Abstract
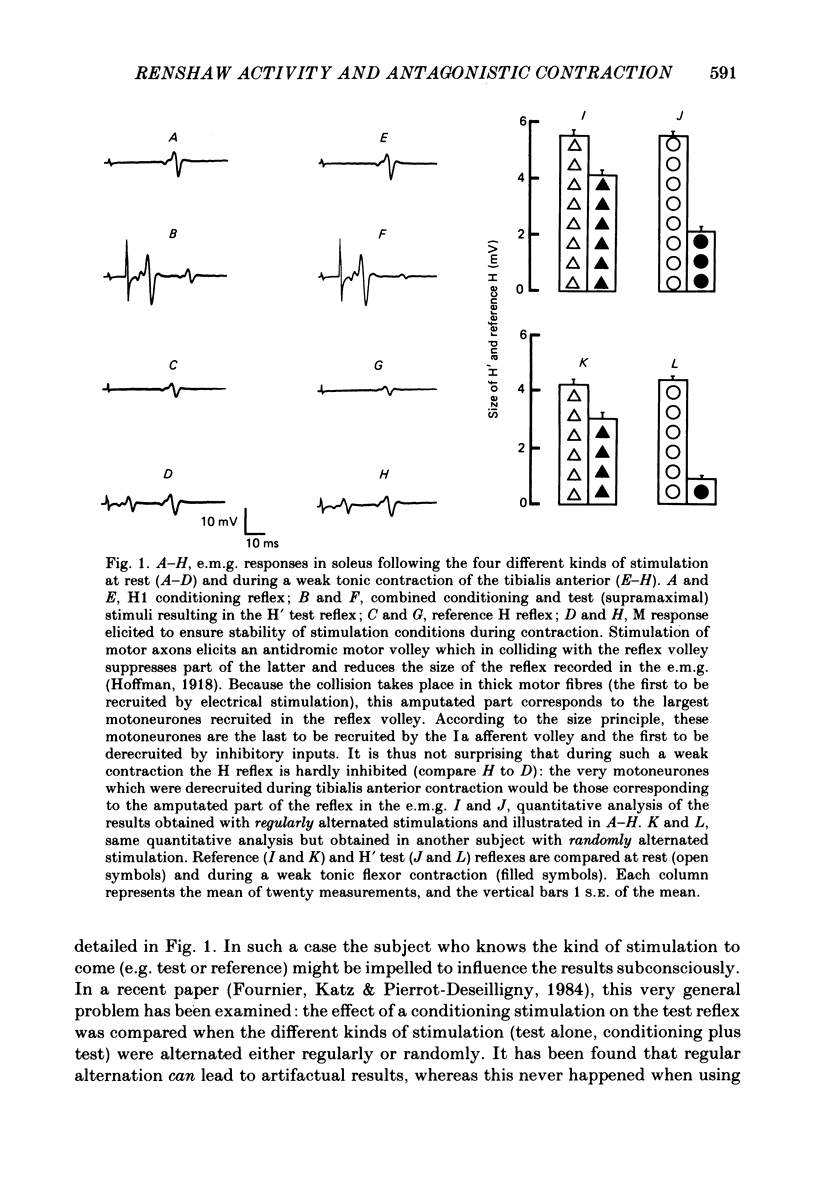
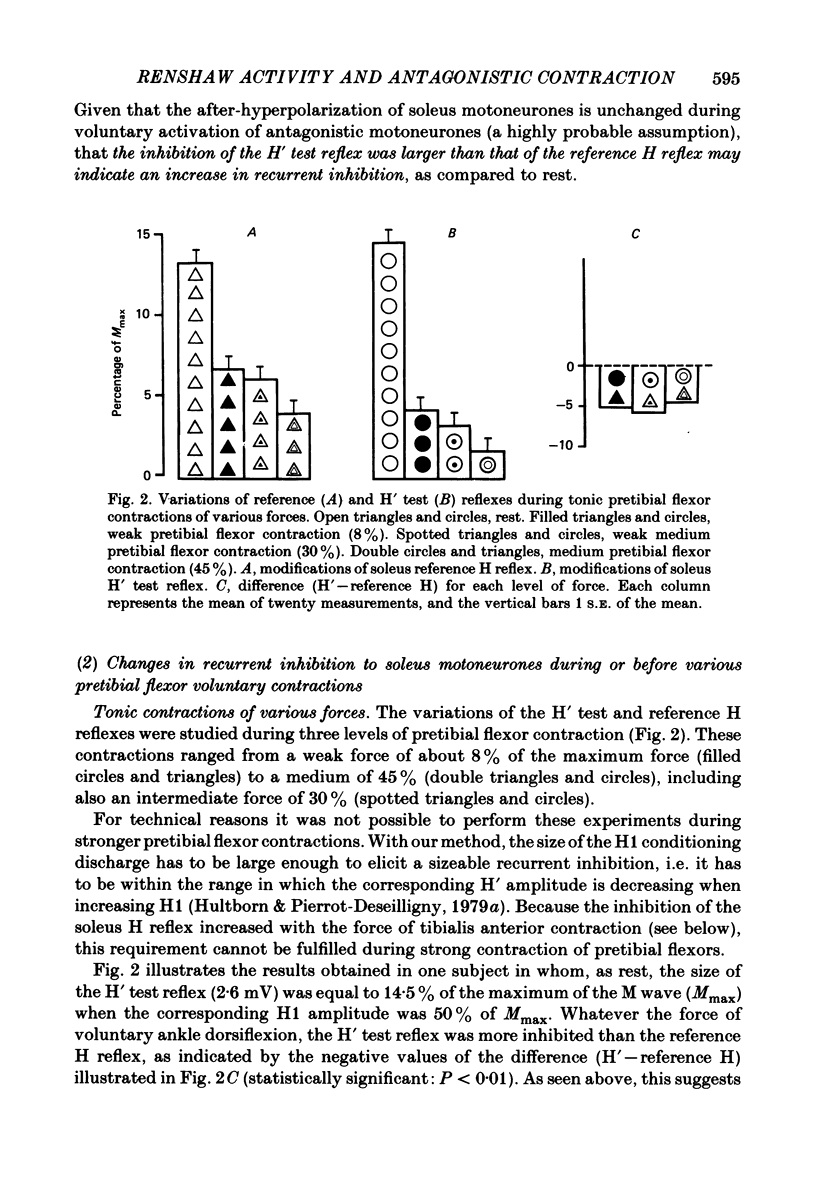
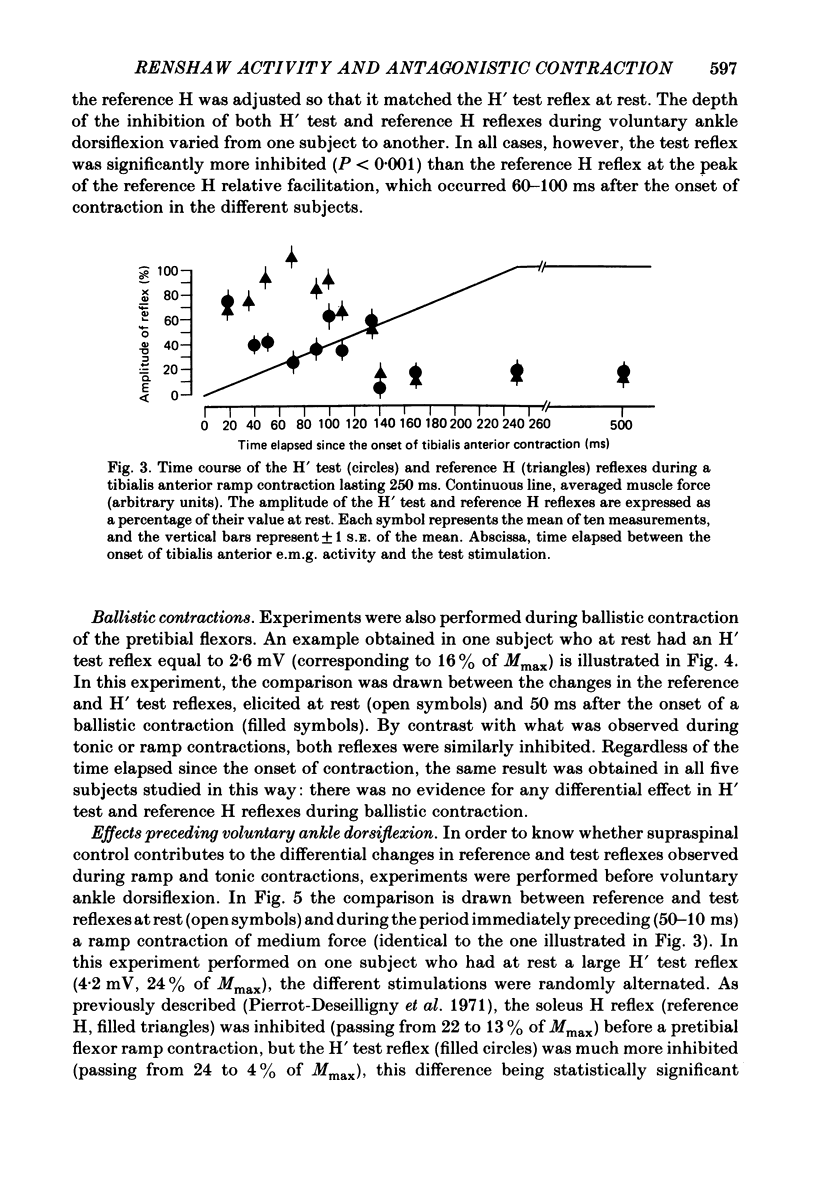
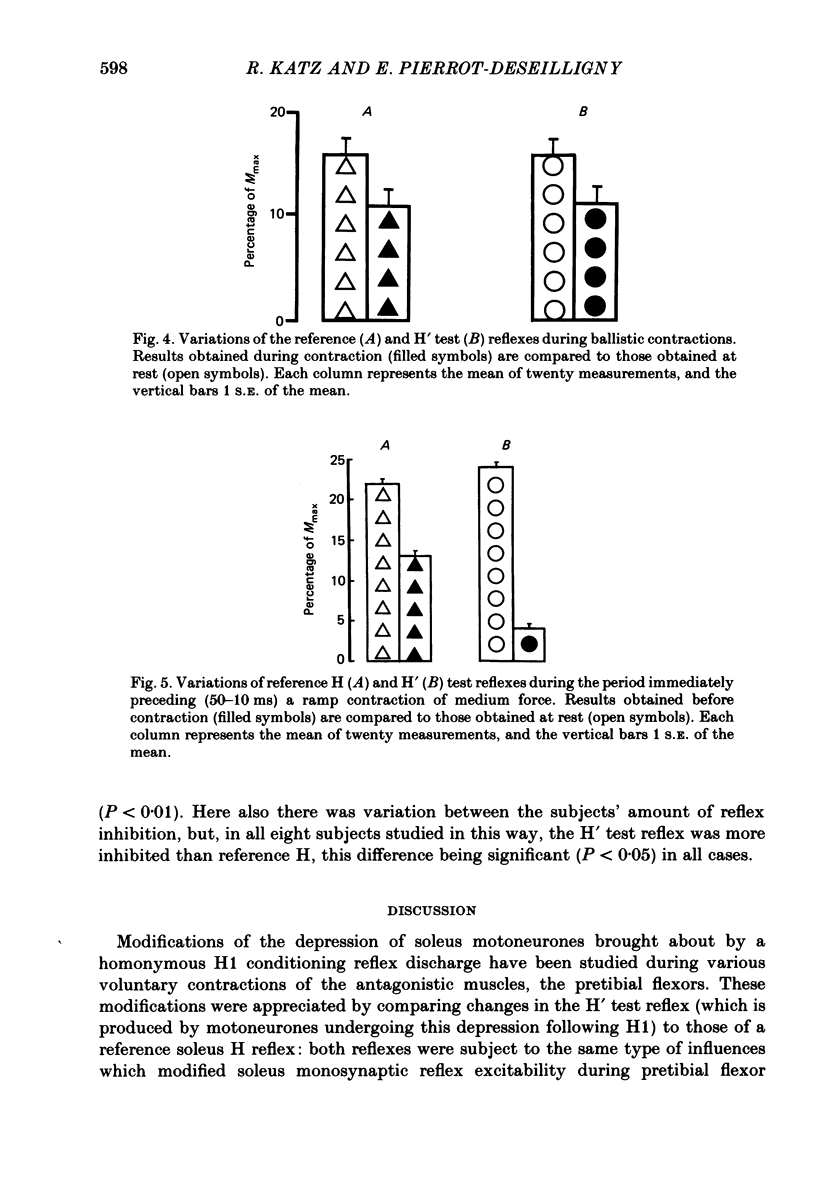
Recurrent inhibition to soleus motoneurones, brought about by a conditioning H-reflex discharge, was estimated in human subjects by a subsequent test H reflex. Changes in recurrent inhibition during voluntary ankle dorsiflexion were evaluated by comparing the amplitude of the test H reflex to a reference H reflex: both reflexes were subjected to the same type of influences which modified soleus monosynaptic reflex excitability during pretibial flexor contraction, but only the test H reflex was subject to the recurrent inhibition evoked by the conditioning H-reflex discharge. During tonic or phasic ramp contractions of the pretibial flexors the inhibition of the test H reflex, as compared to rest, was more marked than that of the reference H reflex. Evidence is presented that this may indicate a facilitation of soleus-coupled Renshaw cells. Since this facilitation of soleus-coupled Renshaw cells was also observed before ramp contraction, it is, at least in part, supraspinal in origin. Within the range of forces studied (8-45% of maximum force) there was no evidence that the facilitation of soleus-coupled Renshaw cells increased along with increased force of the pretibial flexor voluntary contraction. During voluntary phasic ankle dorsiflexion, facilitation of soleus-coupled Renshaw cells was maximum at the moment when soleus motoneurones were most facilitated by the stretch-induced soleus I a discharge. There was no evidence for changes in Renshaw cell excitability during ballistic contractions. It is suggested that this facilitation of soleus-coupled Renshaw cells may be one of the mechanisms preventing the occurrence of a soleus stretch reflex during a voluntary ankle dorsiflexion. Such a mechanism could become important if reciprocal inhibition, via I a inhibitory interneurones, were not strong enough, e.g. because of a weak gamma-drive to the contracting muscles.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bussel B., Pierrot-Deseilligny E. Inhibition of human motoneurons, probably of Renshaw origin, elicited by an orthodromic motor discharge. J Physiol. 1977 Jul;269(2):319–339. doi: 10.1113/jphysiol.1977.sp011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanandan M. S., Eccles R. M., Stenhouse D. Presynaptic inhibition evoked by muscle contraction. J Physiol. 1966 Jul;185(2):471–485. doi: 10.1113/jphysiol.1966.sp007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C., Stark L. Interactions between voluntary and postural mechanisms of thehuman motor system. J Neurophysiol. 1970 May;33(3):365–381. doi: 10.1152/jn.1970.33.3.365. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Wallen G., Löfstedt L. Muscle spindle activity in man during voluntary fast alternating movements. J Neurol Neurosurg Psychiatry. 1975 Jul;38(7):625–635. doi: 10.1136/jnnp.38.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol. 1979 Dec;297(0):229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E. Input-output relations in the pathway of recurrent inhibition to motoneurones in the cat. J Physiol. 1979 Dec;297(0):267–287. doi: 10.1113/jphysiol.1979.sp013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H., Pierrot-Deseilligny E., Wigström H. Recurrent inhibition and afterhyperpolarization following motoneuronal discharge in the cat. J Physiol. 1979 Dec;297(0):253–266. doi: 10.1113/jphysiol.1979.sp013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R., Pierrot-Deseilligny E. Recurrent inhibition of alpha-motoneurons in patients with upper motor neuron lesions. Brain. 1982 Mar;105(Pt 1):103–124. doi: 10.1093/brain/105.1.103. [DOI] [PubMed] [Google Scholar]
- Morin C., Pierrot-Deseilligny E., Hultborn H. Evidence for presynaptic inhibition of muscle spindle Ia afferents in man. Neurosci Lett. 1984 Feb 10;44(2):137–142. doi: 10.1016/0304-3940(84)90071-5. [DOI] [PubMed] [Google Scholar]
- Morin C., Pierrot-Deseilligny E. Role of Ia afferents in the soleus motoneurones. Inhibition during a tibialis anterior voluntary contraction in man. Exp Brain Res. 1977 Apr 21;27(5):509–522. doi: 10.1007/BF00239040. [DOI] [PubMed] [Google Scholar]
- Piercey M. F., Goldfarb J. Discharge patterns of Renshaw cells evoked by volleys in ipsilateral cutaneous and high-threshold muscle afferents and their relationship to reflexes recorded in ventral roots. J Neurophysiol. 1974 Mar;37(2):294–302. doi: 10.1152/jn.1974.37.2.294. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Bergego C., Katz R. Reversal in cutaneous of Ib pathways during human voluntary contraction. Brain Res. 1982 Feb 11;233(2):400–403. doi: 10.1016/0006-8993(82)91213-6. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Katz R., Morin C. Evidence of Ib inhibition in human subjects. Brain Res. 1979 Apr 20;166(1):176–179. doi: 10.1016/0006-8993(79)90660-7. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Lacert P., Cathala H. P. Amplitude et variabilité des réflexes monosynaptiques avant un mouvement volontaire. Physiol Behav. 1971 Oct;7(4):495–508. doi: 10.1016/0031-9384(71)90100-4. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42(3-4):337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Katz R., Bussel B. Influence of voluntary movement and posture on recurrent inhibition in human subjects. Brain Res. 1977 Apr 1;124(3):427–436. doi: 10.1016/0006-8993(77)90944-1. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Hulliger M. Muscle afferent function and its significance for motor control mechanisms during voluntary movements in cat, monkey, and man. Adv Neurol. 1983;39:93–132. [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- Simoyama M., Tanaka R. Reciprocal La inhibition at the onset of voluntary movements in man. Brain Res. 1974 Dec 27;82(2):334–337. doi: 10.1016/0006-8993(74)90615-5. [DOI] [PubMed] [Google Scholar]
- Tanaka R. Reciprocal Ia inhibition during voluntary movements in man. Exp Brain Res. 1974;21(5):529–540. doi: 10.1007/BF00237171. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E., Torebjörk H. E., Wallin B. G. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979 Oct;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]


