Abstract
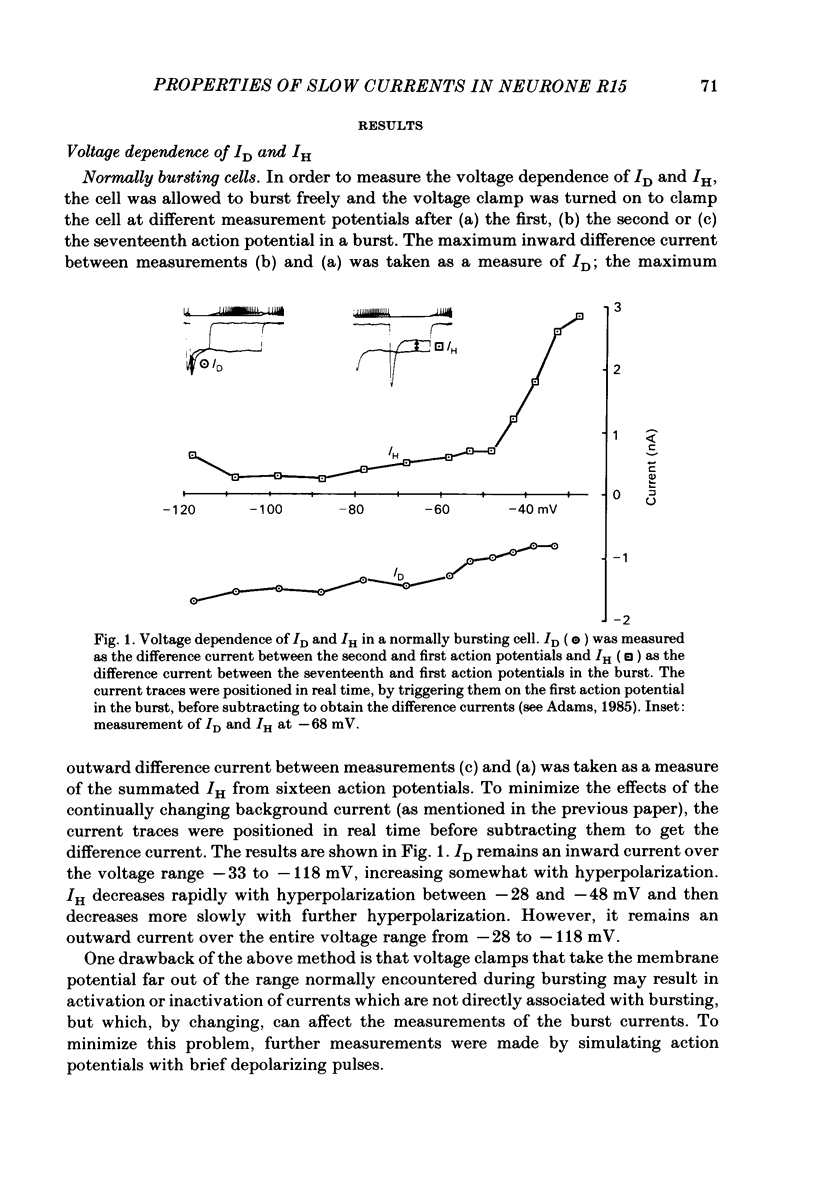
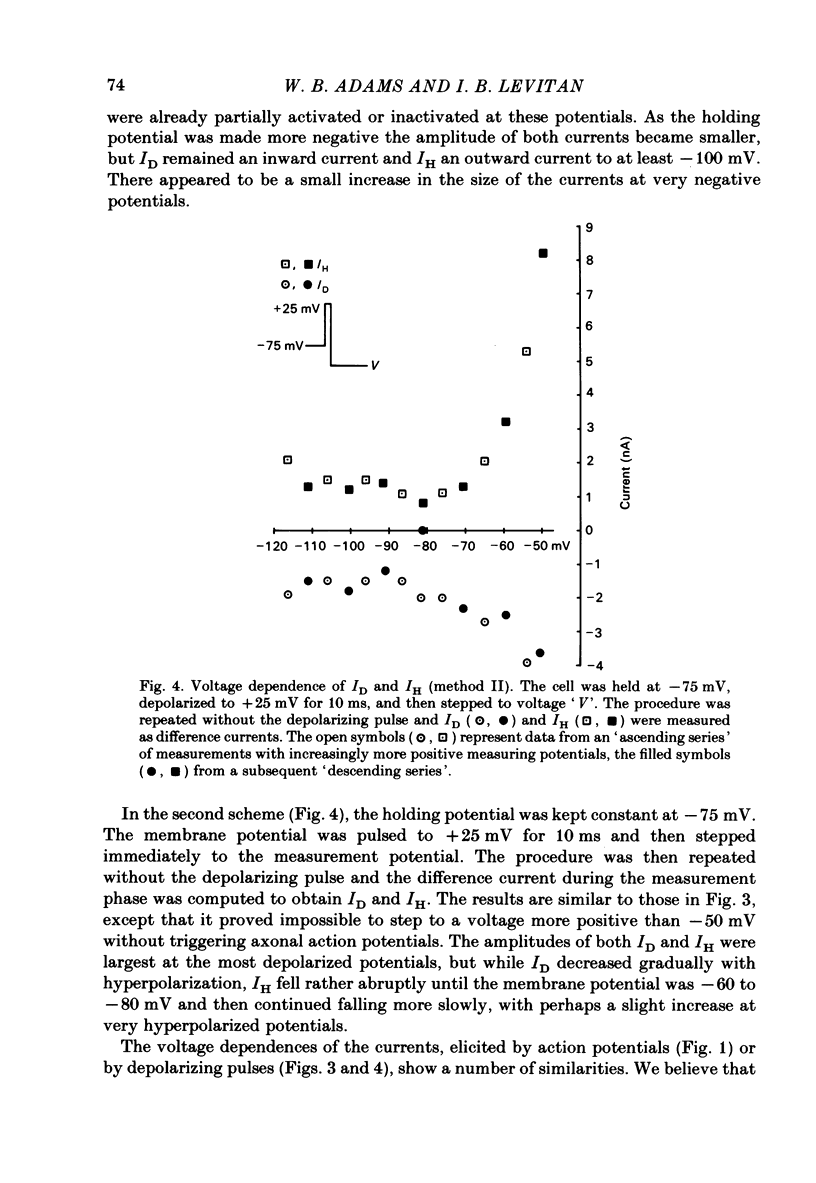
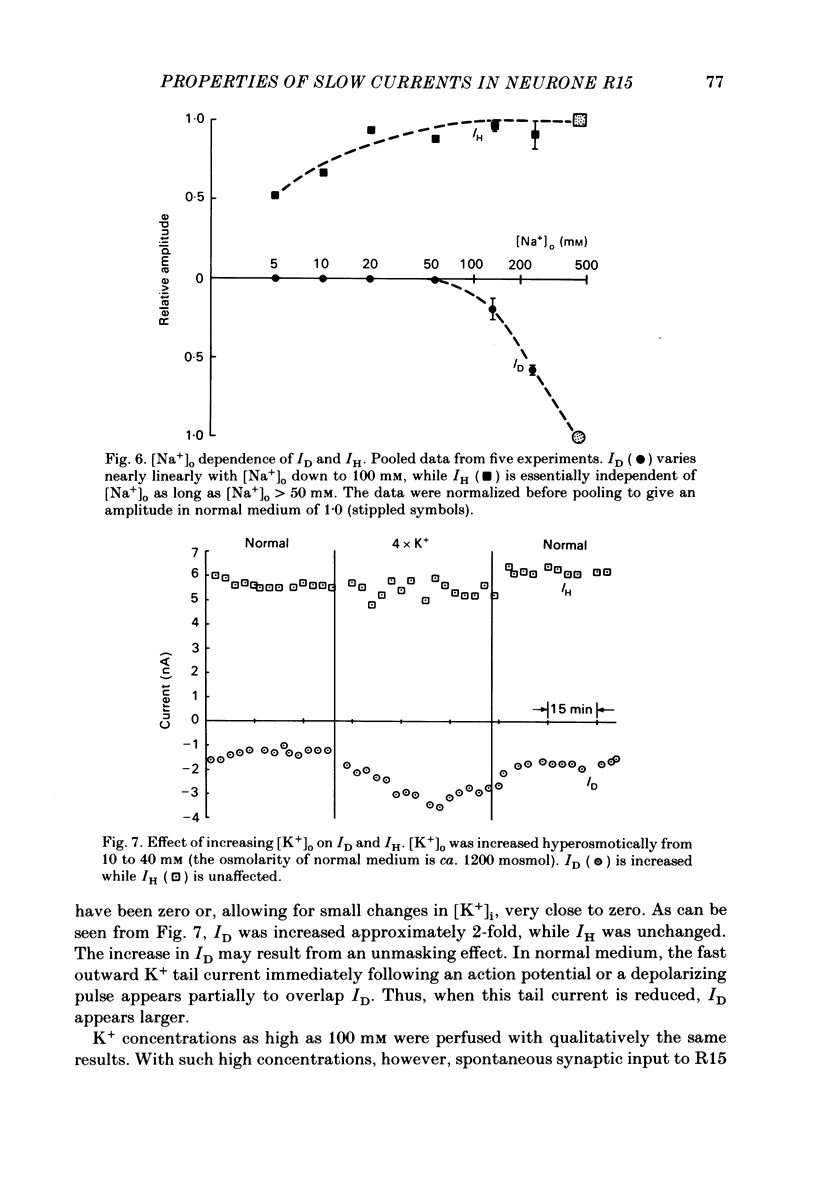
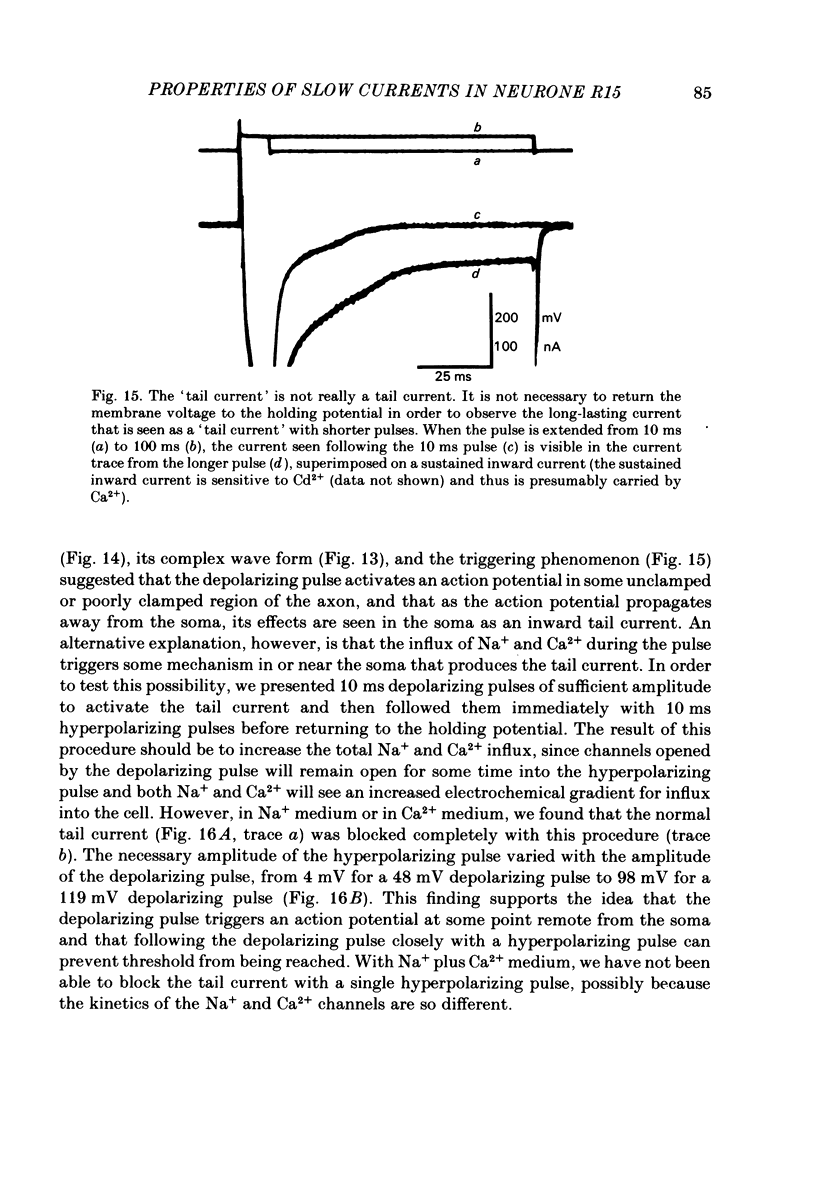
The previous paper described a slow depolarizing tail current, ID, and a slow hyperpolarizing tail current, IH, that are activated by action potentials and by brief depolarizing pulses in Aplysia neurone R15. ID and IH are necessary for the generation of bursting pace-maker activity in this cell. In this paper, the voltage and ion dependence of ID and IH are studied in an effort to determine the charge carriers for the two currents. When the slow currents are activated by brief depolarizing pulses delivered under voltage clamp in normal medium, an increase in the size of the pulse of 5-10 mV is usually sufficient to bring about full activation of ID. The apparent threshold in normal medium is approximately -20 mV. In medium in which K+ channels are blocked, full activation of an inward tail current that resembles ID requires increasing the pulse amplitude by only 1-2 mV. In contrast, IH is activated in a graded fashion over a 40 mV range of pulse amplitudes. After activating the currents with action potentials or with supramaximal pulses, ID remains an inward current and IH an outward current over a range of membrane potentials spanning -20 to -120 mV. In normal medium, ID is dependent on both extracellular Na+ concentration ( [Na+]o) and extracellular Ca2+ concentration ( [Ca2+]o). When K+ channels are blocked, ID can be supported by either [Na+]o or [Ca2+]o. IH depends only on [Ca2+]o as long as [Na+]o is at least 50 mM. Neither ID nor IH is decreased by decreasing the K+ gradient or by application of K+ channel blockers. These treatments increase somewhat the apparent amplitude of ID, probably by unmasking it from the large K+ tail current that follows the depolarizing pulse. A direct comparison in the same cell of the tetraethylammonium sensitivity of IH and of the Ca2+-activated K+ current demonstrates that these two currents flow through separate and distinct populations of channels. We conclude that in R15, ID arises in response to the triggering of an axonal action potential which in turn, through an as yet unknown mechanism, causes an increased influx of Na+ and/or Ca2+. We conclude that the apparent outward current IH, which is responsible for the interburst hyperpolarization in a normally bursting R15, in fact arises from a decrease in a resting inward Ca2+ current, possibly as the result of Ca2+-induced inactivation of Ca2+ channels.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. B., Parnas I., Levitan I. B. Mechanism of long-lasting synaptic inhibition in Aplysia neuron R15. J Neurophysiol. 1980 Dec;44(6):1148–1160. doi: 10.1152/jn.1980.44.6.1148. [DOI] [PubMed] [Google Scholar]
- Adams W. B. Slow depolarizing and hyperpolarizing currents which mediate bursting in Aplysia neurone R15. J Physiol. 1985 Mar;360:51–68. doi: 10.1113/jphysiol.1985.sp015603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale N. T., Wachtel H. Two reciprocating current components underlying slow oscillations in Aplysia bursting neurons. Brain Res. 1980 May;203(1):45–65. doi: 10.1016/0165-0173(80)90003-x. [DOI] [PubMed] [Google Scholar]
- Carpenter D., Gunn R. The dependence of pacemaker discharge of Aplysia neurons upon Na+ and Ca++. J Cell Physiol. 1970 Feb;75(1):121–127. doi: 10.1002/jcp.1040750114. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Time course separation of two inward currents in molluscan neurons. Brain Res. 1977 Jan 7;119(2):487–492. doi: 10.1016/0006-8993(77)90330-4. [DOI] [PubMed] [Google Scholar]
- Eckert R., Ewald D. Inactivation of calcium conductance characterized by tail current measurements in neurones of Aplysia californica. J Physiol. 1983 Dec;345:549–565. doi: 10.1113/jphysiol.1983.sp014996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L., Brehm P. Calcium-mediated control of Ca and K currents. Fed Proc. 1981 Jun;40(8):2226–2232. [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A. Internal effects of divalent cations on potassium permeability in molluscan neurones. J Physiol. 1979 Nov;296:393–410. doi: 10.1113/jphysiol.1979.sp013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A., Thomas M. V. Ionic requirements for membrane oscillations and their dependence on the calcium concentration in a molluscan pace-maker neurone. J Physiol. 1982 Jun;327:185–217. doi: 10.1113/jphysiol.1982.sp014227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Changes in the intracellular concentration of free calcium ions in a pace-maker neurone, measured with the metallochromic indicator dye arsenazo III. J Physiol. 1978 Feb;275:357–376. doi: 10.1113/jphysiol.1978.sp012194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Potassium conductance and internal calcium accumulation in a molluscan neurone. J Physiol. 1980 Nov;308:287–313. doi: 10.1113/jphysiol.1980.sp013472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. External and internal effects of tetraethylammonium on voltage-dependent and Ca-dependent K+ currents components in molluscan pacemaker neurons. Neurosci Lett. 1979 Apr;12(1):87–92. doi: 10.1016/0304-3940(79)91485-x. [DOI] [PubMed] [Google Scholar]
- Johnston D. Voltage, temperature and ionic dependence of the slow outward current in Aplysia burst-firing neurones. J Physiol. 1980 Jan;298:145–157. doi: 10.1113/jphysiol.1980.sp013072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge D., Stephens C. L. Cyclic variation of potassium conductance in a burst-generating neurone in Aplysia. J Physiol. 1973 Nov;235(1):155–181. doi: 10.1113/jphysiol.1973.sp010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. V. Spike aftercurrents in R15 of Aplysia: their relationship to slow inward current and calcium influx. J Neurophysiol. 1984 Feb;51(2):387–403. doi: 10.1152/jn.1984.51.2.387. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B. Calcium current inactivation in identified neurones of Helix aspersa. J Physiol. 1981 Dec;321:273–285. doi: 10.1113/jphysiol.1981.sp013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. J., Zucker R. S. Aequorin response facilitation and intracellular calcium accumulation in molluscan neurones. J Physiol. 1980 Mar;300:167–196. doi: 10.1113/jphysiol.1980.sp013157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B. Ca channel inactivation by intracellular Ca injection into Helix neurones. Nature. 1981 Sep 10;293(5828):158–159. doi: 10.1038/293158a0. [DOI] [PubMed] [Google Scholar]
- Stinnakre J., Tauc L. Calcium influx in active Aplysia neurones detected by injected aequorin. Nat New Biol. 1973 Mar 28;242(117):113–115. doi: 10.1038/newbio242113b0. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D., Gorman A. L. Non-uniform Ca2+ buffer distribution in a nerve cell body. Nature. 1980 Aug 21;286(5775):816–817. doi: 10.1038/286816a0. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]



