Abstract
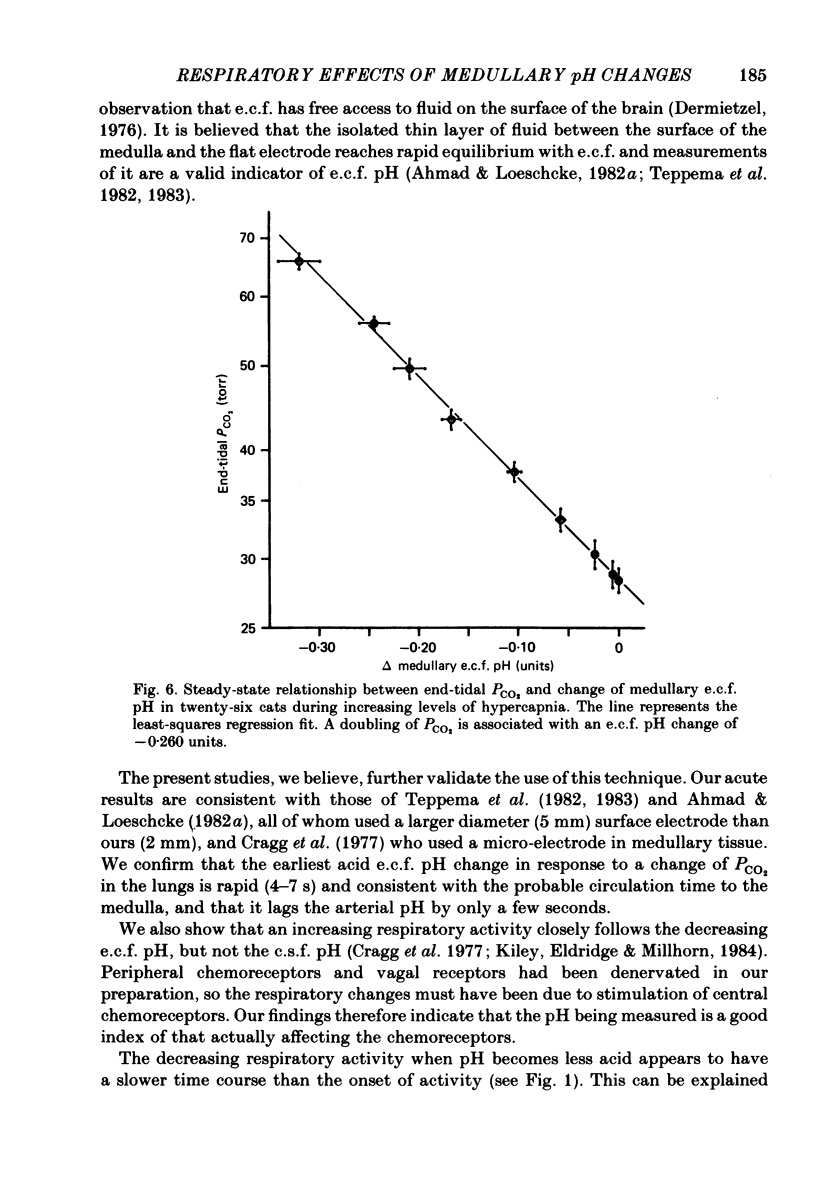
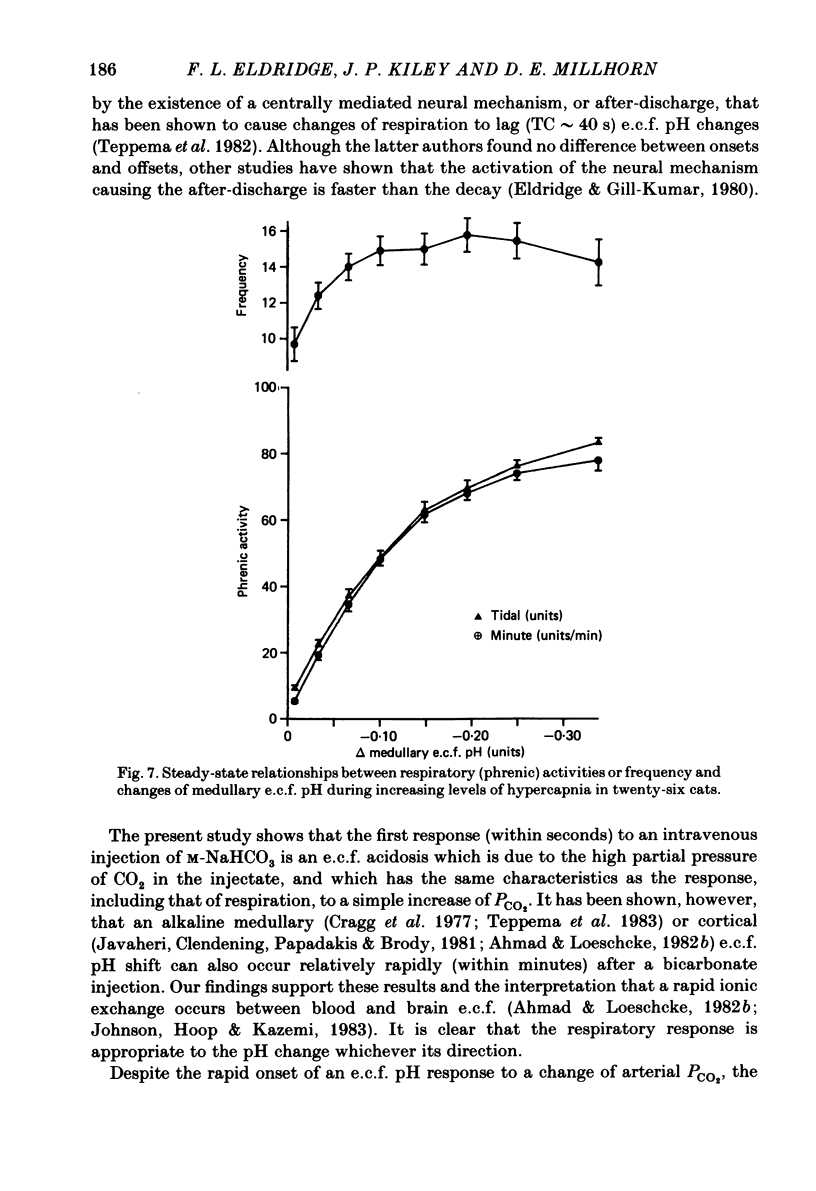
Acute and steady-state responses to hypercapnia of respiratory output, measured as integrated phrenic nerve activity, and medullary extracellular fluid (e.c.f.) pH, measured directly, were determined in paralysed, vagotomized and glomectomized cats. Medullary e.c.f. pH responds within seconds to an acute change of alveolar and arterial PCO2. The respiratory response closely and inversely matches the e.c.f. pH change, but not the cerebrospinal fluid pH change. The medullary e.c.f. pH change following a rapid step-change in end-tidal PCO2 requires at least 5 min for a new steady state to be achieved. Steady-state studies in twenty-six cats show: (a) that the respiratory response to progressive hypercapnic stimulation of the central chemoreceptors is curvilinear (Eldridge, Gill-Kumar & Millhorn, 1981), (b) that the relationship between increasing end-tidal PCO2 and medullary hydrogen ion concentration [(H+]) or changes of pH is linear (r = 0.995); a doubling of PCO2 causes 0.260 units pH change, (c) there is a curvilinear relationship between e.c.f. [H+] and the respiratory response that is the same as that found with CO2. We conclude that medullary e.c.f. pH measured by means of a surface electrode accurately reflects the CO2-induced [H+] stimulus to respiration. The decreasing respiratory responses to identical changes of central chemoreceptor input are due to progressive neuronal saturation of a central pathway between the chemoreceptors and the respiratory controller.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad H. R., Loeschcke H. H. Fast bicarbonate-chloride exchange between plasma and brain extracellular fluid at maintained PCO2. Pflugers Arch. 1982 Dec;395(4):300–305. doi: 10.1007/BF00580793. [DOI] [PubMed] [Google Scholar]
- Ahmad H. R., Loeschcke H. H. Transient and steady state responses of pulmonary ventilation to the medullary extracellular pH after approximately rectangular changes in alveolar PCO2. Pflugers Arch. 1982 Dec;395(4):285–292. doi: 10.1007/BF00580791. [DOI] [PubMed] [Google Scholar]
- Berndt J., Berger W., Berger K., Schmidt M. Untersuchungen zum zentralen chemosensiblen Mechanismus der Atmung. II. Die Steuerung der Atmung durch das extracelluläre pH im Gewebe der Medulla oblongata. Pflugers Arch. 1972;332(2):146–170. doi: 10.1007/BF00589090. [DOI] [PubMed] [Google Scholar]
- Cragg P., Patterson L., Purves M. J. The pH of brain extracellular fluid in the cat. J Physiol. 1977 Oct;272(1):137–166. doi: 10.1113/jphysiol.1977.sp012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P. Central neural respiratory drive and afterdischarge. Respir Physiol. 1980 Apr;40(1):49–63. doi: 10.1016/0034-5687(80)90004-3. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Gill-Kumar P., Millhorn D. E. Input-output relationships of central neural circuits involved in respiration in cats. J Physiol. 1981 Feb;311:81–95. doi: 10.1113/jphysiol.1981.sp013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L., Millhorn D. E., Waldrop T. G. Input-output relationships of the central respiratory controller during peripheral muscle stimulation in cats. J Physiol. 1982 Mar;324:285–295. doi: 10.1113/jphysiol.1982.sp014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between phrenic nerve activity and ventilation. Am J Physiol. 1971 Aug;221(2):535–543. doi: 10.1152/ajplegacy.1971.221.2.535. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L. Relationship between respiratory nerve and muscle activity and muscle force output. J Appl Physiol. 1975 Oct;39(4):567–574. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Fencl V., Miller T. B., Pappenheimer J. R. Studies on the respiratory response to disturbances of acid-base balance, with deductions concerning the ionic composition of cerebral interstitial fluid. Am J Physiol. 1966 Mar;210(3):459–472. doi: 10.1152/ajplegacy.1966.210.3.459. [DOI] [PubMed] [Google Scholar]
- Fukuda Y. Difference between actions of high PCO2 and low [HCO-3] on neurons in the rat medullary chemosensitive areas in vitro. Pflugers Arch. 1983 Sep;398(4):324–330. doi: 10.1007/BF00657242. [DOI] [PubMed] [Google Scholar]
- Javaheri S., Clendening A., Papadakis N., Brody J. S. Changes in brain surface pH during acute isocapnic metabolic acidosis and alkalosis. J Appl Physiol Respir Environ Exerc Physiol. 1981 Aug;51(2):276–281. doi: 10.1152/jappl.1981.51.2.276. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Hoop B., Kazemi H. Movement of CO2 and HCO-3 from blood to brain in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1983 Apr;54(4):989–996. doi: 10.1152/jappl.1983.54.4.989. [DOI] [PubMed] [Google Scholar]
- Loeschcke H. H. Central chemosensitivity and the reaction theory. J Physiol. 1982 Nov;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeschcke H. H., Sugioka K. pH of cerebrospinal fluid in the cisterna Magna and on the surface of the choroid plexus of the 4th ventricle and its effect on ventilation in experimental disturbances of acid base balance. Transients and steady states. Pflugers Arch. 1969;312(4):161–188. doi: 10.1007/BF00586927. [DOI] [PubMed] [Google Scholar]
- Neubauer J. A., Strumpf D. A., Edelman N. H. Regional medullary blood flow during isocapnic hyperpnea in anesthetized cats. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):447–452. doi: 10.1152/jappl.1983.55.2.447. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., FENCL V., HEISEY S. R., HELD D. ROLE OF CEREBRAL FLUIDS IN CONTROL OF RESPIRATION AS STUDIED IN UNANESTHETIZED GOATS. Am J Physiol. 1965 Mar;208:436–450. doi: 10.1152/ajplegacy.1965.208.3.436. [DOI] [PubMed] [Google Scholar]
- Pontén U., Siesjö B. K. Gradients of CO2 tension in the brain. Acta Physiol Scand. 1966 Jun;67(2):129–140. doi: 10.1111/j.1748-1716.1966.tb03294.x. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Mercer R. R., Eldridge F. L. Servo control of end-tidal CO2 in paralyzed animals. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jul;45(1):133–136. doi: 10.1152/jappl.1978.45.1.133. [DOI] [PubMed] [Google Scholar]
- Teppema L. J., Barts P. W., Folgering H. T., Evers J. A. Effects of respiratory and (isocapnic) metabolic arterial acid-base disturbances on medullary extracellular fluid pH and ventilation in cats. Respir Physiol. 1983 Sep;53(3):379–395. doi: 10.1016/0034-5687(83)90127-5. [DOI] [PubMed] [Google Scholar]
- Teppema L. J., Vis A., Evers J. A., Folgering H. T. Dynamics of brain extracellular fluid pH and phrenic nerve activity in cats after end-tidal CO2 forcing. Respir Physiol. 1982 Dec;50(3):359–380. doi: 10.1016/0034-5687(82)90029-9. [DOI] [PubMed] [Google Scholar]
- Vis A., Folgering H. The dynamic effect of PETCO, on vertebral bloodflow in cats. Respir Physiol. 1980 Nov;42(2):131–143. doi: 10.1016/0034-5687(80)90110-3. [DOI] [PubMed] [Google Scholar]


