clinic organisation, human and technical resources, or financial constraints.
Lockdowns during the COVID-19 pandemic increased the use of telemedicine for TB care, including virtual directly observed therapy (DOT), and the use of multimonth TB drug dispensing to minimise facility visits and the risk of nosocomial transmission.2 13 17 In parallel, differentiated service delivery for HIV was accelerated in sub-Saharan Africa during the pandemic and integrated TB preventive therapy in some places.18 We observed that telehealth, virtual DOT, multimonth dispensing and differentiated service delivery were used to sustain TB care provision during the pandemic and were maintained at some clinics since then. This reflects the advantages of decentralised, patient-centred models of care delivery. Indeed, flexible models of delivering healthcare are clinically effective with the advantage of being more compatible with the everyday life of people living with HIV and TB.19 20 Furthermore, remote and less frequent access to care reduces travel costs, stigma of coming to HIV and TB clinics, and the workload for healthcare workers.21 22
The decrease in TB diagnoses that we observed during the pandemic was in line with the global trends reported by the WHO and others.1 2 23 Indeed, during the pandemic, the massive disruption in TB services was shown to have reversed the global efforts to control the TB epidemic by roughly 10 years.3 Furthermore, a modelling study estimated a 20% increase in TB mortality in the years following the pandemic.24 Our findings suggest that TB diagnosis improved in the years after the pandemic, indicating that TB care activities have resumed. Continuous monitoring of the number of TB diagnoses at the clinic level could prompt investigations into the potential drivers of changes in TB caseloads and thereby help identify appropriate interventions in the care cascade.
Although the IeDEA collaborating clinics are not fully representative of HIV care providers, this study provides a broad overview of the lingering effects of COVID-19 on TB services in ART clinics in Africa and the Asia-Pacific, including small and rural facilities that are less studied. We also acknowledge that our data are self-reported, involving some risk of recall or social-desirability bias. Furthermore, our study did not assess regional differences in how governments restricted services during the pandemic. Indeed, restrictions, IPC measures and even lockdowns may have varied in intensity and feasibility over time and places, influencing how care could or could not be delivered at different points in time. Our study could not measure the impact of COVID-19 on clinical TB outcomes, but we assessed the trends in TB diagnoses—a key indicator of TB control—in addition to site-level factors. This allowed assessing how service disruptions can affect disease management, and therefore, transmission risks.
In conclusion, this study assessed the lingering effects of the COVID-19 pandemic on the provision of TB services in low- and middle-income countries. COVID-19 severely disrupted TB services during the pandemic, but care provision partly resumed after the pandemic. Important IPC measures were either adopted or reinforced during the pandemic; some were maintained thereafter. Telehealth, multimonth TB drug dispensing and other models of differentiated service delivery were implemented to support care delivery during the pandemic. However, few sites maintained these services afterwards. Our study showed the overall resilience and adaptability of health systems in low- and middle-income settings. However, further efforts are needed to sustain approaches that reduce the risk of transmission of infections in ART clinics, including TB, and to promote client-centred care.
Acknowledgements
We would like to thank all the participating clinics and all those who completed the questionnaire. Further, we would like to thank the IeDEA TB & Lung Health Working Group and the Site Assessment Working Group for their input and feedback on the survey and the manuscript.
Footnotes
Funding: IeDEA is supported by the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center: Asia-Pacific, U01AI069907; Central Africa, U01AI096299; East Africa, U01AI069911; NA-ACCORD, U01AI069918; Southern Africa, U01AI069924; West Africa, U01AI069919. Informatics resources are supported by the Harmonist project, R24AI24872. This work is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned.
Handling editor: Naomi Clare Lee
Data availability free text: Complete data for this study cannot be posted in a supplemental file or a public repository because of legal and ethical restrictions. The Principles of Collaboration of this multinational consortium and the regulatory requirements of the different countries’ IRBs require the submission and approval of individual project concept sheets that describe the planned analyses. Specifically, while the data held by the IeDEA consortium may be available to other investigators, proposed use must be based on a concept note that is approved by the regional Steering Groups and the IeDEA Executive Committee (Chairperson: Annette Sohn, MD; email: annette.sohn@treatasia.org).
Patient consent for publication: Not applicable.
Ethics approval: The Cantonal Ethics Committee of Bern (Switzerland) reviewed and approved the study (no. PB_2016–00273).
Provenance and peer review: Not commissioned; externally peer-reviewed.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting or dissemination plans of this research. Refer to the Methods section for further details.
Data availability statement
Data are available upon reasonable request.
References
- 1.World Health Organization Global tuberculosis report 2023. 2023. [29-Apr-2024]. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 Available. Accessed.
- 2.Dheda K, Perumal T, Moultrie H, et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med. 2022;10:603–22. doi: 10.1016/S2213-2600(22)00092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmer AJ, Klinton JS, Oga-Omenka C, et al. Tuberculosis in times of COVID-19. J Epidemiol Community Health. 2022;76:310–6. doi: 10.1136/jech-2021-217529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brazier E, Ajeh R, Maruri F, et al. Service delivery challenges in HIV care during the first year of the COVID-19 pandemic: results from a site assessment survey across the global IeDEA consortium. J Int AIDS Soc. 2022;25:e26036. doi: 10.1002/jia2.26036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzon D, Zignol M, Bastard M, et al. The impact of the COVID-19 pandemic on the global tuberculosis epidemic. Front Immunol. 2023;14:1234785. doi: 10.3389/fimmu.2023.1234785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles MK, Lindegren ML, Wester CW, et al. Implementation of Tuberculosis Intensive Case Finding, Isoniazid Preventive Therapy, and Infection Control (“Three I’s”) and HIV-Tuberculosis Service Integration in Lower Income Countries. PLoS One. 2016;11:e0153243. doi: 10.1371/journal.pone.0153243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marti M, Zürcher K, Enane LA, et al. Impact of the COVID-19 pandemic on TB services at ART programmes in low- and middle-income countries: a multi-cohort survey. J Int AIDS Soc. 2022;25:e26018. doi: 10.1002/jia2.26018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zürcher K, Cox SR, Ballif M, et al. Integrating services for HIV and multidrug-resistant tuberculosis: A global cross-sectional survey among ART clinics in low- and middle-income countries. PLOS Glob Public Health . 2022;2:e0000180. doi: 10.1371/journal.pgph.0000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaxman S, Gelman A, Neill D, et al. Fast hierarchical Gaussian processes. 2015.
- 12.Shrinivasan R, Rane S, Pai M. India’s syndemic of tuberculosis and COVID-19. BMJ Glob Health. 2020;5:e003979. doi: 10.1136/bmjgh-2020-003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Migliori GB, Thong PM, Akkerman O, et al. Worldwide effects of coronavirus disease pandemic on tuberculosis services, January–April 2020 - Volume 26, number 11—November 2020 - emerging infectious diseases journal. CDC. 2020. https://wwwnc.cdc.gov/eid/article/26/11/20-3163_article Available. [DOI] [PMC free article] [PubMed]
- 14.Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV. 2020;7:e629–40. doi: 10.1016/S2352-3018(20)30211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachega JB, Kapata N, Sam-Agudu NA, et al. Minimizing the impact of the triple burden of COVID-19, tuberculosis and HIV on health services in sub-Saharan Africa. Int J Infect Dis. 2021;113 Suppl 1:S16–21. doi: 10.1016/j.ijid.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiau S, Krause KD, Valera P, et al. The Burden of COVID-19 in People Living with HIV: A Syndemic Perspective. AIDS Behav. 2020;24:2244–9. doi: 10.1007/s10461-020-02871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keene C, Mohr-Holland E, Cassidy T, et al. How COVID-19 could benefit tuberculosis and HIV services in South Africa. Lancet Respir Med. 2020;8:844–6. doi: 10.1016/S2213-2600(20)30311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimsrud A, Wilkinson L. Acceleration of differentiated service delivery for HIV treatment in sub-Saharan Africa during COVID-19. J Int AIDS Soc. 2021;24:e25704. doi: 10.1002/jia2.25704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alipanah N, Jarlsberg L, Miller C, et al. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15:e1002595. doi: 10.1371/journal.pmed.1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng EH, Holmes CB, Moshabela M, et al. Personalized public health: An implementation research agenda for the HIV response and beyond. PLoS Med. 2019;16:e1003020. doi: 10.1371/journal.pmed.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimsrud A, Bygrave H, Doherty M, et al. Reimagining HIV service delivery: the role of differentiated care from prevention to suppression. J Int AIDS Soc. 2016;19:21484. doi: 10.7448/IAS.19.1.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christ B, van DJ, Ballif M, et al. Differentiated antiretroviral therapy delivery in rural Zimbabwe: availability, needs and challenges. OSF Preprints. 2020. https://osf.io/zpq2e/ Available.
- 23.Kiarie H, Temmerman M, Nyamai M, et al. The COVID-19 pandemic and disruptions to essential health services in Kenya: a retrospective time-series analysis. Lancet Glob Health. 2022;10:e1257–67. doi: 10.1016/S2214-109X(22)00285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e1132–41. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.