Abstract
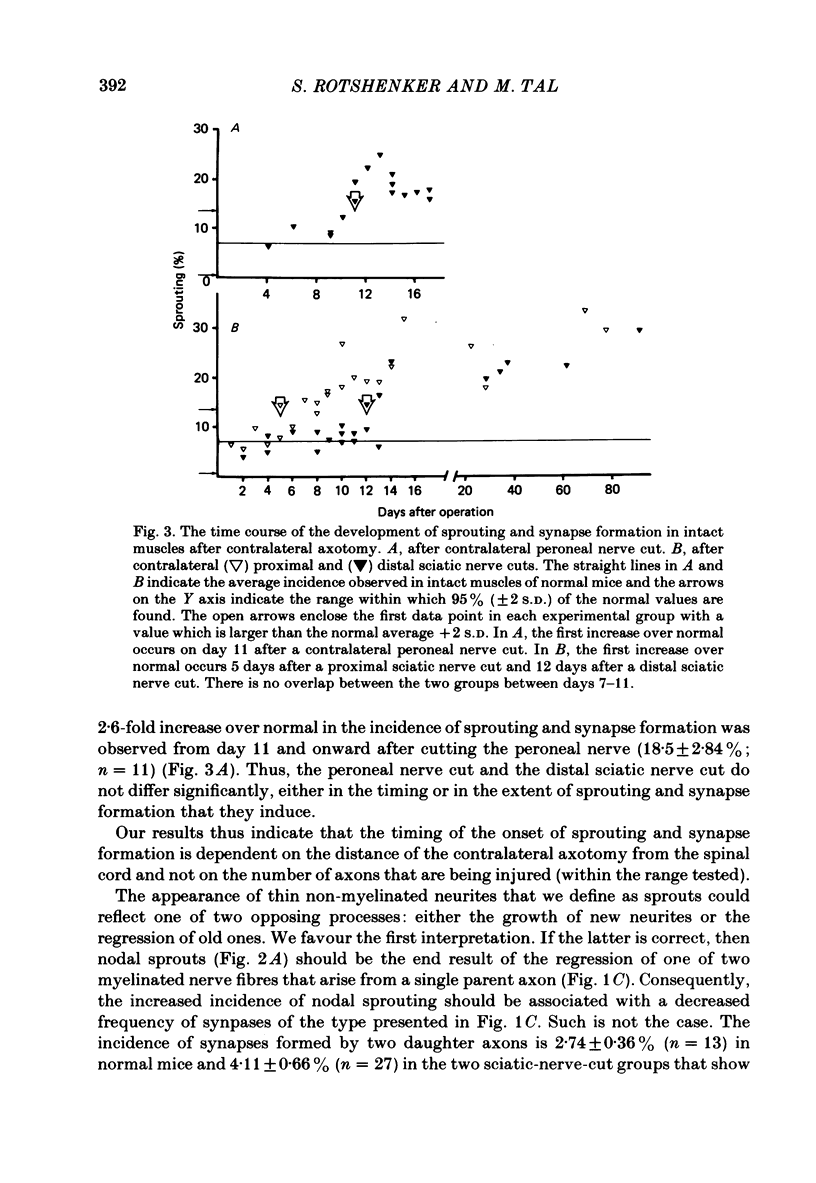
The pattern of innervation to intact peroneal and extensor digitorum longus muscles of normal and experimental young adult mice was studied by light microscopy after staining neuromuscular junctions by a combined silver-cholinesterase stain. Spontaneous sprouting and synapse formation occur in intact muscles of normal mice. In about 7% of the junctions, sprouts contribute to the innervation of muscle fibres already innervated by their parent axons. Axotomy of the sciatic nerve in one hind limb is followed by an average 3-fold increase over normal in the incidence of sprouting and synapse formation in the intact muscles of the opposite hind limb. The time to onset of sprouting and synapse formation becomes shorter as the site of the contralateral axotomy is placed closer to the spinal cord. A significant increase over normal in the incidence of sprouting is first observed 5 days after a proximal sciatic nerve cut and only 12 days after a distal sciatic nerve cut. The timing of sprouting is independent of the difference in the number of axons that are involved in the contralateral axotomies at different sites. These findings suggest that, in the intact muscles of normal mice, sprouting and synapse formation is an ongoing process which can be enhanced by contralateral axotomy. As in frogs (Rotshenker, 1979, 1982) the underlying mechanism may be the transneuronal induction of sprouting and synapse formation.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. C., Holland R. L., Hopkins W. G. Motor nerve sprouting. Annu Rev Neurosci. 1981;4:17–42. doi: 10.1146/annurev.ne.04.030181.000313. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Nodal and terminal sprouting from motor nerves in fast and slow muscles of the mouse. J Physiol. 1980 Sep;306:493–510. doi: 10.1113/jphysiol.1980.sp013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardasis C. A., Padykula H. A. Ultrastructural evidence indicating reorganization at the neuromuscular junction in the normal rat soleus muscle. Anat Rec. 1981 May;200(1):41–59. doi: 10.1002/ar.1092000105. [DOI] [PubMed] [Google Scholar]
- Cardasis C. A. Ultrastructural evidence of continued reorganization at the aging (11-26 months) rat soleus neuromuscular junction. Anat Rec. 1983 Nov;207(3):399–415. doi: 10.1002/ar.1092070303. [DOI] [PubMed] [Google Scholar]
- Dennis M. J. Development of the neuromuscular junction: inductive interactions between cells. Annu Rev Neurosci. 1981;4:43–68. doi: 10.1146/annurev.ne.04.030181.000355. [DOI] [PubMed] [Google Scholar]
- Elizalde A., Huerta M., Stefani E. Selective reinnervation of twitch and tonic muscle fibres of the frog. J Physiol. 1983 Jul;340:513–524. doi: 10.1113/jphysiol.1983.sp014777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim M. A., Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982 Aug;11(4):641–656. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- Goshgarian H. G. A rapid silver impregnation for central and peripheral nerve fibers in paraffin and frozen sections. Exp Neurol. 1977 Oct;57(1):296–301. doi: 10.1016/0014-4886(77)90065-6. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H. Local re-innervation in partially denervated muscle; a histophysiological study. Aust J Exp Biol Med Sci. 1950 Jul;28(4):383–397. doi: 10.1038/icb.1950.39. [DOI] [PubMed] [Google Scholar]
- McIsaac G., Kiernan J. A. Complete staining of neuromuscular innervation with bromoindigo and silver. Stain Technol. 1974 Jul;49(4):211–214. doi: 10.3109/10520297409116980. [DOI] [PubMed] [Google Scholar]
- Riley D. A. Ultrastructural evidence for axon retraction during the spontaneous elimination of polyneuronal innervation of the rat soleus muscle. J Neurocytol. 1981 Jun;10(3):425–440. doi: 10.1007/BF01262414. [DOI] [PubMed] [Google Scholar]
- Ring G., Reichert F., Rotshenker S. Sprouting in intact sartorius muscles of the frog following contralateral axotomy. Brain Res. 1983 Feb 7;260(2):313–316. doi: 10.1016/0006-8993(83)90687-x. [DOI] [PubMed] [Google Scholar]
- Rotshenker S., Reichert F. Motor axon sprouting and site of synapse formation in intact innervated skeletal muscle of the frog. J Comp Neurol. 1980 Sep 15;193(2):413–422. doi: 10.1002/cne.901930208. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Synapse formation in intact innervated cutaneous-pectoris muscles of the frog following denervation of the opposite muscle. J Physiol. 1979 Jul;292:535–547. doi: 10.1113/jphysiol.1979.sp012870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S. Transneuronal and peripheral mechanisms for the induction of motor neuron sprouting. J Neurosci. 1982 Oct;2(10):1359–1368. doi: 10.1523/JNEUROSCI.02-10-01359.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach J. H. Neuromuscular junctions and alpha-bungarotoxin-binding sites in denervated and contralateral cat skeletal muscles. J Physiol. 1981;313:513–528. doi: 10.1113/jphysiol.1981.sp013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffery A. R. Growth and degeneration of motor end-plates in normal cat hind limb muscles. J Anat. 1971 Nov;110(Pt 2):221–247. [PMC free article] [PubMed] [Google Scholar]
- Wernig A., Carmody J. J., Anzil A. P., Hansert E., Marciniak M., Zucker H. Persistence of nerve sprouting with features of synapse remodelling in soleus muscles of adult mice. Neuroscience. 1984 Jan;11(1):241–253. doi: 10.1016/0306-4522(84)90227-6. [DOI] [PubMed] [Google Scholar]
- Wernig A., Pécot-Dechavassine M., Stover H. Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare. J Neurocytol. 1980 Jun;9(3):278–303. doi: 10.1007/BF01181538. [DOI] [PubMed] [Google Scholar]



