Abstract
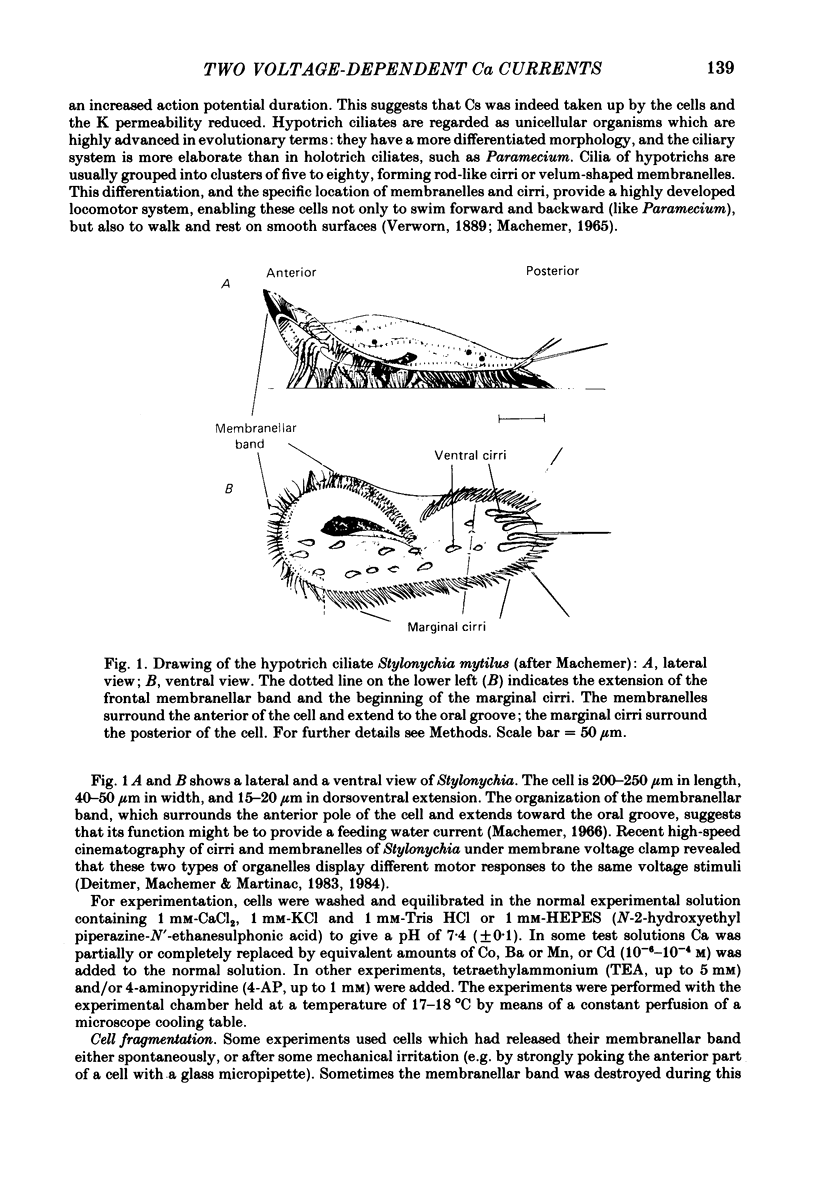
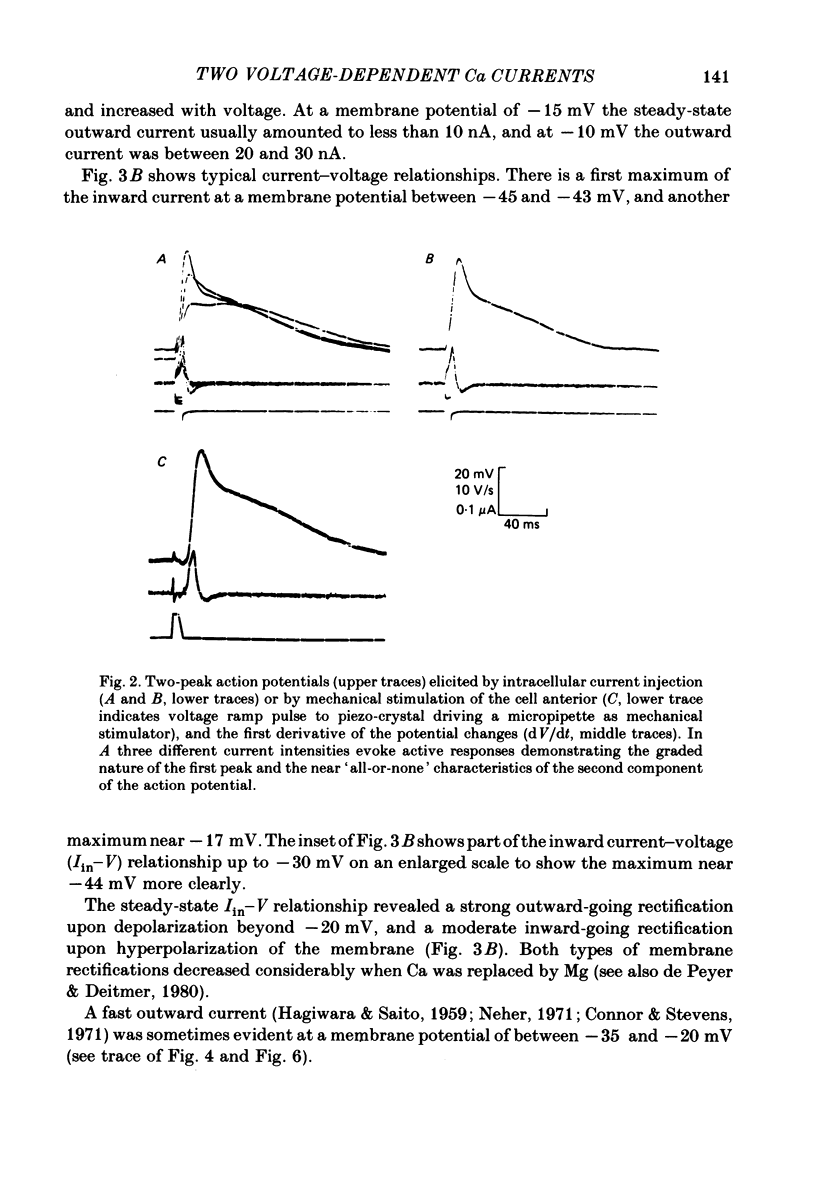
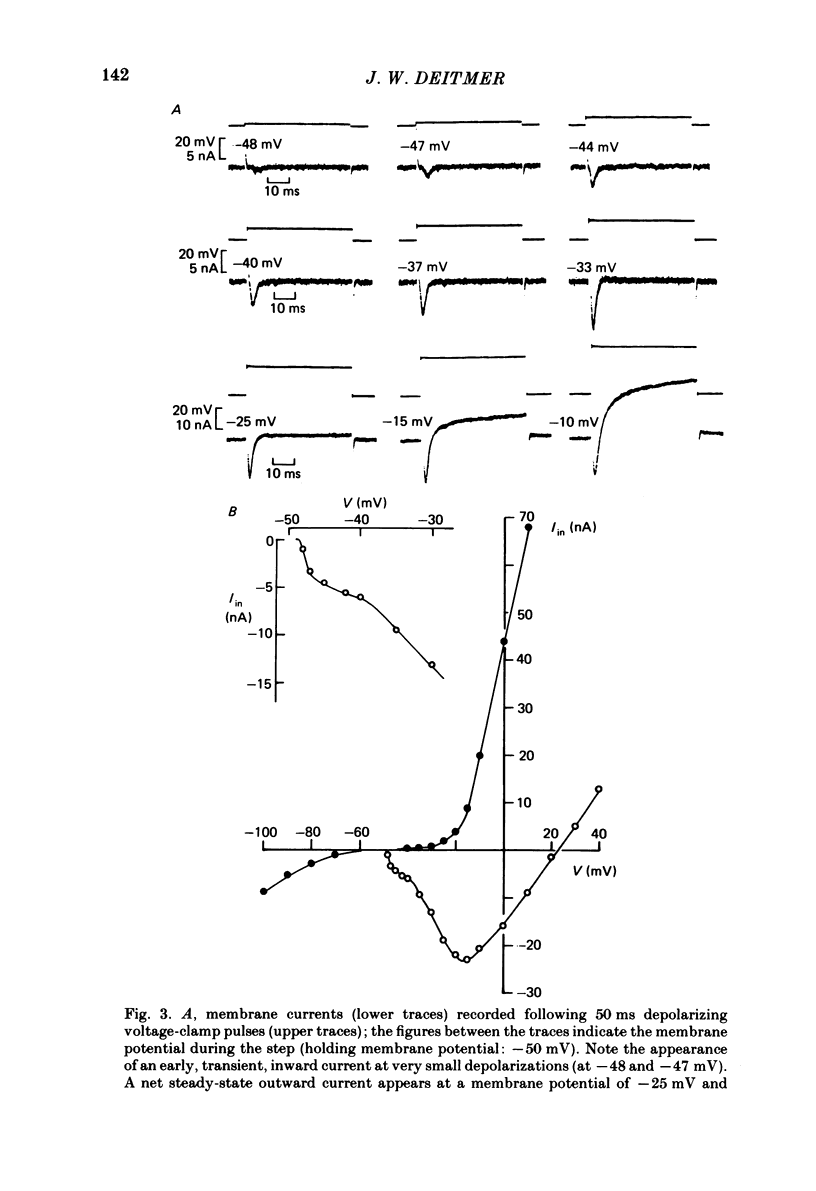
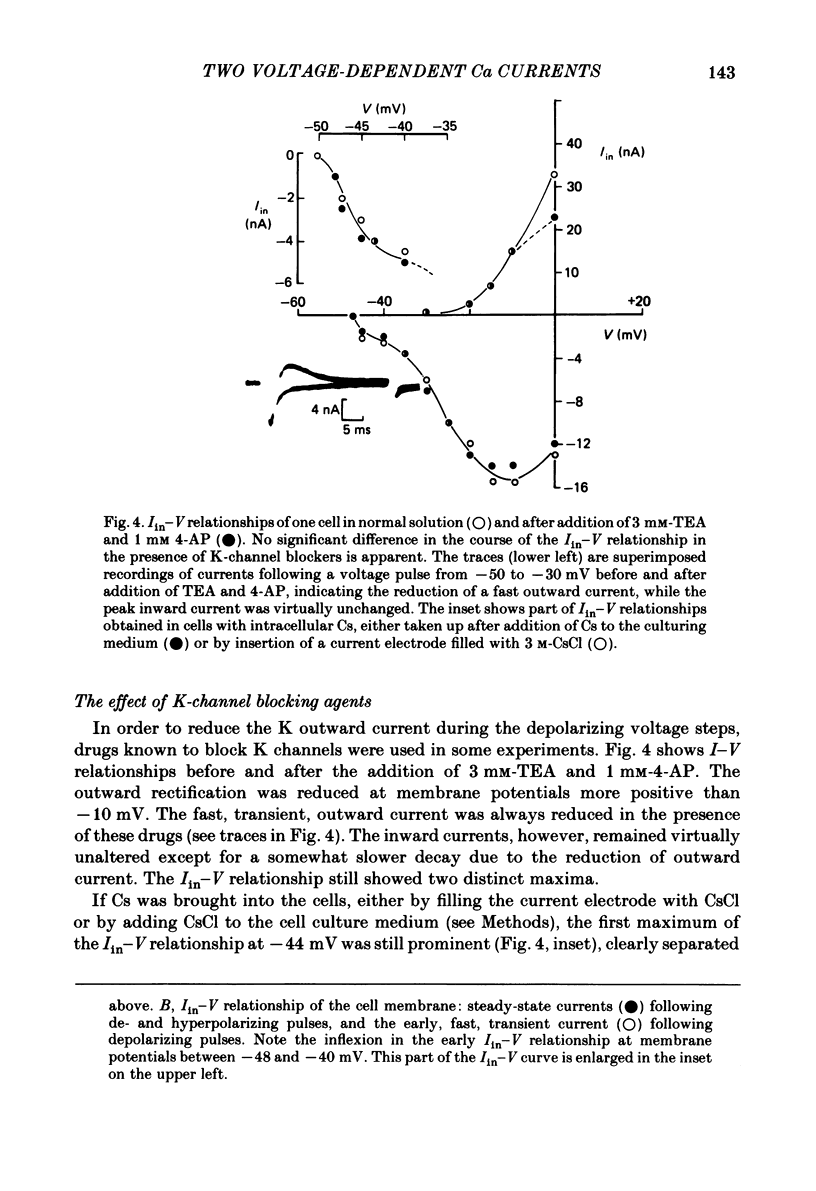
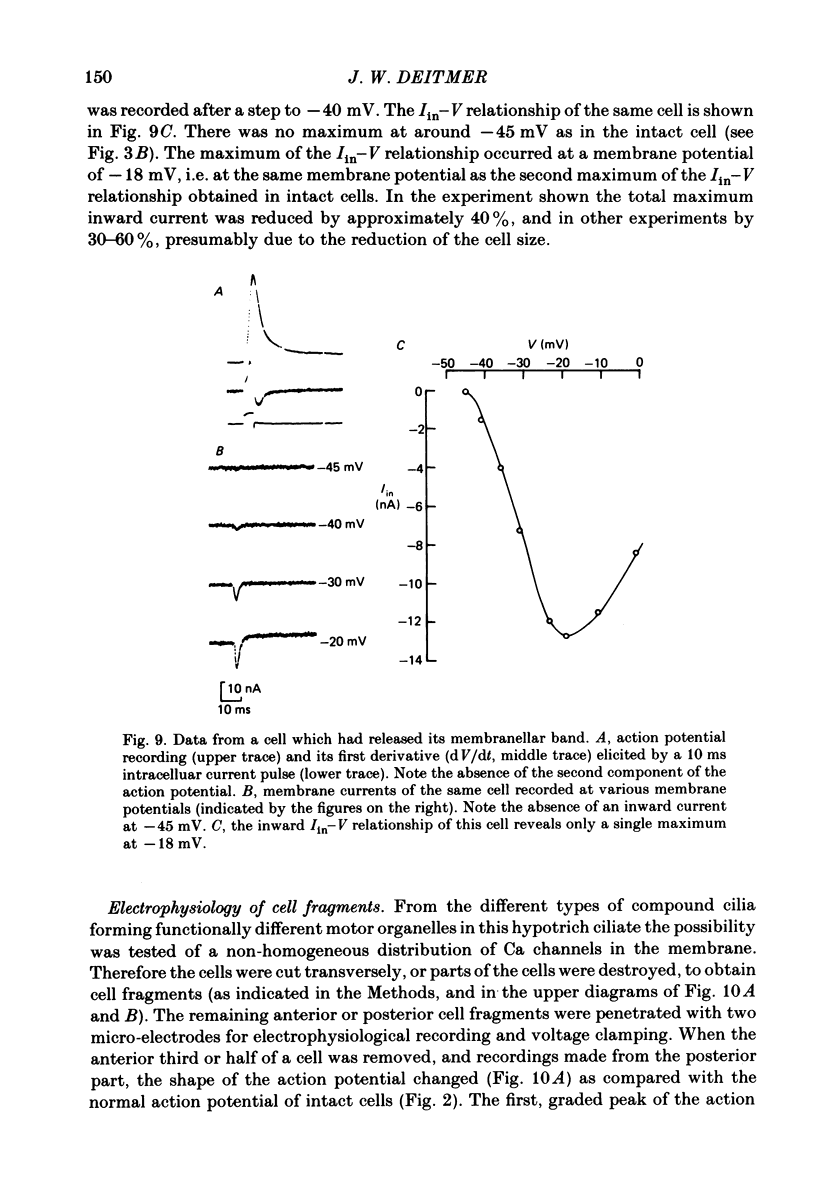
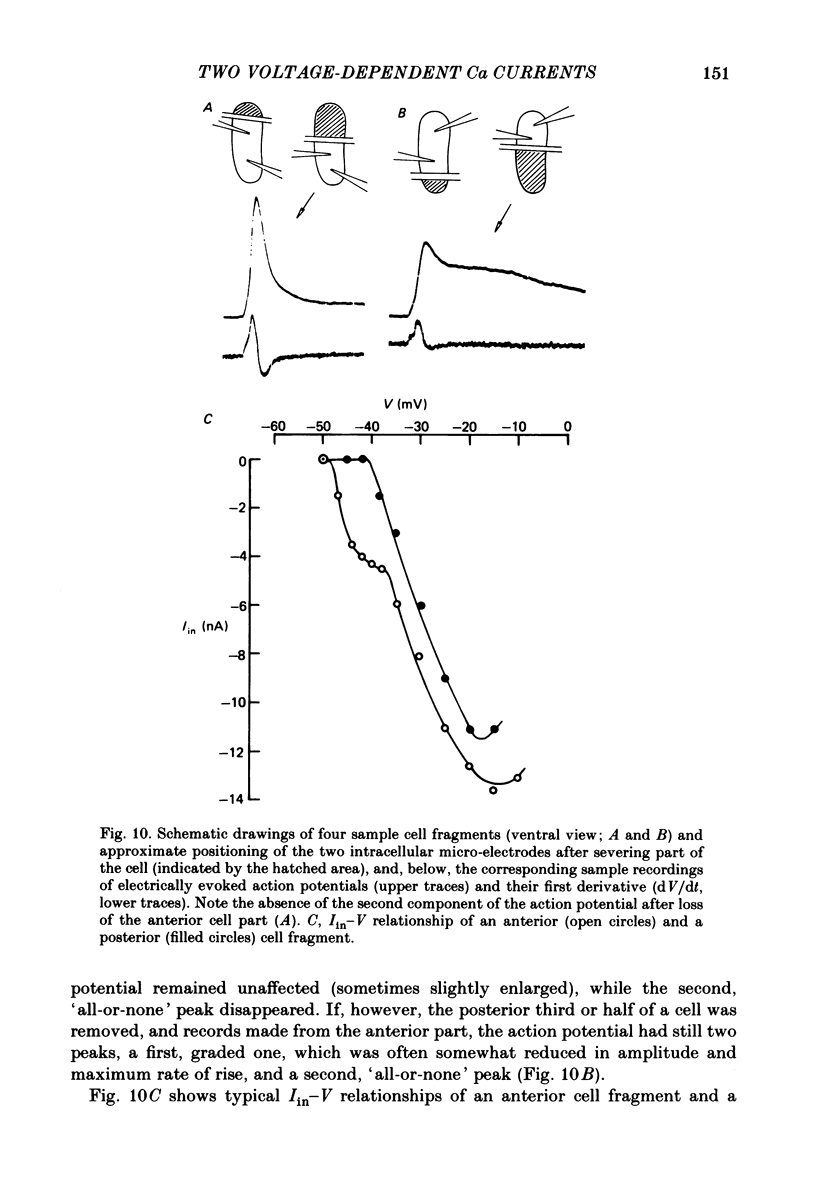
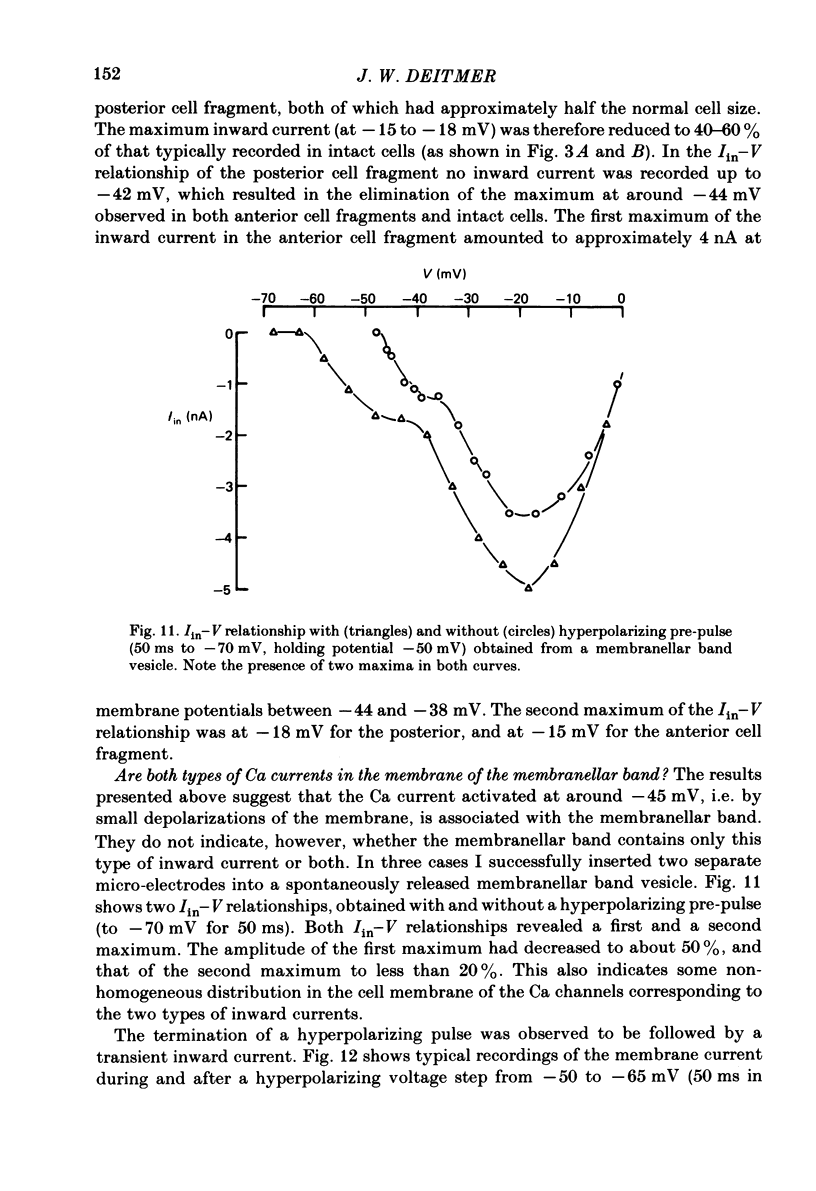
Action potentials and voltage-dependent membrane currents have been investigated in the fresh-water hypotrich ciliate Stylonychia mytilus, using two intracellular micro-electrodes. The inward current-voltage (Iin-V) relationship has two maxima, the first around -45 mV, and the second around -17 mV (resting and holding membrane potential being -50 mV). The shape of the Iin-V relationship is virtually unaltered in the presence of the K-channel blockers tetraethylammonium, 4-aminopyridine or internal Cs. The inward currents exhibit a differential sensitivity to both external CO and Cd; the inward current activated at potentials greater than or equal to -40 mV is more sensitive to these divalent cations than the inward current activated at around -45 mV. This suggests the presence of two different types of Ca inward currents. Both types of inward currents are present when Ca is replaced by Ba (or Sr). The small inward current recorded between -48 and -40 mV relaxes similarly in Ca and in Ba solutions. The larger inward current, recorded at -30 or -20 mV, relaxes rapidly in Ca solution but only slowly and incompletely in Ba solution. A two-pulse protocol revealed that for both types of inward currents inactivation may depend partially upon the influx and/or intracellular accumulation of the charge-carrying divalent cation. There appears to be a significant difference in the degree of inactivation of the two types of inward currents, however, when Ba is the charge carrier. When the cell spontaneously released, or was induced to release its membranellar band (row of compound cilia), the second, "all-or-none' component of the action potential, and the maximum of the Iin-V relationship at -45 mV disappeared. The first, graded peak of the action potential and the larger maximum of the Iin-V relationship remained essentially unaltered. The smaller Ca current and the action potential shoulder also disappeared when the anterior half of the cell (with most of the membranellar band) was severed, but not when the posterior half was cut off. When recording from a membranellar band vesicle both types of inward currents were present. The results suggest that the two components of the action potential may correspond to the two types of Ca currents. These Ca currents are separable by their localization in the membrane. The smaller Ca current appears to be restricted to the membranellar band.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Stanfield P. R. Calcium inactivation in skeletal muscle fibres of the stick insect, Carausius morosus. J Physiol. 1982 Sep;330:349–372. doi: 10.1113/jphysiol.1982.sp014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Brehm P., Eckert R., Tillotson D. Calcium-mediated inactivation of calcium current in Paramecium. J Physiol. 1980 Sep;306:193–203. doi: 10.1113/jphysiol.1980.sp013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad J., Eckert R., Ewald D. Kinetics of calcium-dependent inactivation of calcium current in voltage-clamped neurones of Aplysia californica. J Physiol. 1984 Feb;347:279–300. doi: 10.1113/jphysiol.1984.sp015066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Localization of calcium channels in Paramecium caudatum. J Physiol. 1977 Sep;271(1):119–133. doi: 10.1113/jphysiol.1977.sp011993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Eckert R., Brehm P. Ionic mechanisms of excitation in Paramecium. Annu Rev Biophys Bioeng. 1979;8:353–383. doi: 10.1146/annurev.bb.08.060179.002033. [DOI] [PubMed] [Google Scholar]
- Fox A. P. Voltage-dependent inactivation of a calcium channel. Proc Natl Acad Sci U S A. 1981 Feb;78(2):953–956. doi: 10.1073/pnas.78.2.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Voltage-gated Ca2+ channel in mouse myeloma cells. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2240–2242. doi: 10.1073/pnas.80.8.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., SAITO N. Voltage-current relations in nerve cell membrane of Onchidium verruculatum. J Physiol. 1959 Oct;148:161–179. doi: 10.1113/jphysiol.1959.sp006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen R. D., Saimi Y. A mutation that alters properties of the calcium channel in Paramecium tetraurelia. J Physiol. 1984 Jun;351:397–410. doi: 10.1113/jphysiol.1984.sp015252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Takahashi K. Comparison of properties of calcium channels between the differentiated 1-cell embryo and the egg cell of ascidians. J Physiol. 1984 Feb;347:327–344. doi: 10.1113/jphysiol.1984.sp015068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C., Saimi Y. The physiological basis of taxes in Paramecium. Annu Rev Physiol. 1982;44:519–534. doi: 10.1146/annurev.ph.44.030182.002511. [DOI] [PubMed] [Google Scholar]
- Machemer H. Korrelation zwischen Membranpotential und Fortbewegung bei Stylonychia (Hypotricha) Naturwissenschaften. 1970 Aug;57(8):398–399. doi: 10.1007/BF00599991. [DOI] [PubMed] [Google Scholar]
- Machemer H., Ogura A. Ionic conductances of membranes in ciliated and deciliated Paramecium. J Physiol. 1979 Nov;296:49–60. doi: 10.1113/jphysiol.1979.sp012990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machemer H., de Peyer J. E. Analysis of ciliary beating frequency under voltage clamp control of the membrane. Prog Clin Biol Res. 1982;80:205–210. doi: 10.1002/cm.970020739. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura A., Takahashi K. Artificial deciliation causes loss of calcium-dependent responses in Paramecium. Nature. 1976 Nov 11;264(5582):170–172. doi: 10.1038/264170a0. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B. Calcium current inactivation in identified neurones of Helix aspersa. J Physiol. 1981 Dec;321:273–285. doi: 10.1113/jphysiol.1981.sp013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B., Ward T. A. The effects of injection of calcium ions and calcium chelators on calcium channel inactivation in Helix neurones. J Physiol. 1983 Jan;334:189–212. doi: 10.1113/jphysiol.1983.sp014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimi Y., Kung C. A Ca-induced Na-current in Paramecium. J Exp Biol. 1980 Oct;88:305–325. doi: 10.1242/jeb.88.1.305. [DOI] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Peyer J. E., Deitmer J. W. Divalent cations as charge carriers during two functionally different membrane currents in the ciliate Stylonychia. J Exp Biol. 1980 Oct;88:73–89. doi: 10.1242/jeb.88.1.73. [DOI] [PubMed] [Google Scholar]


