Abstract
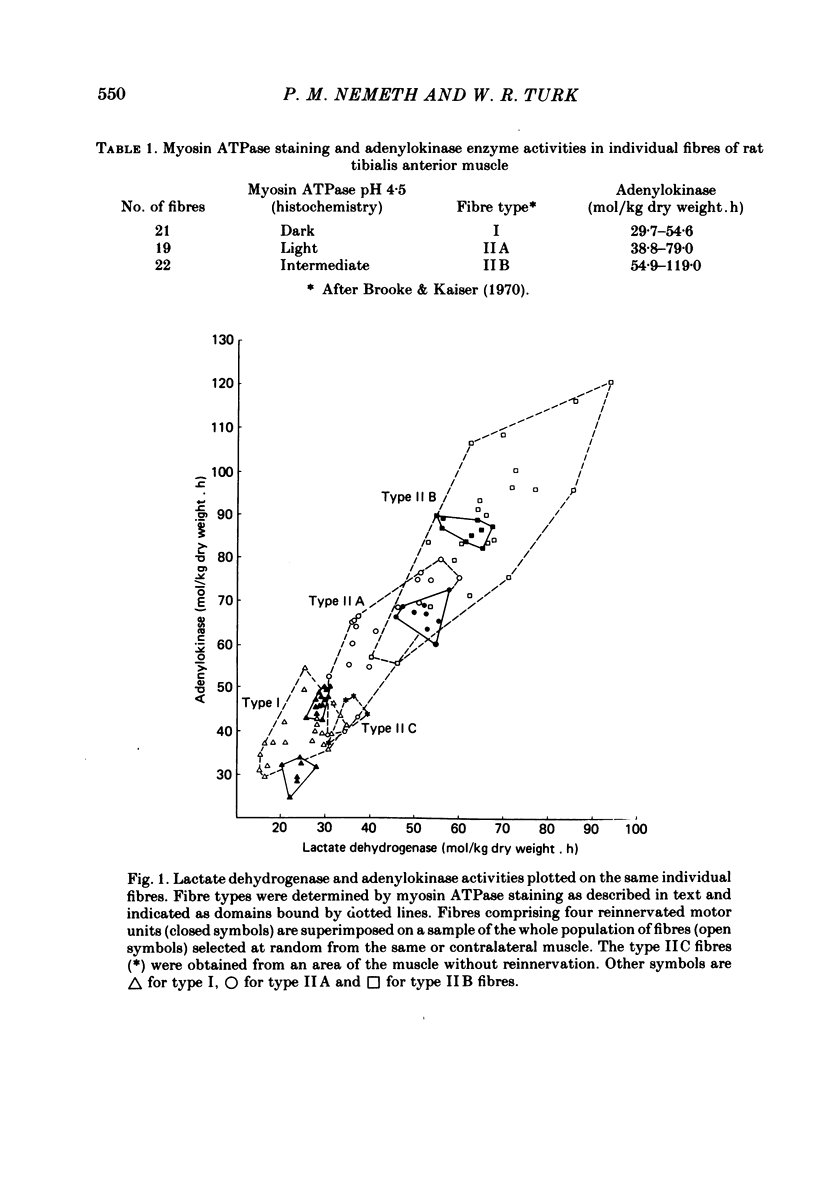
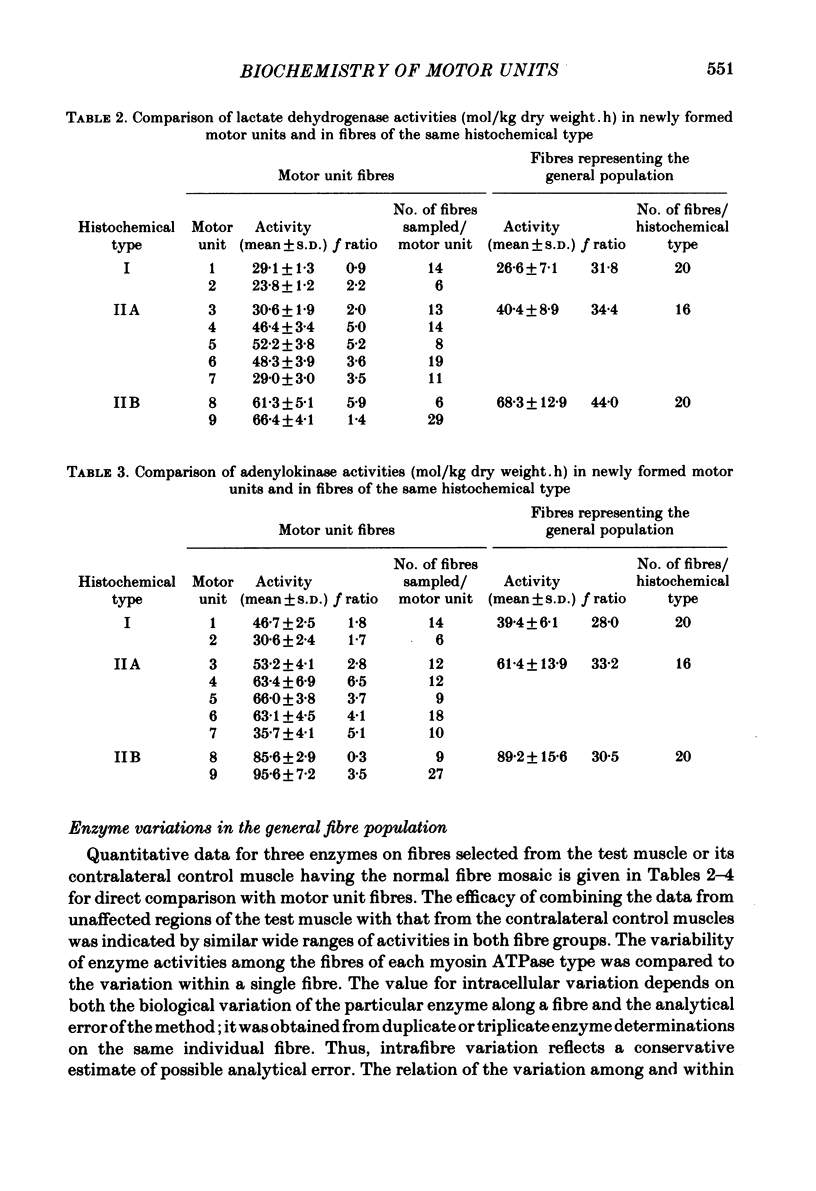
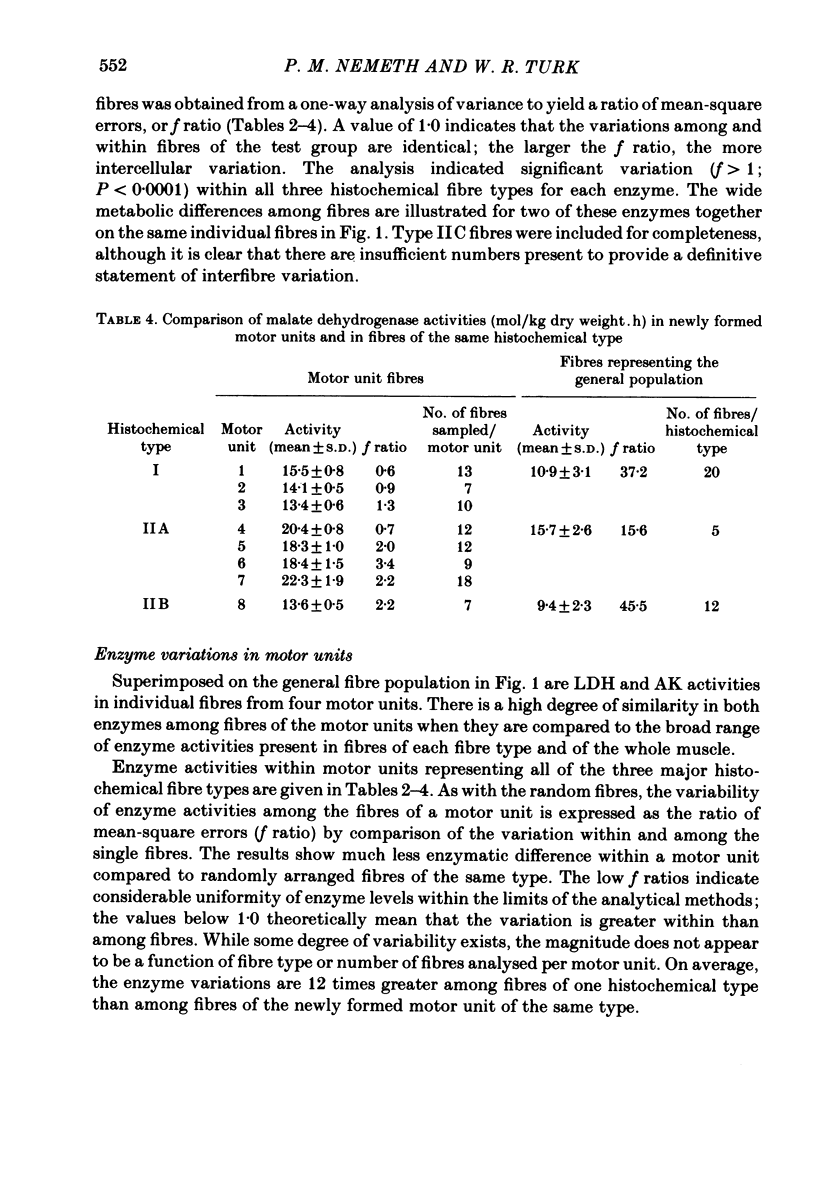
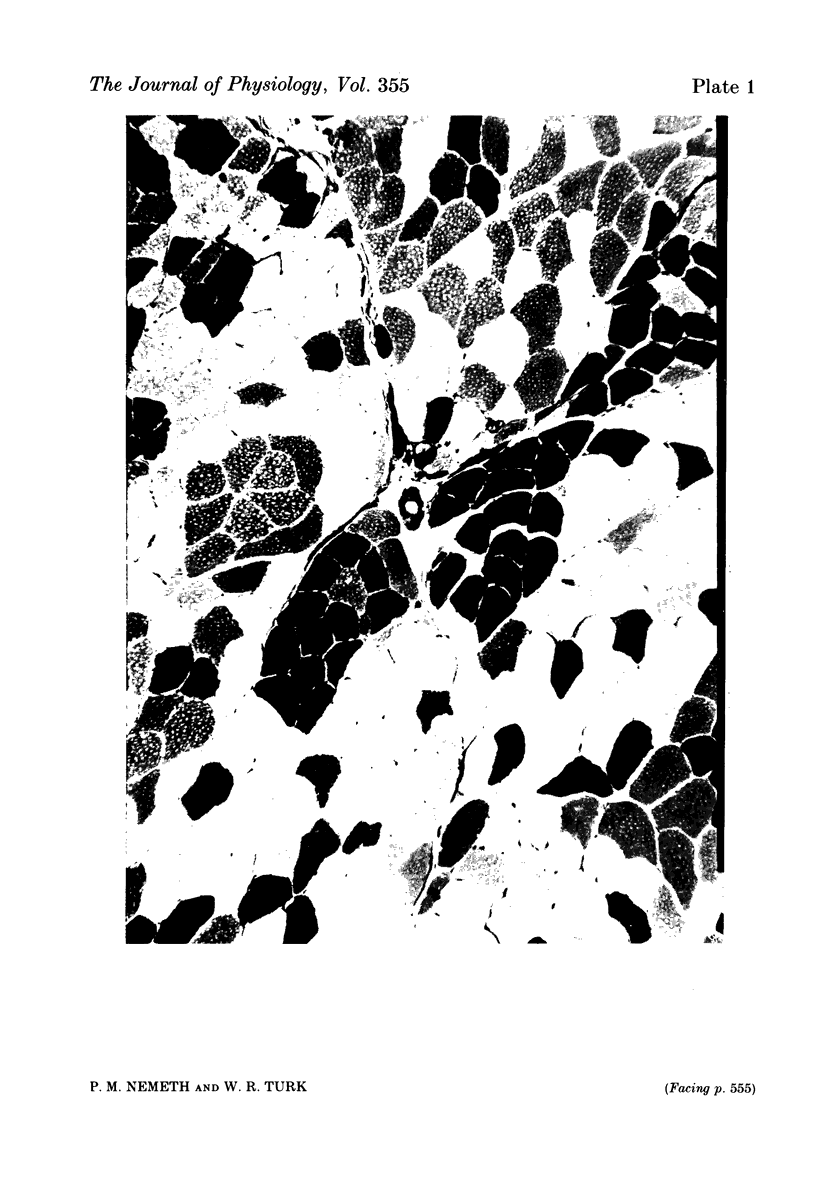
A partial crush was applied surgically to the common peroneal nerves of rats, producing motor deficits lasting 4 weeks; the tibialis anterior muscles supplied by the crushed nerves were removed 5 weeks after recovery along with the contralateral control muscles. Myosin ATPase staining following pre-incubation at pH 4.5 was used to determine fibre types and to demonstrate areas of fibre-type grouping in the reinnervated areas of the muscles. Enzyme activities of lactate dehydrogenase (LDH), adenylokinase (AK) and malate dehydrogenase (MDH) were measured using micro-analytical techniques on the individual fibres within the histochemically identical groups and on fibres of the same types selected from areas of the test muscle or the contralateral control which appeared normal. The results show that the degree of enzymatic variation among single fibres reinnervated by a common axon is very small when compared to the general fibre population and, moreover, to fibres of the same histochemical type. Enzyme variability within the newly formed motor units was only slightly greater than the variability reported for normal motor units (Nemeth, Pette & Vrbová, 1981). The results indicate that skeletal muscle fibres originally having great differences in levels of enzyme activity, as demonstrated in the general fibre population, acquire considerable enzymatic similarity following reinnervation by a common motor neurone.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Three "myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970 Sep;18(9):670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Buchegger A., Nemeth P. M., Pette D., Reichmann H. Effects of chronic stimulation on the metabolic heterogeneity of the fibre population in rabbit tibialis anterior muscle. J Physiol. 1984 May;350:109–119. doi: 10.1113/jphysiol.1984.sp015191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi M. M., Hintz C. S., Coyle E. F., Martin W. H., 3rd, Ivy J. L., Nemeth P. M., Holloszy J. O., Lowry O. H. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. Am J Physiol. 1983 Mar;244(3):C276–C287. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- Chi M. M., Lowry C. V., Lowry O. H. An improved enzymatic cycle for nicotinamide-adenine dinucleotide phosphate. Anal Biochem. 1978 Aug 15;89(1):119–129. doi: 10.1016/0003-2697(78)90732-7. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz C. S., Coyle E. F., Kaiser K. K., Chi M. M., Lowry O. H. Comparison of muscle fiber typing by quantitative enzyme assays and by myosin ATPase staining. J Histochem Cytochem. 1984 Jun;32(6):655–660. doi: 10.1177/32.6.6202737. [DOI] [PubMed] [Google Scholar]
- Hintz C. S., Lowry C. V., Kaiser K. K., McKee D., Lowry O. H. Enzyme levels in individual rat muscle fibers. Am J Physiol. 1980 Sep;239(3):C58–C65. doi: 10.1152/ajpcell.1980.239.3.C58. [DOI] [PubMed] [Google Scholar]
- Karpati G., Engel W. K. "Type grouping" in skeletal muscles after experimental reinnervation. Neurology. 1968 May;18(5):447–455. doi: 10.1212/wnl.18.5.447. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L., Abbruzzese M. Mapping of motor units in experimentally reinnervated rat muscle. Interpretation of histochemical and atrophic fibre patterns in neurogenic lesions. J Neurol Neurosurg Psychiatry. 1970 Jun;33(3):319–329. doi: 10.1136/jnnp.33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E., Edström L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):415–423. doi: 10.1136/jnnp.31.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. V., Kimmey J. S., Felder S., Chi M. M., Kaiser K. K., Passonneau P. N., Kirk K. A., Lowry O. H. Enzyme patterns in single human muscle fibers. J Biol Chem. 1978 Nov 25;253(22):8269–8277. [PubMed] [Google Scholar]
- Mommaerts W. F., Buller A. J., Seraydarian K. The modification of some biochemical properties of muscle by cross-innervation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):128–133. doi: 10.1073/pnas.64.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth P. M., Pette D., Vrbová G. Comparison of enzyme activities among single muscle fibres within defined motor units. J Physiol. 1981 Feb;311:489–495. doi: 10.1113/jphysiol.1981.sp013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth P., Pette D. Succinate dehydrogenase activity in fibres classified by myosin ATPase in three hind limb muscles of rat. J Physiol. 1981 Nov;320:73–80. doi: 10.1113/jphysiol.1981.sp013935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prewitt M. A., Salafsky B. Effect of cross innervation on biochemical characteristics of skeletal muscles. Am J Physiol. 1967 Jul;213(1):295–300. doi: 10.1152/ajplegacy.1967.213.1.295. [DOI] [PubMed] [Google Scholar]
- Spamer C., Pette D. Activities of malate dehydrogenase, 3-hydroxyacyl-CoA dehydrogenase and fructose-1,6-diphosphatase with regard to metabolic subpopulations of fast- and slow-twitch fibres in rabbit muscles. Histochemistry. 1979 Feb 26;60(1):9–19. doi: 10.1007/BF00495725. [DOI] [PubMed] [Google Scholar]
- Spamer C., Pette D. Activity patterns of phosphofructokinase, glyceraldehydephosphate dehydrogenase, lactate dehydrogenase and malate dehydrogenase in microdissected fast and slow fibres from rabbit psoas and soleus muscle. Histochemistry. 1977 Jun 8;52(3):201–216. doi: 10.1007/BF00495857. [DOI] [PubMed] [Google Scholar]



