Abstract
The role of the A1 and A2 noradrenergic cell groups of the caudal medulla in regulating the activity of paraventricular nucleus neurosecretory cells was examined with electrophysiological methods in anaesthetized male Sprague-Dawley rats. Antidromically identified neurosecretory cells were classified as vasopressin or oxytocin secreting on the basis of spontaneous firing patterns and responsivity to baroreceptor activation. The effect on cell firing of single pulses (25-200 microA) delivered to either the A1 or A2 cell group areas was then examined using peri-stimulus histograms. Stimulation of the A1 region enhanced the activity of 78% of putative vasopressin-secreting neurones tested (n = 18), but failed to affect the activity of the majority (73%) of putative oxytocin-secreting units (n = 15). A2 stimulation enhanced the firing rate of both putative vasopressin- (60%, total n = 14) and putative oxytocin-secreting (70%, total n = 27) neurones. Destruction of the paraventricular nucleus catecholamine terminal plexus by pre-treatment with the neurotoxin 6-hydroxydopamine abolished the facilitatory effects of both A1 and A2 stimulation. These findings suggest that noradrenergic afferents of medullary origin facilitate the activity of paraventricular nucleus neurosecretory cells. The role of the projection from the A1 cell group appears to differ from that of the A2 group, however, in that its effects are specific to putative vasopressin-secreting units.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade R., Aghajanian G. K. Single cell activity in the noradrenergic A-5 region: responses to drugs and peripheral manipulations of blood pressure. Brain Res. 1982 Jun 17;242(1):125–135. doi: 10.1016/0006-8993(82)90502-9. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M., Fox K., Kruk Z. L., Millar J. Quantitative ionophoresis of catecholamines using multibarrel carbon fibre microelectrodes. J Neurosci Methods. 1981 Dec;4(4):385–406. doi: 10.1016/0165-0270(81)90008-x. [DOI] [PubMed] [Google Scholar]
- Arnauld E., Cirino M., Layton B. S., Renaud L. P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology. 1983;36(3):187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Supraoptic neurosecretory cells: adrenergic and cholinergic sensitivity. Science. 1971 Jan 15;171(3967):208–210. doi: 10.1126/science.171.3967.208. [DOI] [PubMed] [Google Scholar]
- Bhargava K. P., Kulshrestha V. K., Srivastava Y. P. Central cholinergic and adrenergic mechanisms in the release of antidiuretic hormone. Br J Pharmacol. 1972 Apr;44(4):617–627. doi: 10.1111/j.1476-5381.1972.tb07301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges T. E., Hillhouse E. W., Jones M. T. The effect of dopamine on neurohypophysial hormone release in vivo and from the rat neural lobe and hypothalamus in vitro. J Physiol. 1976 Sep;260(3):647–666. doi: 10.1113/jphysiol.1976.sp011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote J. H., Sato Y. Reflex regulation of sympathetic activity in the spontaneously hypertensive rat. Circ Res. 1977 Jun;40(6):571–577. doi: 10.1161/01.res.40.6.571. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Harris M. C., Tribollet E. Excitation of phasically firing hypothalamic supraoptic neurones by carotid occlusion in rats. J Physiol. 1976 May;257(2):337–354. doi: 10.1113/jphysiol.1976.sp011372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- Furness J. B., Heath J. W., Costa M. Aqueous aldehyde (Faglu) methods for the fluorescence histochemical localization of catecholamines and for ultrastructural studies of central nervous tissue. Histochemistry. 1978 Sep 28;57(4):285–295. doi: 10.1007/BF00492664. [DOI] [PubMed] [Google Scholar]
- Harris M. C., Dreifuss J. J., Legros J. J. Excitation of phasically firing supraoptic neurones during vasopressin release. Nature. 1975 Nov 6;258(5530):80–82. doi: 10.1038/258080b0. [DOI] [PubMed] [Google Scholar]
- Harris M. C. Effects of chemoreceptor and baroreceptor stimulation on the discharge of hypothalamic supraoptic neurones in rats. J Endocrinol. 1979 Jul;82(1):115–125. doi: 10.1677/joe.0.0820115. [DOI] [PubMed] [Google Scholar]
- Hoffman W. E., Phillips M. I., Schmid P. The role of catecholamines in central antidiuretic and pressor mechanisms. Neuropharmacology. 1977 Sep;16(9):563–569. doi: 10.1016/0028-3908(77)90025-9. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Fuxe K., Hökfelt T., Goldstein M. Resistance of central phenylethanolamine-n-methyl transferase containing neurons to 6-hydroxydopamine. Med Biol. 1976 Dec;54(6):421–426. [PubMed] [Google Scholar]
- Kimura T., Share L., Wang B. C., Crofton J. T. The role of central adrenoreceptors in the control of vasopressin release and blood pressure. Endocrinology. 1981 May;108(5):1829–1836. doi: 10.1210/endo-108-5-1829. [DOI] [PubMed] [Google Scholar]
- Kostrzewa R. M., Jacobowitz D. M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974 Sep;26(3):199–288. [PubMed] [Google Scholar]
- Kühn E. R. Cholinergic and adrenergic release mechanism for vasopressin in the male rat: a study with injections of neurotransmitters and blocking agents into the third ventricle. Neuroendocrinology. 1974;16(5-6):255–264. doi: 10.1159/000122572. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Wakerley J. B. Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J Physiol. 1974 Oct;242(2):533–554. doi: 10.1113/jphysiol.1974.sp010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy A. D., Neil J. J. The role of descending monoaminergic systems in central control of blood pressure. Fed Proc. 1981 Nov;40(13):2778–2785. [PubMed] [Google Scholar]
- McNeill T. H., Sladek J. R., Jr Simultaneous monoamine histofluorescence and neuropeptide immunocytochemistry: II. Correlative distribution of catecholamine varicosities and magnocellular neurosecretory neurons in the rat supraoptic and paraventricular nuclei. J Comp Neurol. 1980 Oct 15;193(4):1023–1033. doi: 10.1002/cne.901930414. [DOI] [PubMed] [Google Scholar]
- Miller T. R., Handelman W. A., Arnold P. E., McDonald K. M., Molinoff P. B., Schrier R. W. Effect of central catecholamine depletion on the osmotic and nonosmotic stimulation of vasopressin (antidiuretic hormone) in the rat. J Clin Invest. 1979 Dec;64(6):1599–1607. doi: 10.1172/JCI109621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton A. S., Paterson A. T. A microinjection study of the control of antidiuretic hormone release by the supraoptic nucleus of the hypothalamus in the cat. J Physiol. 1974 Sep;241(3):607–628. doi: 10.1113/jphysiol.1974.sp010674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. D., Guyenet P. G. An electrophysiological study of the forebrain projection of nucleus commissuralis: preliminary identification of presumed A2 catecholaminergic neurons. Brain Res. 1983 Mar 21;263(2):211–222. doi: 10.1016/0006-8993(83)90314-1. [DOI] [PubMed] [Google Scholar]
- Moos F., Richard P. The inhibitory role of beta-noradrenergic receptors in oxytocin release during suckling. Brain Res. 1979 Jun 29;169(3):595–599. doi: 10.1016/0006-8993(79)90411-6. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Dyball R. E., Cross B. A. Responses of antidromically identified supraoptic and paraventricular units to acetylcholine, noradrenaline and glutamate applied iontophoretically. Brain Res. 1971 Dec 24;35(2):573–575. doi: 10.1016/0006-8993(71)90504-x. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Urban I., Cross B. A. Microelectrophoresis of cholinergic and aminergic drugs on paraventricular neurons. Am J Physiol. 1972 Aug;223(2):310–318. doi: 10.1152/ajplegacy.1972.223.2.310. [DOI] [PubMed] [Google Scholar]
- Olsson K. Studies on central regulation of secretion of antidiuretic hormone (ADH) in the goat. Acta Physiol Scand. 1969 Dec;77(4):465–474. doi: 10.1111/j.1748-1716.1969.tb04590.x. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B., Dyball R. E. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Ranck J. B., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Reid I. A., Nolan P. L., Wolf J. A., Keil L. C. Suppression of vasopressin secretion by clonidine: effect of alpha-adrenoceptor antagonists. Endocrinology. 1979 May;104(5):1403–1406. doi: 10.1210/endo-104-5-1403. [DOI] [PubMed] [Google Scholar]
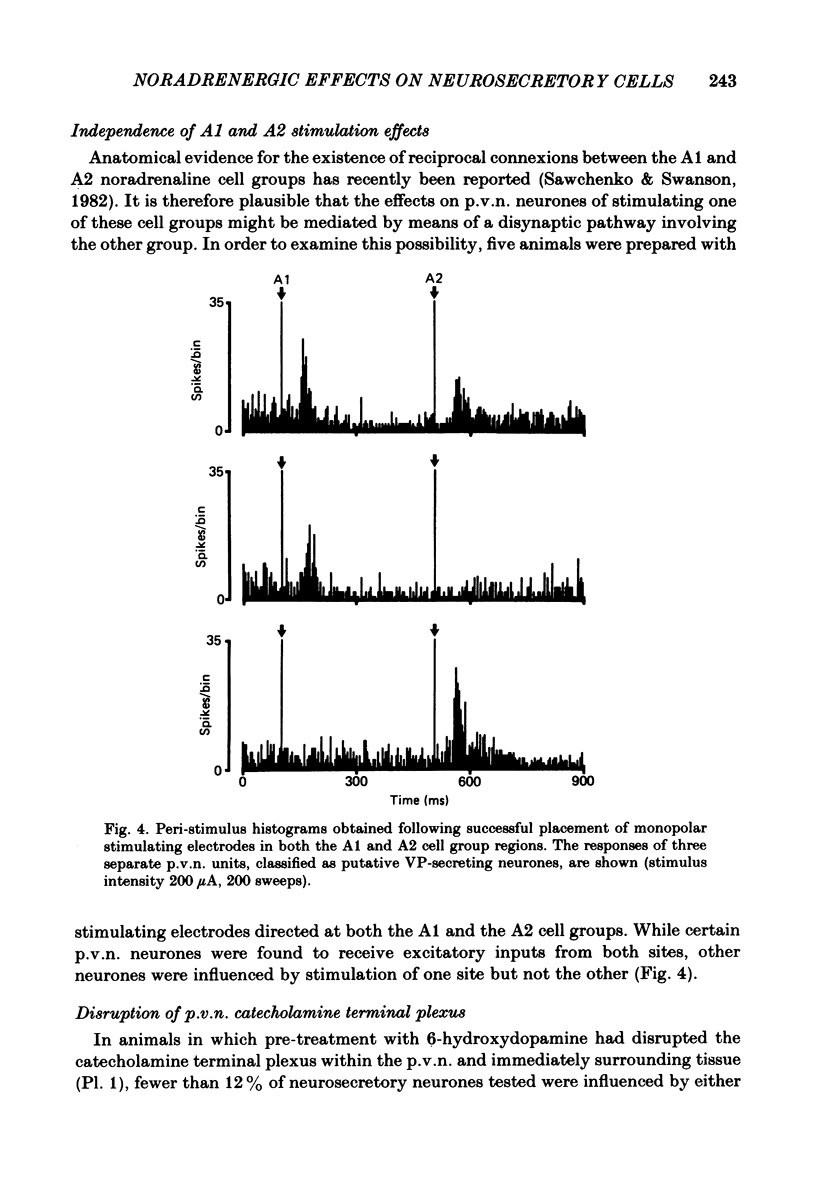
- Sawchenko P. E., Swanson L. W. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982 Nov;257(3):275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Share L., Levy M. N. Carotid sinus pulse pressure, a determinant of plasma antidiuretic hormone concentration. Am J Physiol. 1966 Sep;211(3):721–724. doi: 10.1152/ajplegacy.1966.211.3.721. [DOI] [PubMed] [Google Scholar]
- Silverman A. J., Hou-Yu A., Oldfield B. J. Ultrastructural identification of noradrenergic nerve terminals and vasopressin-containing neurons of the paraventricular nucleus in the same thin section. J Histochem Cytochem. 1983 Sep;31(9):1151–1156. doi: 10.1177/31.9.6350438. [DOI] [PubMed] [Google Scholar]
- Svensson T. H., Bunney B. S., Aghajanian G. K. Inhibition of both noradrenergic and serotonergic neurons in brain by the alpha-adrenergic agonist clonidine. Brain Res. 1975 Jul 11;92(2):291–306. doi: 10.1016/0006-8993(75)90276-0. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Clarke G., Dreifuss J. J., Lincoln D. W. The role of central adrenergic receptors in the reflex release of oxytocin. Brain Res. 1978 Feb 17;142(1):69–84. doi: 10.1016/0006-8993(78)90177-4. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- Vandeputte-Van Messon G., Peeters G. Effect of intraventricular administration of noradrenaline on water diuresis in goats. J Endocrinol. 1975 Sep;66(3):375–383. doi: 10.1677/joe.0.0660375. [DOI] [PubMed] [Google Scholar]
- Vandesande F., Dierickx K. Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretroy system of the rat. Cell Tissue Res. 1975 Dec 2;164(2):153–162. doi: 10.1007/BF00218970. [DOI] [PubMed] [Google Scholar]
- van den Pol A. N. The magnocellular and parvocellular paraventricular nucleus of rat: intrinsic organization. J Comp Neurol. 1982 Apr 20;206(4):317–345. doi: 10.1002/cne.902060402. [DOI] [PubMed] [Google Scholar]



