Abstract
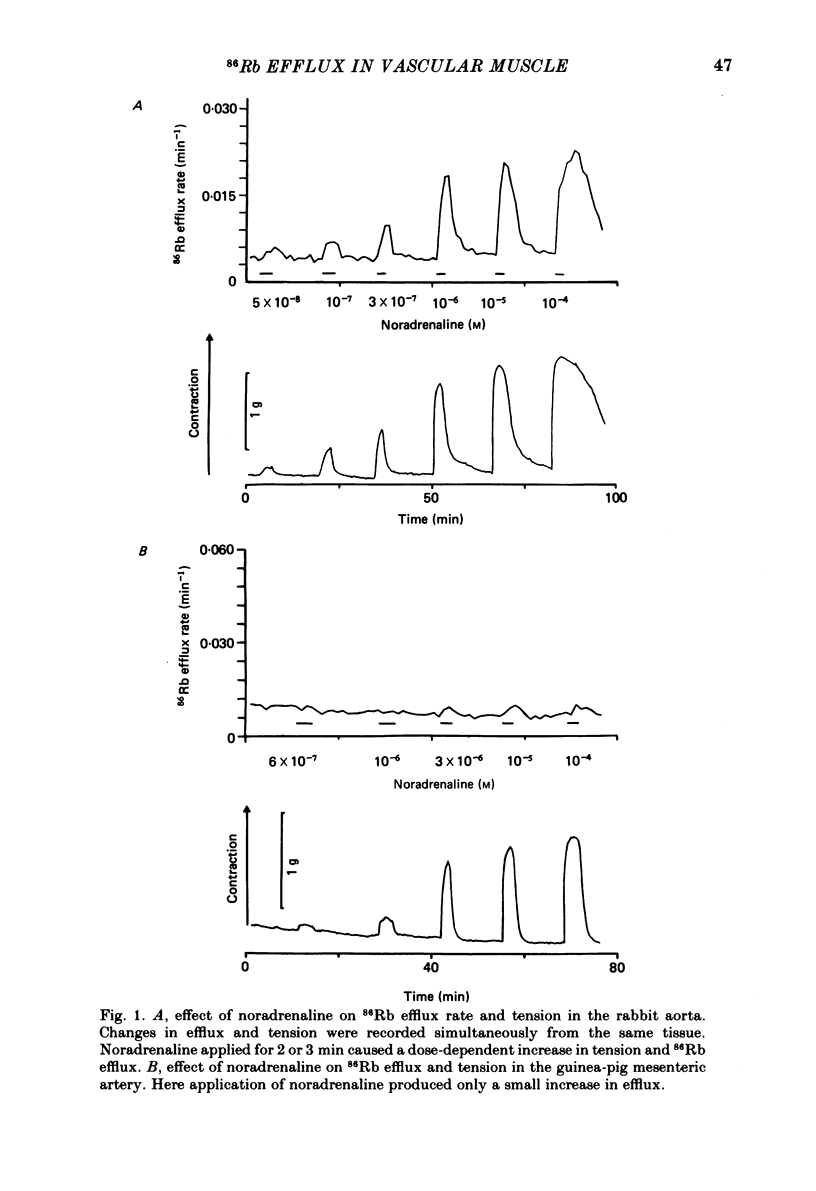
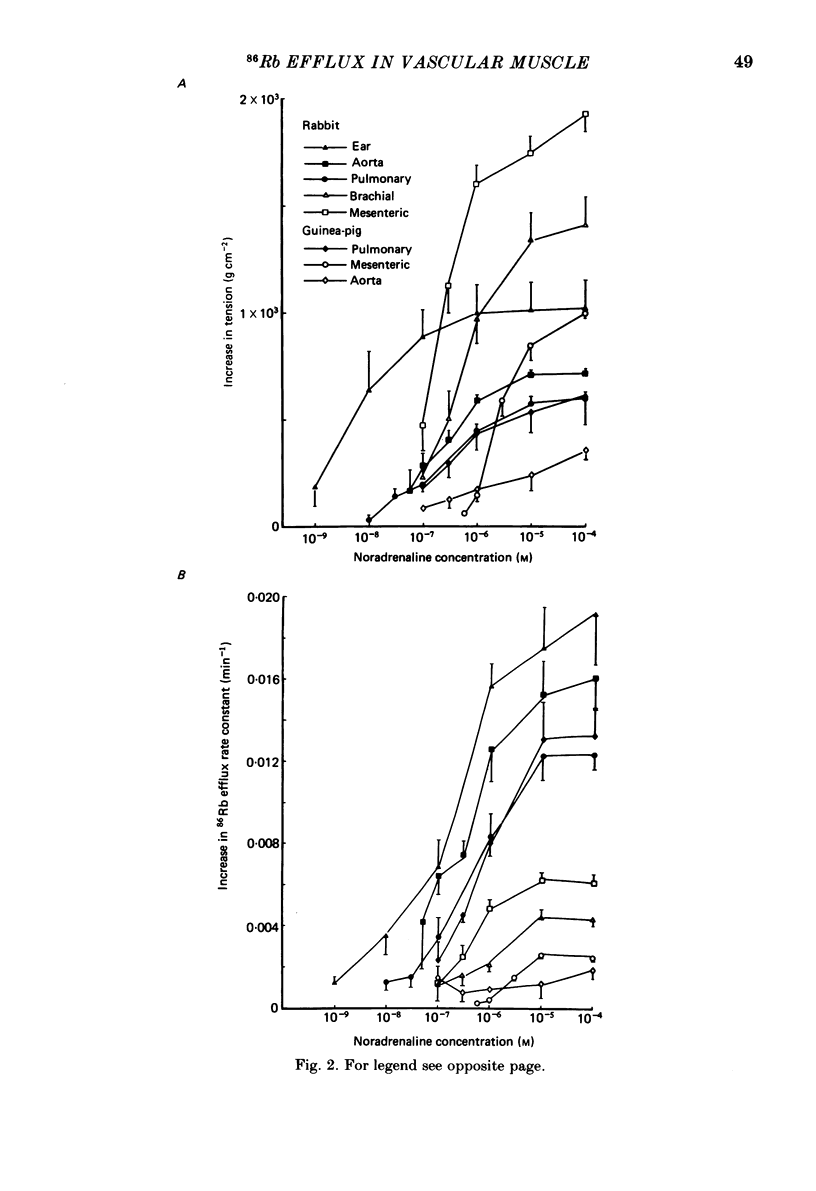
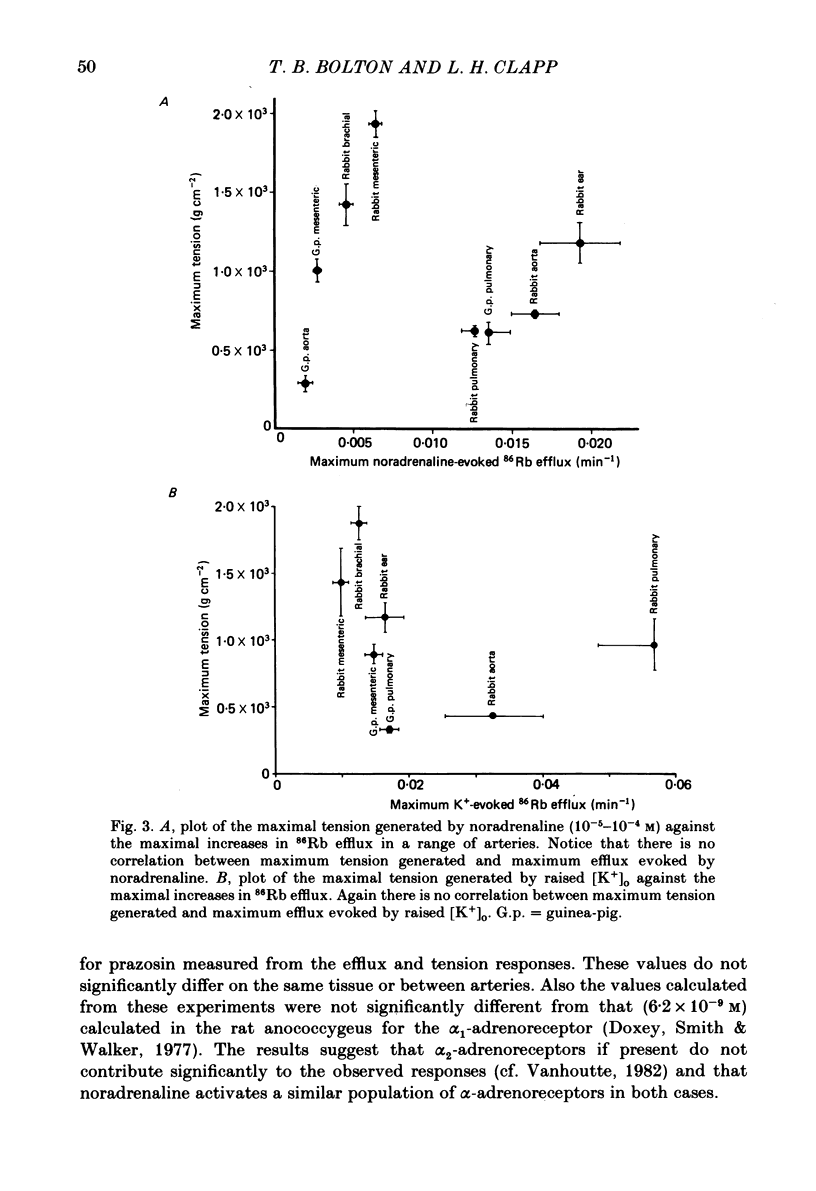
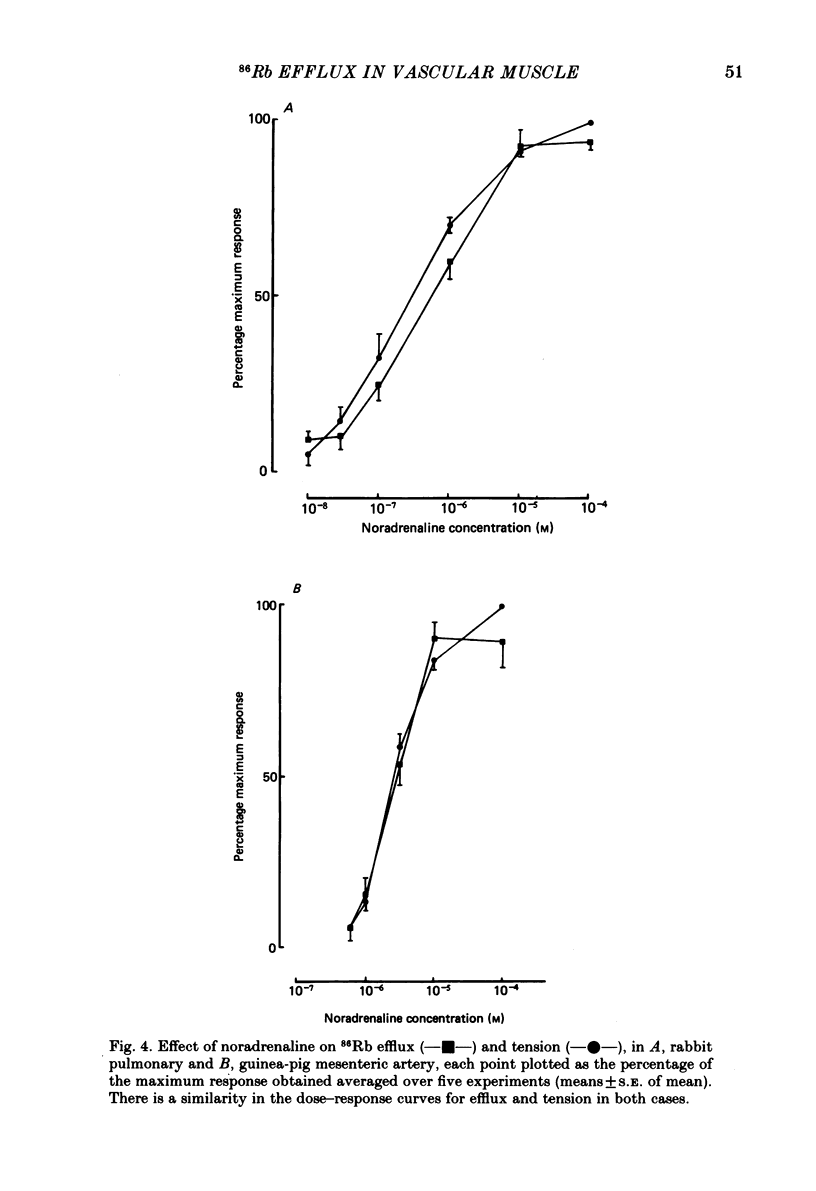
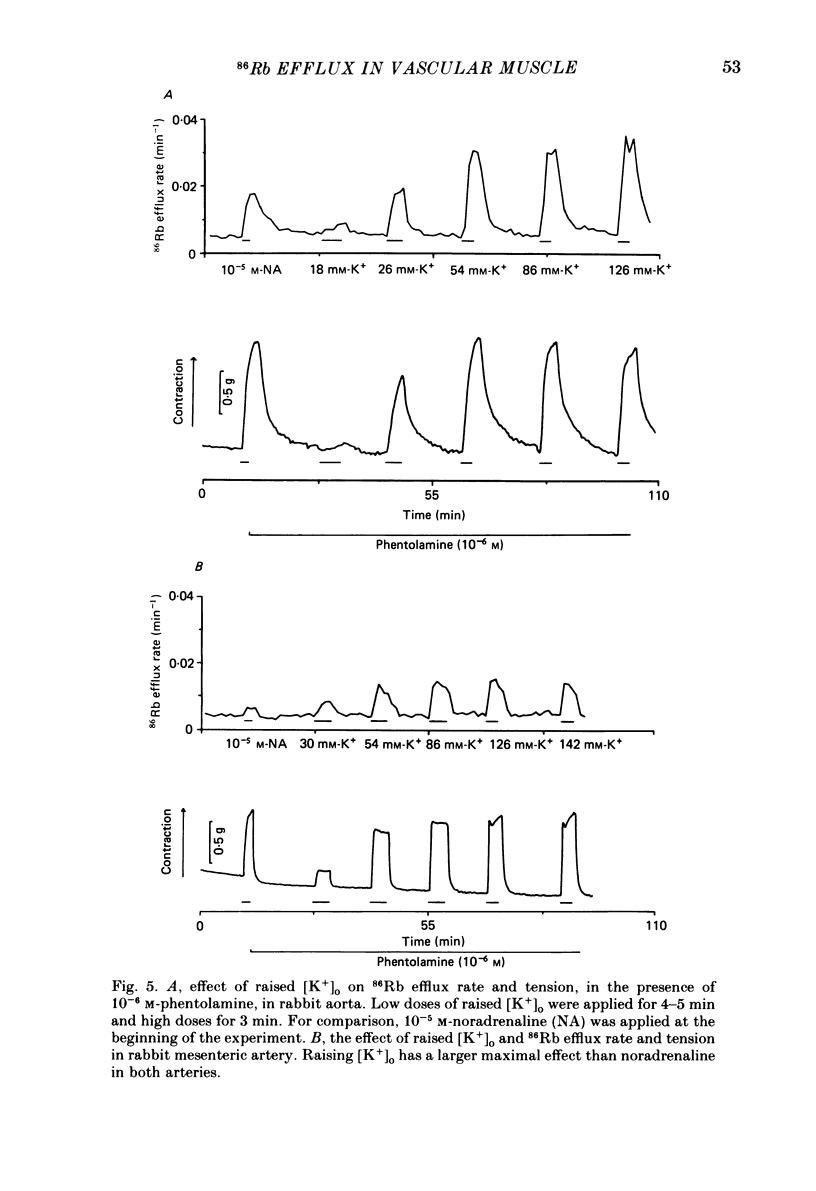
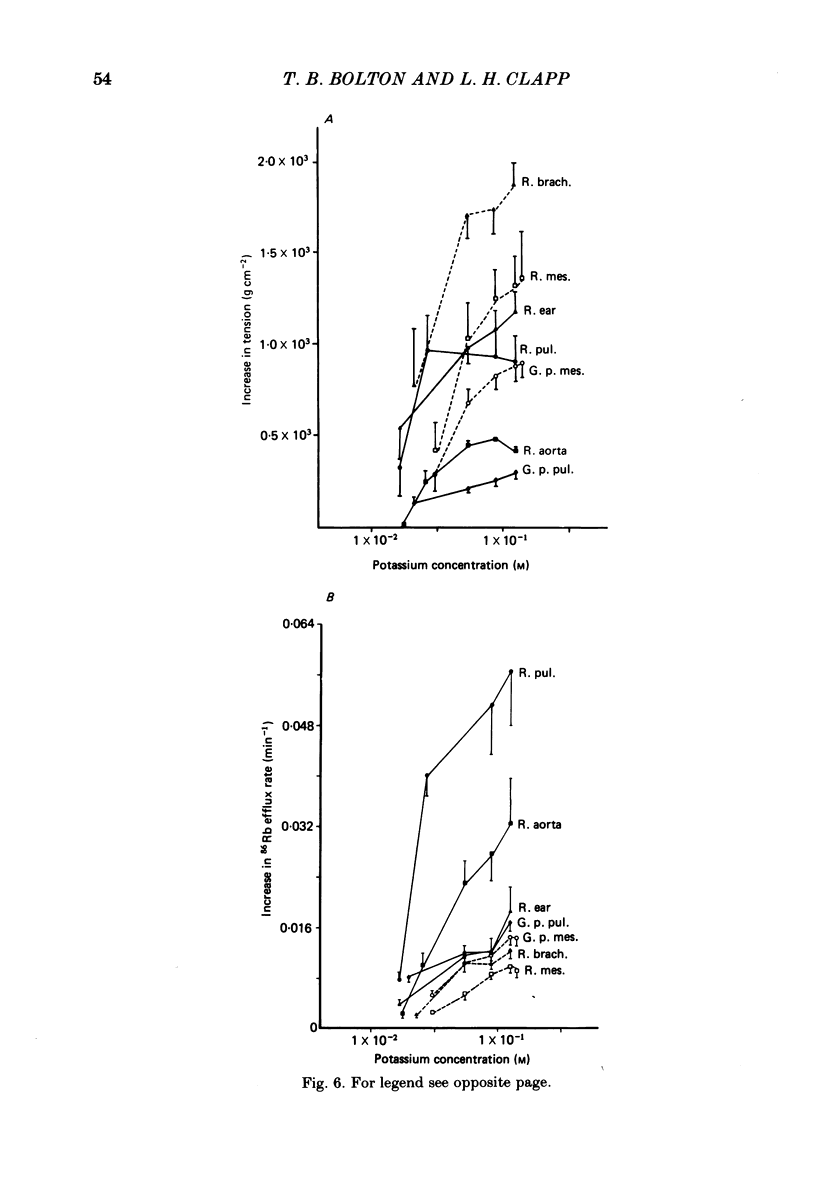
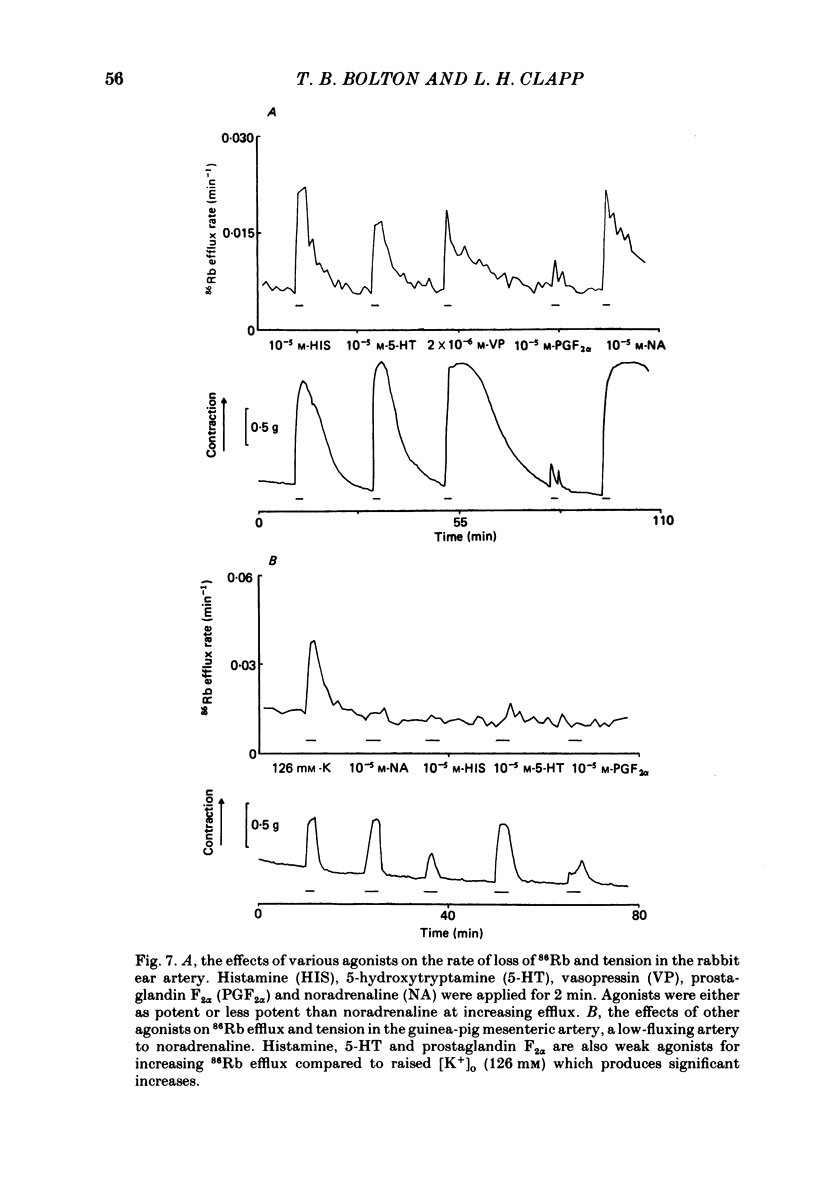
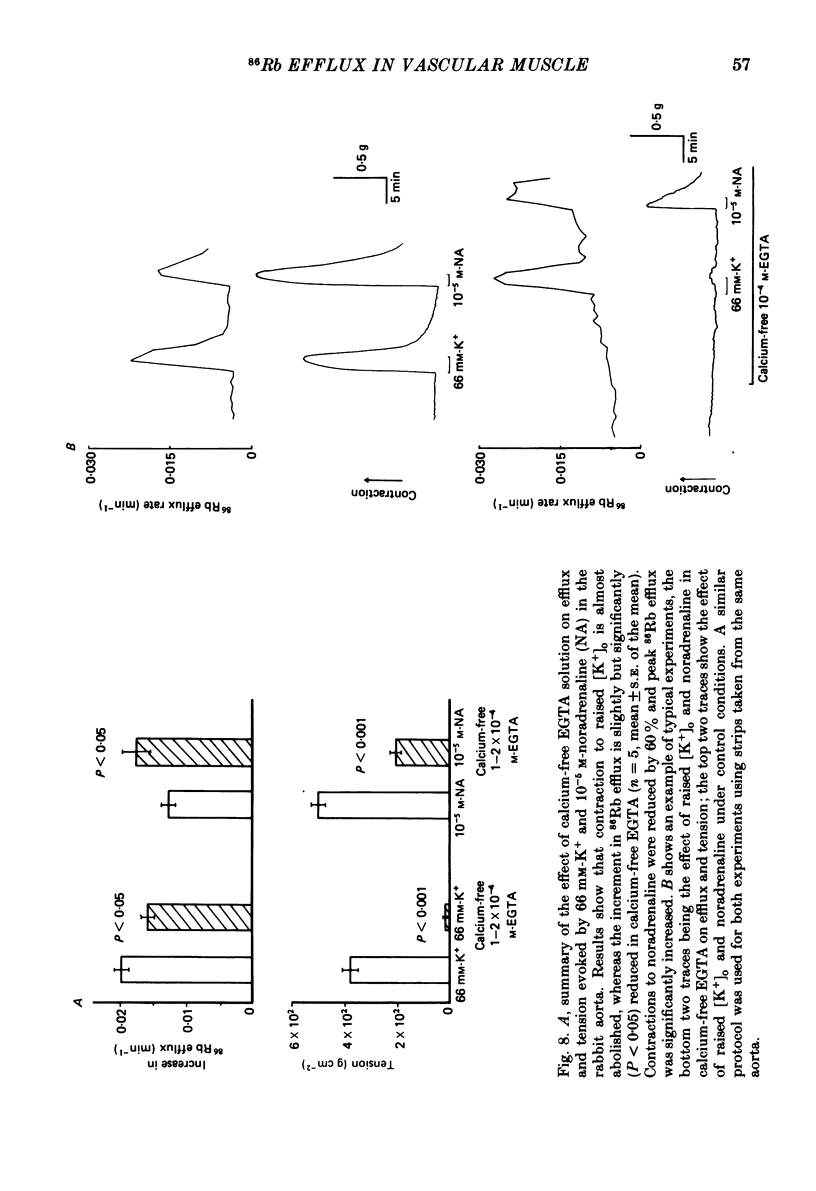
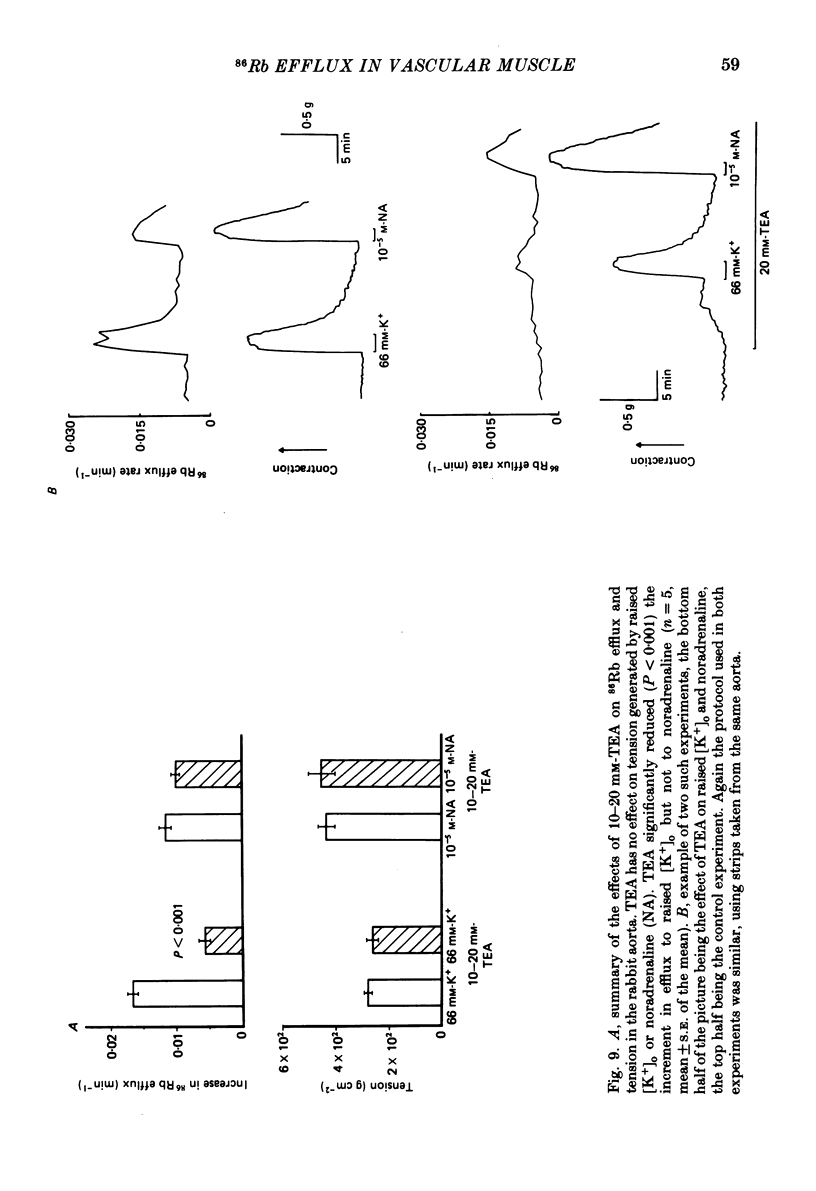
The effects of noradrenaline and of raised external potassium ([K+]o) on the efflux of 86Rb or 42K and on tension were studied in preparations taken from eight different arteries under various conditions. There was a 10-fold variation in the maximum 86Rb efflux evoked by noradrenaline (10(-5)-10(-4) M) in the arteries studied, even though tension generated was comparable. Arterial contractions were either accompanied by large increases in 86Rb efflux, e.g. rabbit ear artery and aorta, guinea-pig and rabbit pulmonary artery, or by small increases, e.g. rabbit and guinea-pig mesenteric artery, rabbit brachial artery and guinea-pig abdominal aorta. Raising [K+]o also had a diverse effect on 86Rb and 42K efflux: arteries giving small increases in efflux to noradrenaline also gave small increases in efflux to raised [K+]o. The maximum efflux evoked by raised [K+]o was on average three times greater than the maximum efflux evoked by noradrenaline in the arteries studied. The heterogeneity of the efflux response could not be explained by the quantitative heterogeneity of the efflux response could not be explained by the quantitative differences in the effects of noradrenaline or of raised [K+]o on membrane potential or, in the case of noradrenaline, by differences in the alpha-receptors. In arteries in which the noradrenaline-evoked 86Rb efflux was small, histamine, 5-hydroxytryptamine, vasopressin and angiotensin also had little effect. Conversely, where noradrenaline produced a large increase in 86Rb efflux those other stimulants had comparable effects. Removal of extracellular calcium only slightly reduced the increment in 86Rb efflux evoked by 66 mM-external K+ in the rabbit aorta even though contractions were virtually abolished under these conditions. In the case of 10(-5) M-noradrenaline, 40% of the contraction remained and its effect on efflux was significantly increased (P less than 0.05) in calcium-free conditions. Essentially similar results were obtained using 42K. Tetraethylammonium (10-20 mM) produced a significant and substantial reduction (P less than 0.001) in the 86Rb efflux evoked by raised [K+]o while only slightly affecting the noradrenaline-evoked efflux in the rabbit aorta. It was concluded from these efflux experiments on vascular muscle that the channels through which potassium can escape, opened by depolarization and by activation of alpha-receptors with noradrenaline, are from different populations, and that their properties vary from one artery to another. We have been unable to detect any substantial calcium-activated component in 42K or 86Rb efflux responses to raised [K+]o or to noradrenaline.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohr D. F. Vascular smooth muscle updated. Circ Res. 1973 Jun;32(6):665–672. doi: 10.1161/01.res.32.6.665. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Cambridge D., Davey M. J., Massingham R. Prazosin, a selective antagonist of post-synaptic alpha-adrenoceptors [proceedings]. Br J Pharmacol. 1977 Mar;59(3):514P–515P. [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey J. C., Smith C. F., Walker J. M. Selectivity of blocking agents for pre-and postsynaptic alpha-adrenoceptors. Br J Pharmacol. 1977 May;60(1):91–96. doi: 10.1111/j.1476-5381.1977.tb16752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew G. M., Whiting S. B. Evidence for two distinct types of postsynaptic alpha-adrenoceptor in vascular smooth muscle in vivo. Br J Pharmacol. 1979 Oct;67(2):207–215. doi: 10.1111/j.1476-5381.1979.tb08668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler G. Relationship between noradrenaline-induced depolarization and contraction in vascular smooth muscle. Blood Vessels. 1978;15(1-3):46–54. doi: 10.1159/000158152. [DOI] [PubMed] [Google Scholar]
- Haeusler G., Thorens S. Effects of tetraethylammonium chloride on contractile, membrane and cable properties of rabbit artery muscle. J Physiol. 1980 Jun;303:203–224. doi: 10.1113/jphysiol.1980.sp013281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler G. alpha-Adrenoceptor mediated contractile and electrical responses of vascular smooth muscle. J Cardiovasc Pharmacol. 1982;4 (Suppl 1):S97–100. doi: 10.1097/00005344-198200041-00020. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Watanabe M. The effect of tetraethylammonium chloride on potassium permeability in the smooth muscle cell membrane of canine trachea. J Physiol. 1981 Jul;316:33–46. doi: 10.1113/jphysiol.1981.sp013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima T. Effects of vasopressin on smooth muscle cells of guinea-pig mesenteric vessels. Br J Pharmacol. 1981 Apr;72(4):673–684. doi: 10.1111/j.1476-5381.1981.tb09148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Mechanical response with reversed electrical response to noradrenaline by Ca-deprived arterial smooth muscle. J Physiol. 1972 Jul;224(1):21–34. doi: 10.1113/jphysiol.1972.sp009879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Wakade A. R. Release of noradrenaline from the cat spleen by potassium. J Physiol. 1968 Feb;194(3):595–608. doi: 10.1113/jphysiol.1968.sp008427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. The effects of acetylcholine on the membrane and contractile properties of smooth muscle cells of the rabbit superior mesenteric artery. Br J Pharmacol. 1978 Dec;64(4):493–501. doi: 10.1111/j.1476-5381.1978.tb17310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Studies of the electrical excitability of aorta smooth muscle of rabbit. J Physiol. 1979 Aug;293:11–21. doi: 10.1113/jphysiol.1979.sp012876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- SU C., BEVAN J. A., URSILLO R. C. ELECTRICAL QUIESCENCE OF PULMONARY ARTERY SMOOTH MUSCLE DURING SYMPATHOMIMETIC STIMULATION. Circ Res. 1964 Jul;15:26–27. doi: 10.1161/01.res.15.1.20. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Starke K., Endo T., Taube H. D. Relative pre- and postsynaptic potencies of alpha-adrenoceptor agonists in the rabbit pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(1):55–78. doi: 10.1007/BF00510821. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Effects of endogenous and exogenous noradrenaline on the smooth muscle of guinea-pig mesenteric vein. J Physiol. 1981 Dec;321:495–512. doi: 10.1113/jphysiol.1981.sp013999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans P. B., Kwa H. Y., van Zwieten P. A. Possible subdivision of postsynaptic alpha-adrenoceptors mediating pressor responses in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):189–193. doi: 10.1007/BF00500284. [DOI] [PubMed] [Google Scholar]
- Trapani A., Matsuki N., Abel P. W., Hermsmeyer K. Norepinephrine produces tension through electromechanical coupling in rabbit ear artery. Eur J Pharmacol. 1981 Jun 10;72(1):87–91. doi: 10.1016/0014-2999(81)90301-0. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M. Heterogeneity of postjunctional vascular alpha-adrenoceptors and handling of calcium. J Cardiovasc Pharmacol. 1982;4 (Suppl 1):S91–S96. doi: 10.1097/00005344-198200041-00019. [DOI] [PubMed] [Google Scholar]


