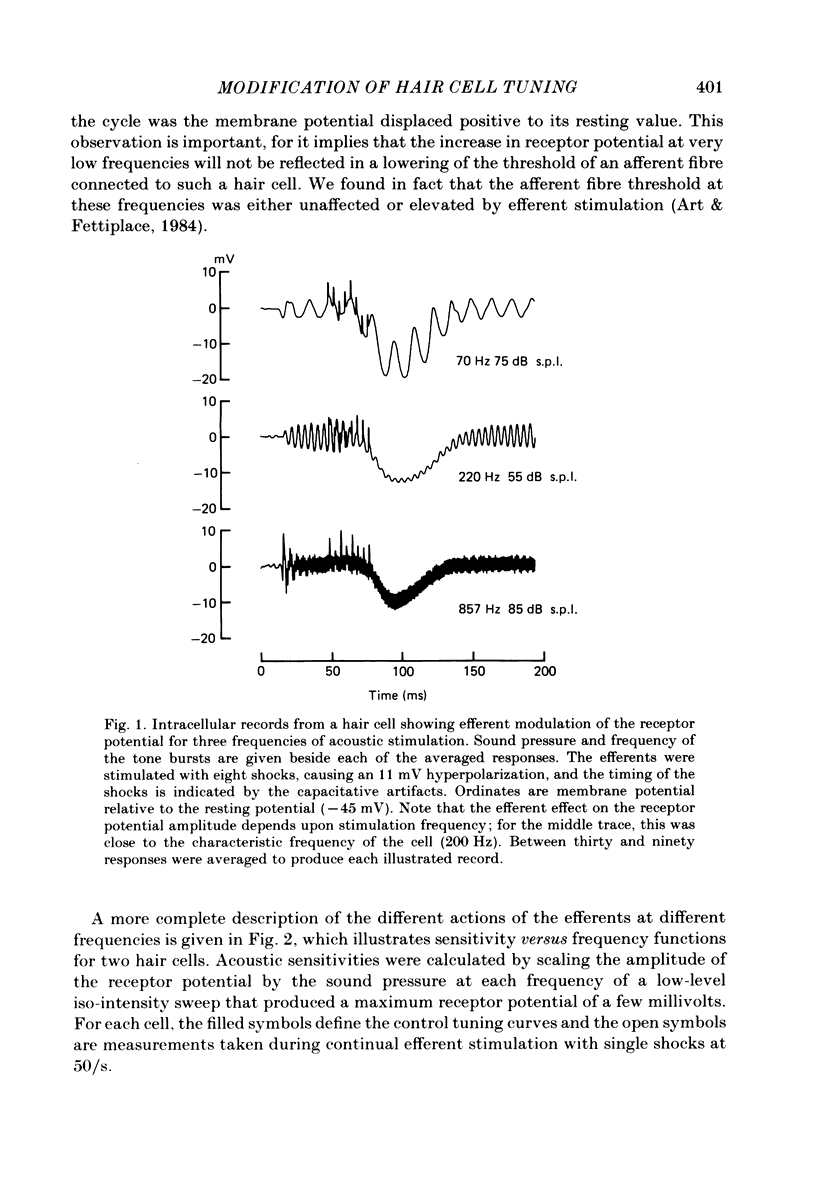
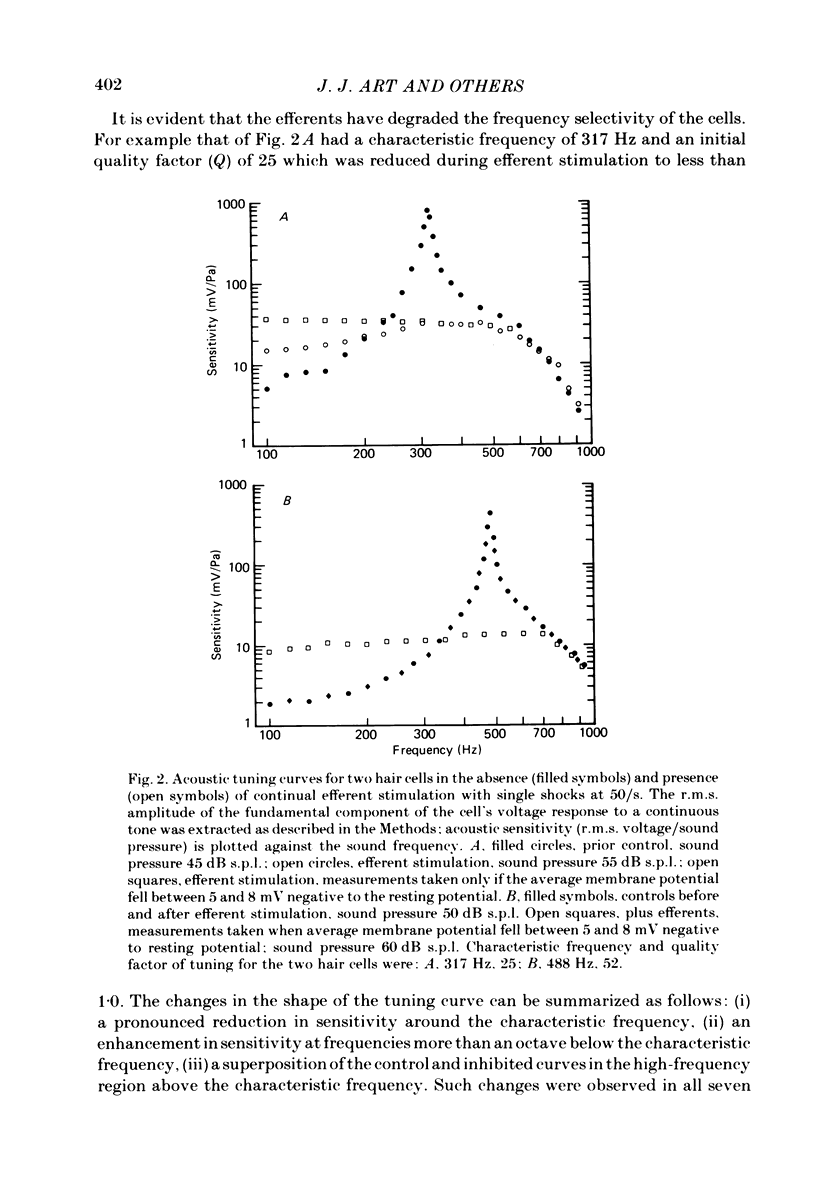
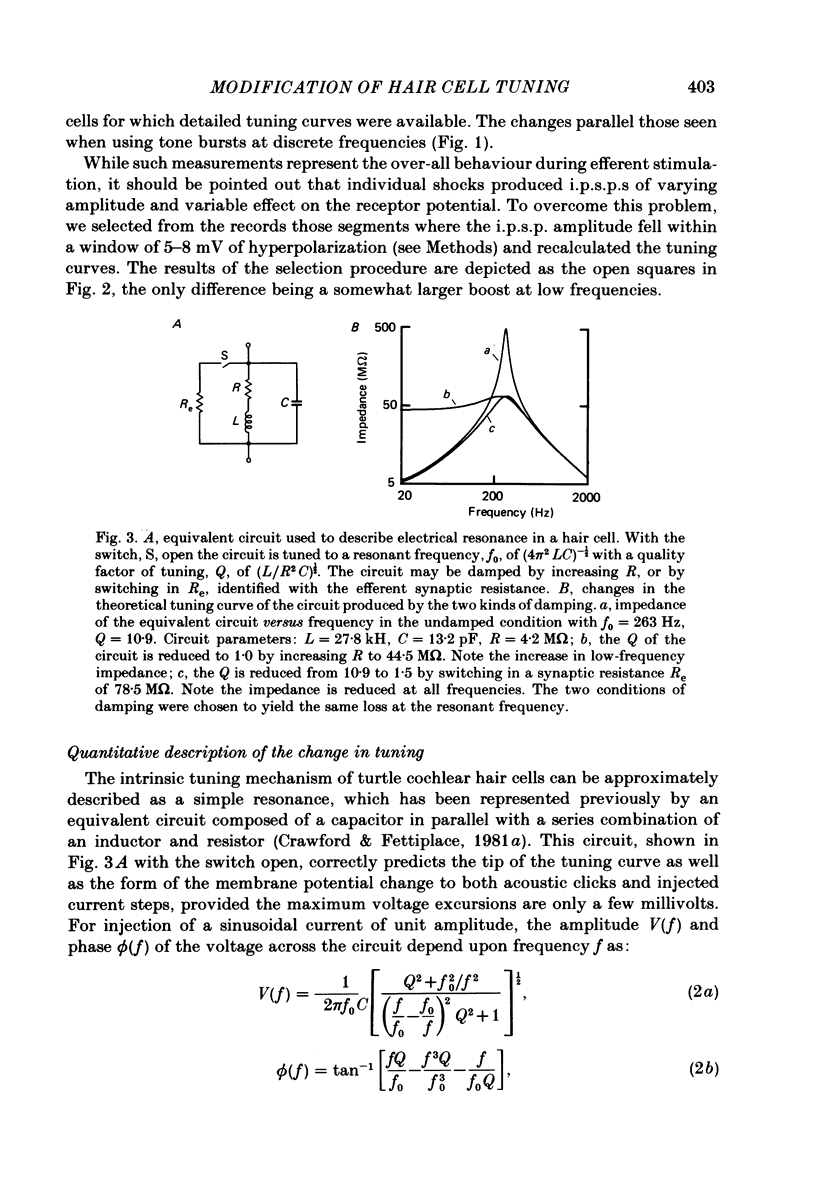
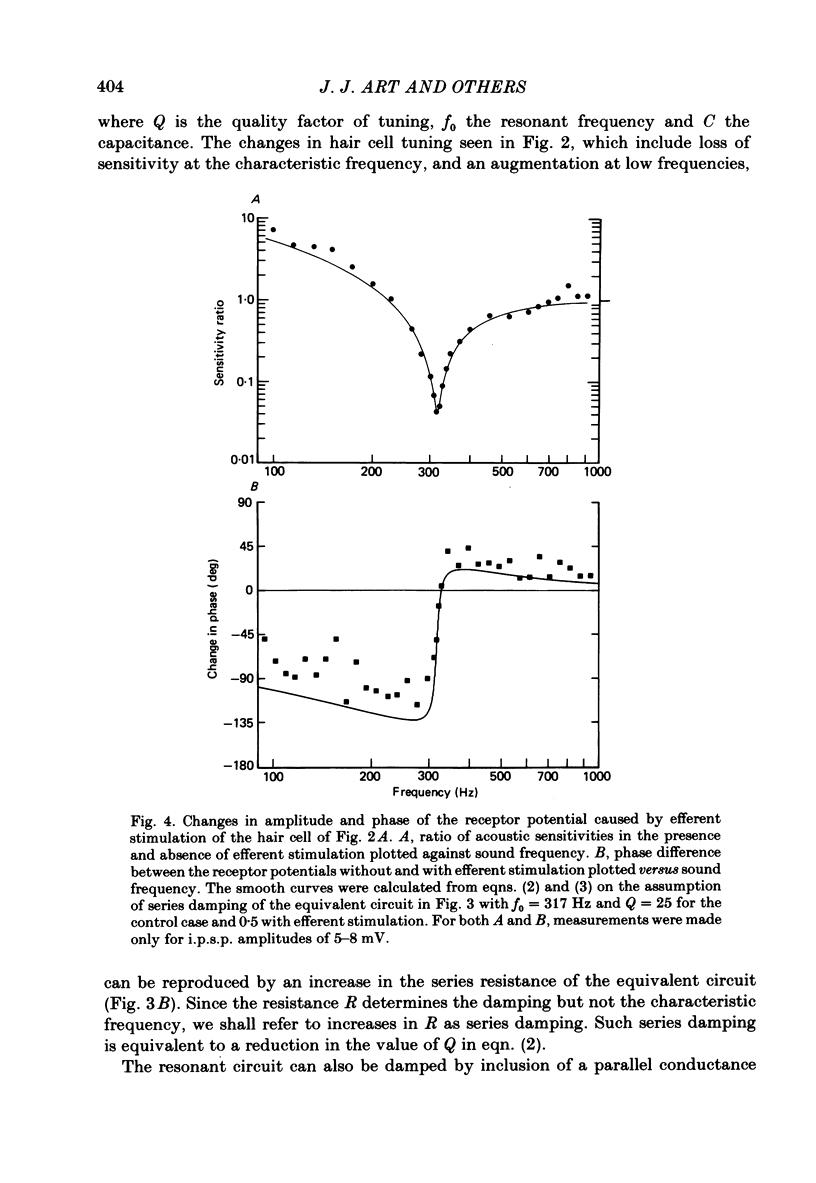
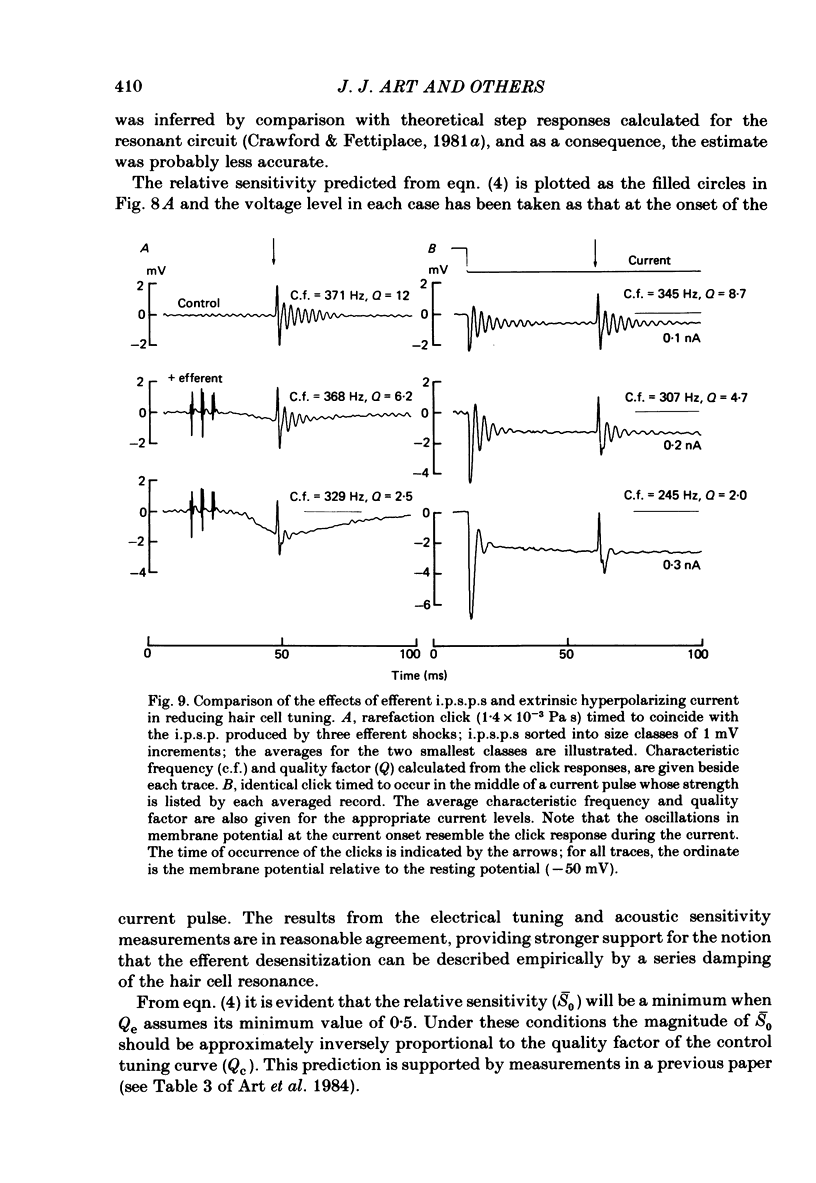
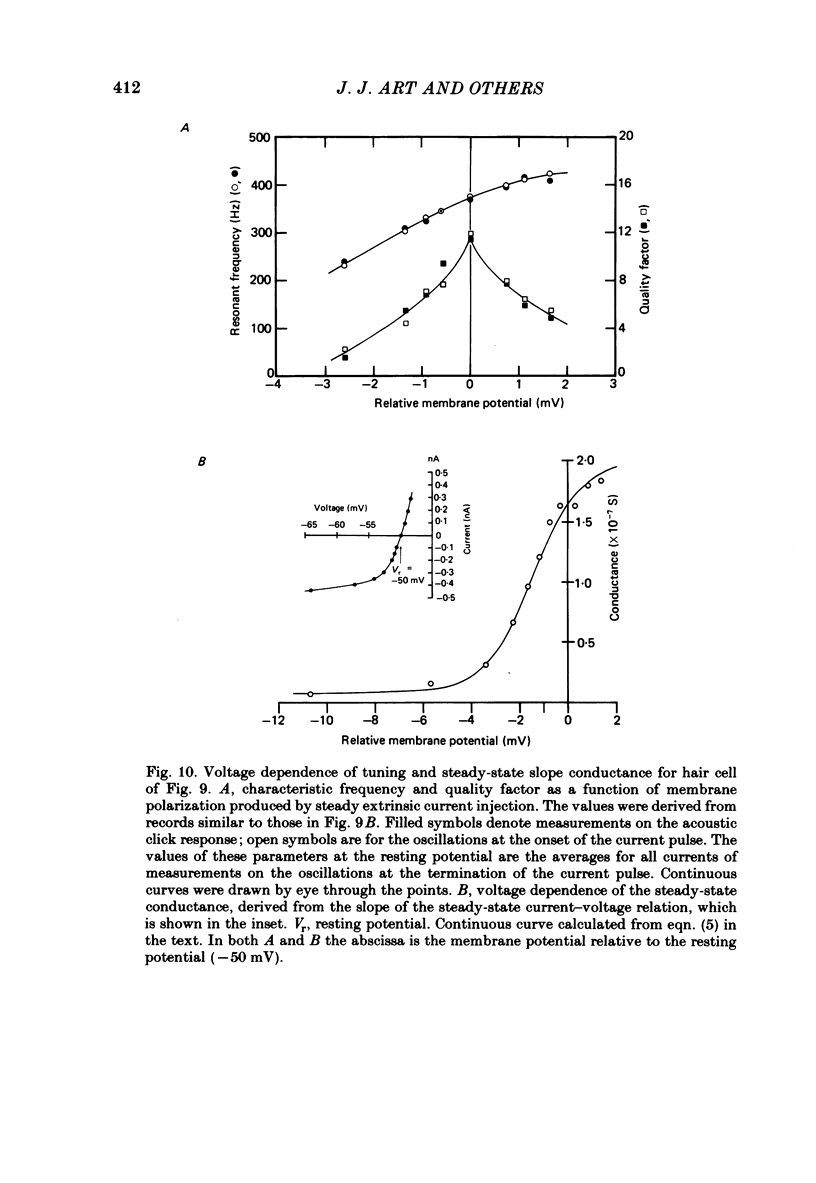
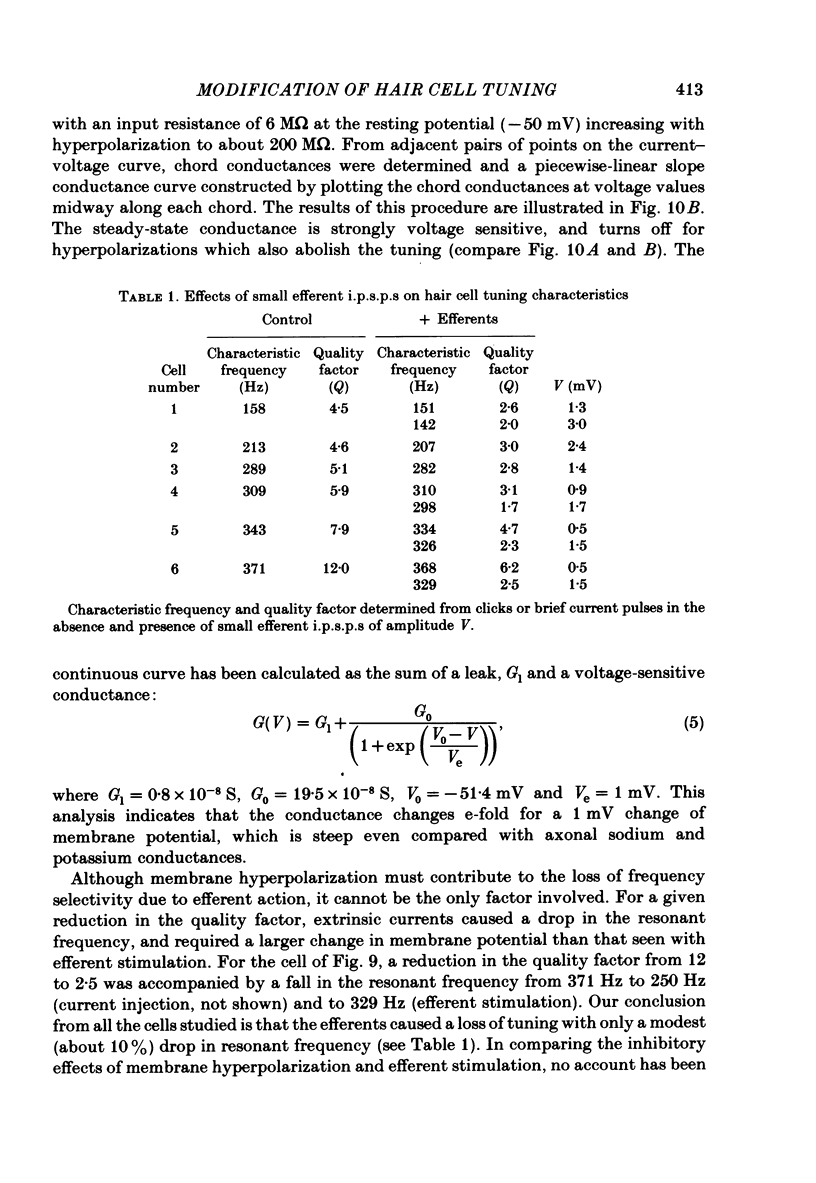
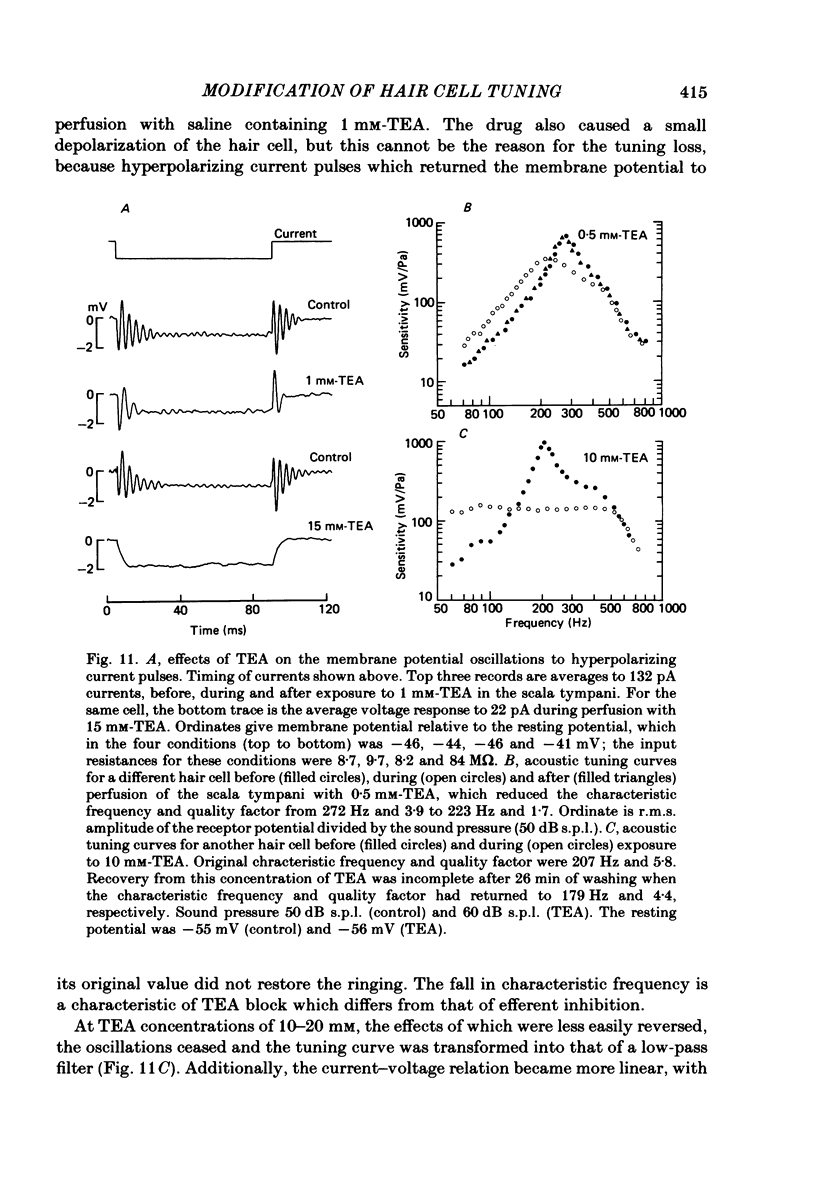
Abstract
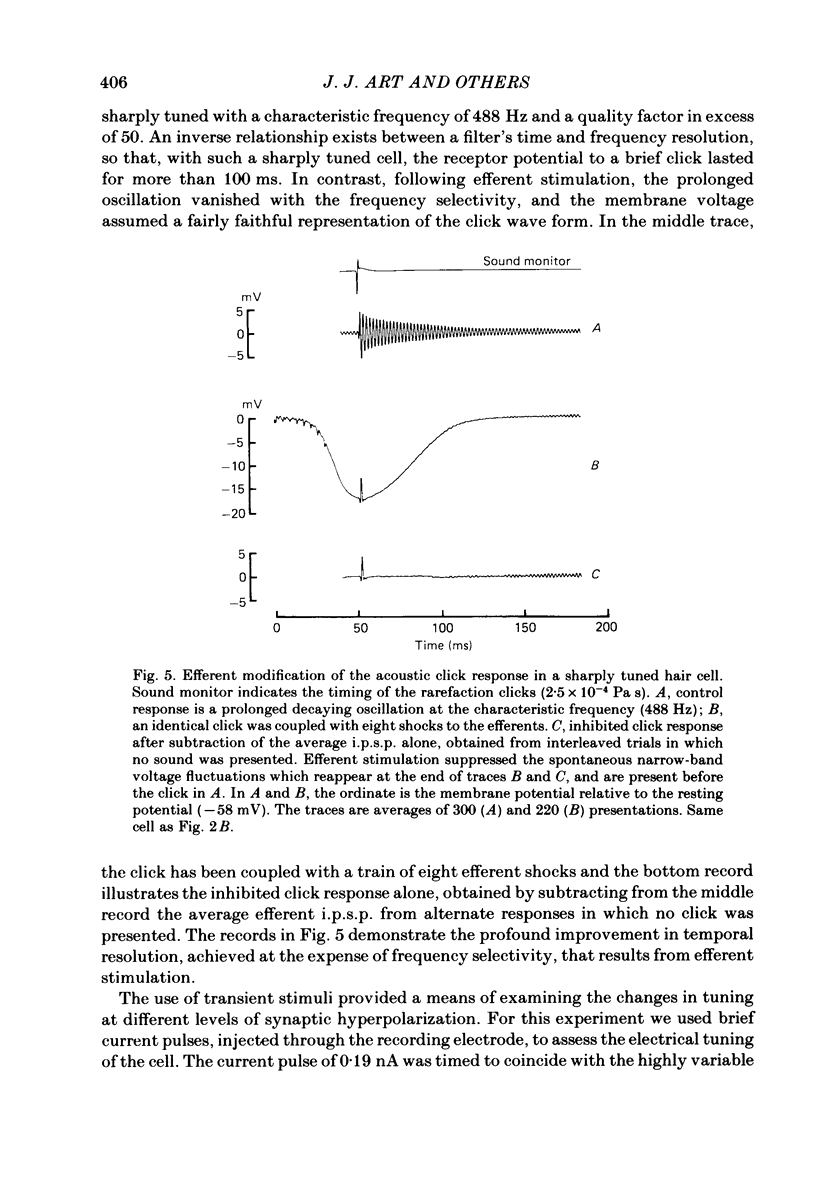
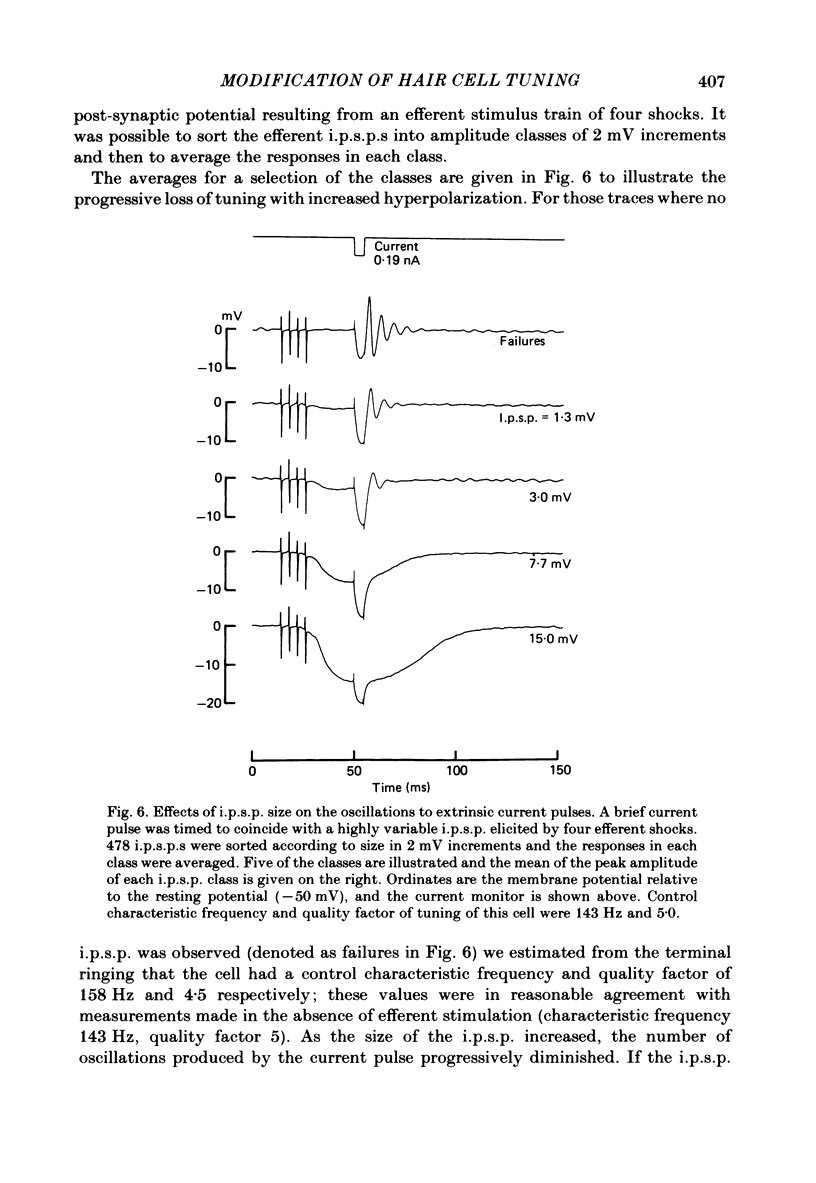
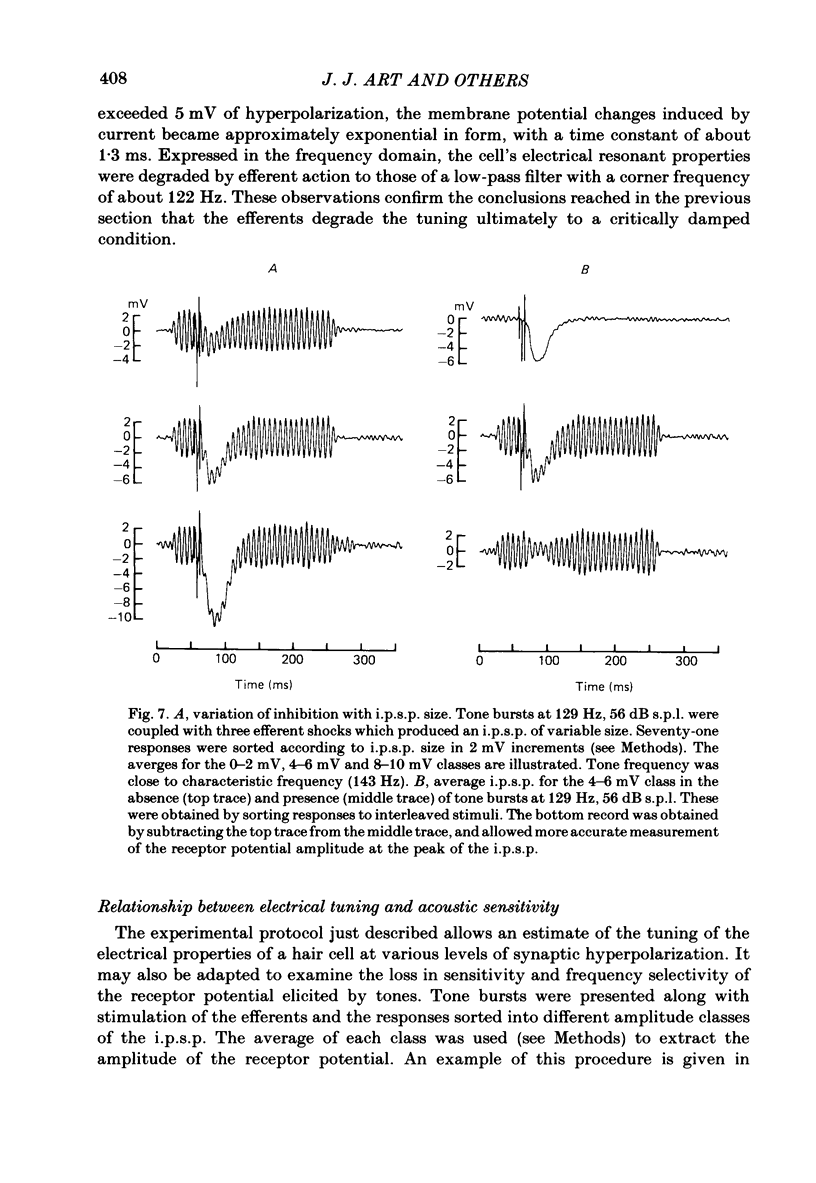
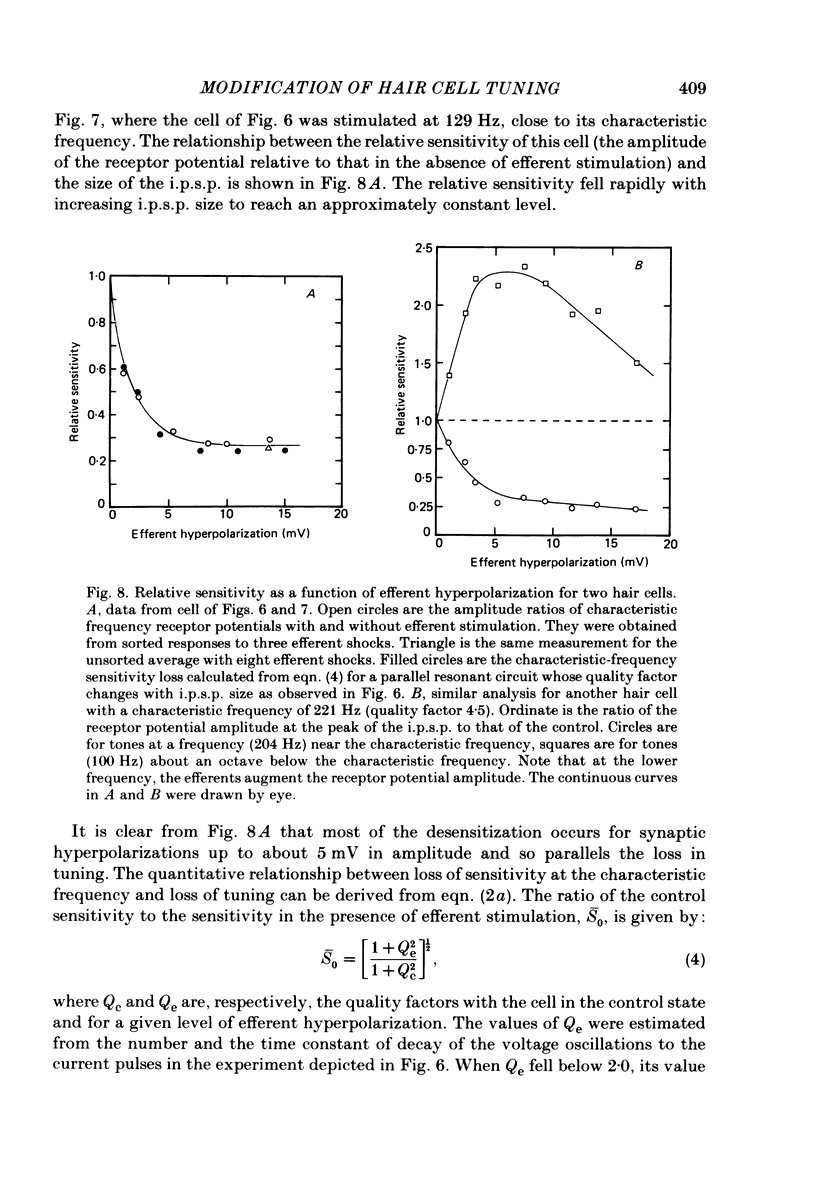
Intracellular recordings were made from turtle cochlear hair cells in order to study the changes in their tuning properties resulting from electrical stimulation of the efferent axons. Efferent stimulation caused a reduction in the amplitude of the receptor potential at the hair cell's most sensitive or characteristic frequency, an increased amplitude at frequencies more than an octave below the characteristic frequency, and no change at very high frequencies. These differential effects resulted in a broadening of each cell's tuning curve, which, during maximal efferent stimulation degenerated from a sharply tuned resonance to a critically damped low-pass filter. Efferent alterations in tuning were also inferred from the oscillations in membrane potential produced by acoustic clicks or extrinsic currents. The quality factor (Q) of tuning, derived from the decay of the oscillations, was progressively reduced with synaptic hyperpolarizations up to about 5 mV in amplitude. A consequence of efferent action was that the wave forms of transient pressure changes were more faithfully encoded as changes in hair cell membrane potential. Hyperpolarization of a hair cell by steady current injection resulted in a lowering of its characteristic frequency and quality factor, and an increase in steady-state resistance. By comparison, for a given reduction in quality factor, efferent stimulation was associated with a smaller change in characteristic frequency. This difference is expected if the resonance is also damped by the shunting action of the synaptic conductance. Perfusion with perilymphs containing 0.5-15 mM of the potassium channel blocker, tetraethylammonium bromide (TEA) reduced the hair cell's frequency selectivity, whether assayed acoustically or with extrinsic currents. Lower TEA concentrations abolished the efferent inhibitory post-synaptic potential with only a minor change in tuning. TEA produced other effects different from efferent stimulation including (i) a lowering of the characteristic frequency, and (ii) a highly asymmetric receptor potential. These observations suggest that the efferents do not simply block membrane conductances associated with tuning. We conclude that the efferent modification of the shape of the tuning curve may be a composite result of the synaptic conductance and the hyperpolarization of the hair cell membrane.
Full text
PDF






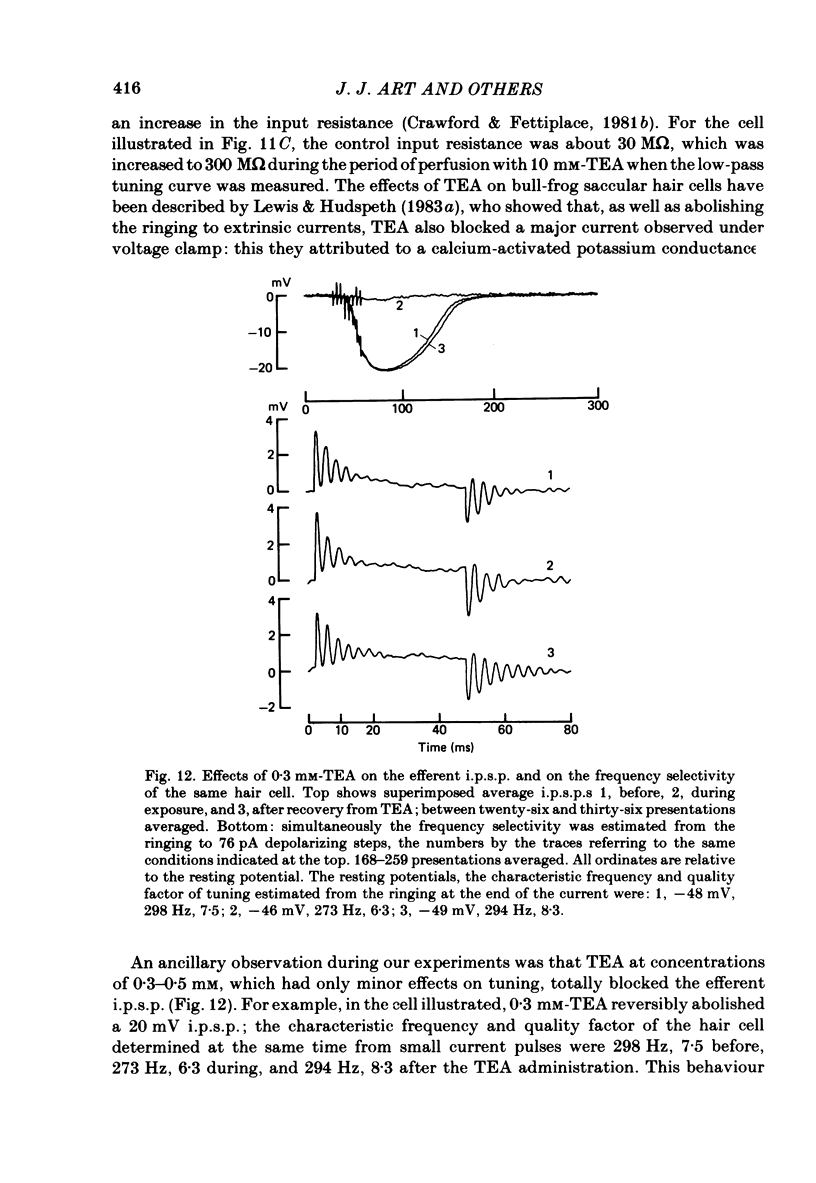
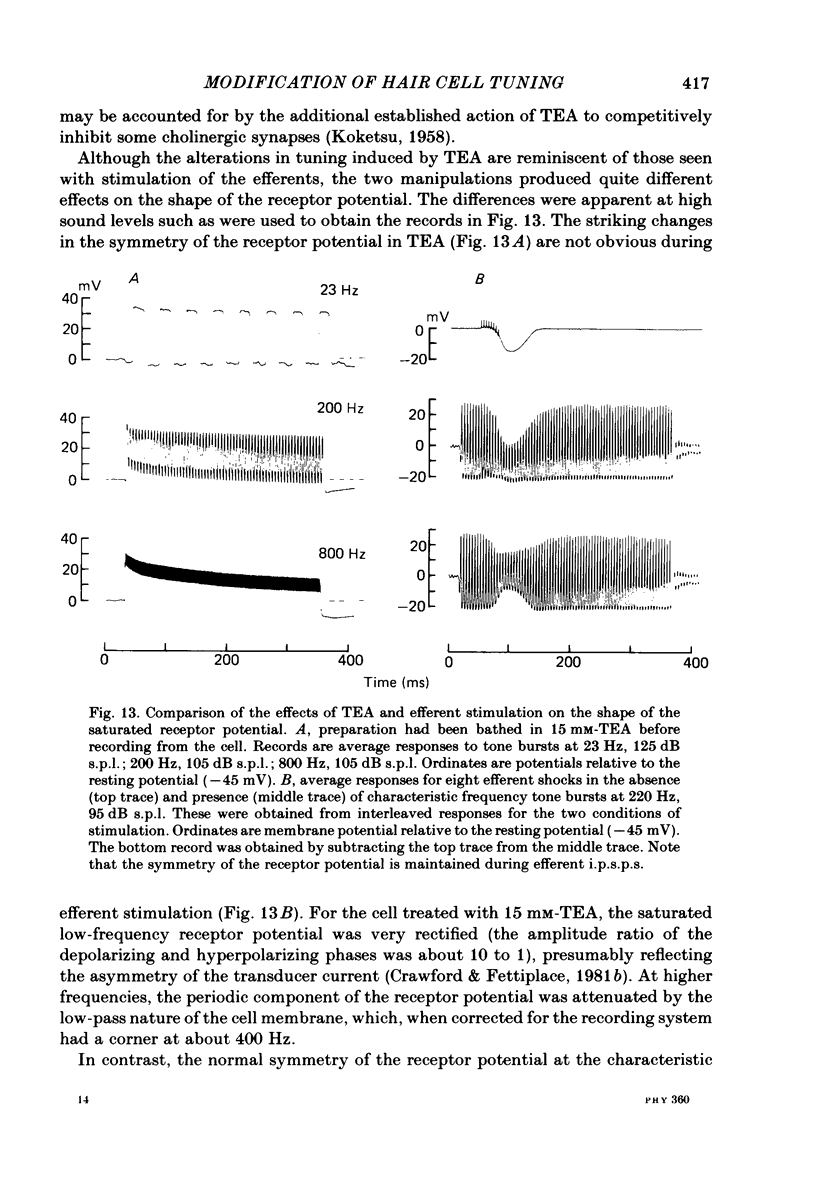




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Art J. J., Crawford A. C., Fettiplace R., Fuchs P. A. Efferent regulation of hair cells in the turtle cochlea. Proc R Soc Lond B Biol Sci. 1982 Oct 22;216(1204):377–384. doi: 10.1098/rspb.1982.0081. [DOI] [PubMed] [Google Scholar]
- Art J. J., Fettiplace R. Efferent desensitization of auditory nerve fibre responses in the cochlea of the turtle Pseudemys scripta elegans. J Physiol. 1984 Nov;356:507–523. doi: 10.1113/jphysiol.1984.sp015480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Art J. J., Fettiplace R., Fuchs P. A. Synaptic hyperpolarization and inhibition of turtle cochlear hair cells. J Physiol. 1984 Nov;356:525–550. doi: 10.1113/jphysiol.1984.sp015481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F. Frequency tuning in a frog vestibular organ. Nature. 1983 Aug 11;304(5926):536–538. doi: 10.1038/304536a0. [DOI] [PubMed] [Google Scholar]
- Corey D. P., Hudspeth A. J. Ionic basis of the receptor potential in a vertebrate hair cell. Nature. 1979 Oct 25;281(5733):675–677. doi: 10.1038/281675a0. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. An electrical tuning mechanism in turtle cochlear hair cells. J Physiol. 1981 Mar;312:377–412. doi: 10.1113/jphysiol.1981.sp013634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The frequency selectivity of auditory nerve fibres and hair cells in the cochlea of the turtle. J Physiol. 1980 Sep;306:79–125. doi: 10.1113/jphysiol.1980.sp013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt J. E., Robertson D. Ionic mechanism of the efferent olivo-cochlear inhibition studied by cochlear perfusion in the cat. J Physiol. 1975 May;247(2):407–428. doi: 10.1113/jphysiol.1975.sp010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fex J. Efferent inhibition in the cochlea related to hair-cell dc activity: study of postsynaptic activity of the crossed olivocochlear fibres in the cat. J Acoust Soc Am. 1967 Mar;41(3):666–675. doi: 10.1121/1.1910395. [DOI] [PubMed] [Google Scholar]
- Flock A., Russell I. Inhibition by efferent nerve fibres: action on hair cells and afferent synaptic transmission in the lateral line canal organ of the burbot Lota lota. J Physiol. 1976 May;257(1):45–62. doi: 10.1113/jphysiol.1976.sp011355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOKETSU K. Action of tetraethylammonium chloride on neuromuscular transmission in frogs. Am J Physiol. 1958 Apr;193(1):213–218. doi: 10.1152/ajplegacy.1958.193.1.213. [DOI] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke R., Galley N. Efferent innervation of vestibular and auditory receptors. Physiol Rev. 1974 Apr;54(2):316–357. doi: 10.1152/physrev.1974.54.2.316. [DOI] [PubMed] [Google Scholar]
- Lewis R. S., Hudspeth A. J. Voltage- and ion-dependent conductances in solitary vertebrate hair cells. Nature. 1983 Aug 11;304(5926):538–541. doi: 10.1038/304538a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T. F. Bidirectional transduction in vertebrate hair cells: a mechanism for coupling mechanical and electrical processes. Hear Res. 1982 Aug;7(3):353–360. doi: 10.1016/0378-5955(82)90045-4. [DOI] [PubMed] [Google Scholar]



