Abstract
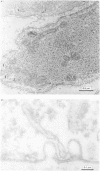
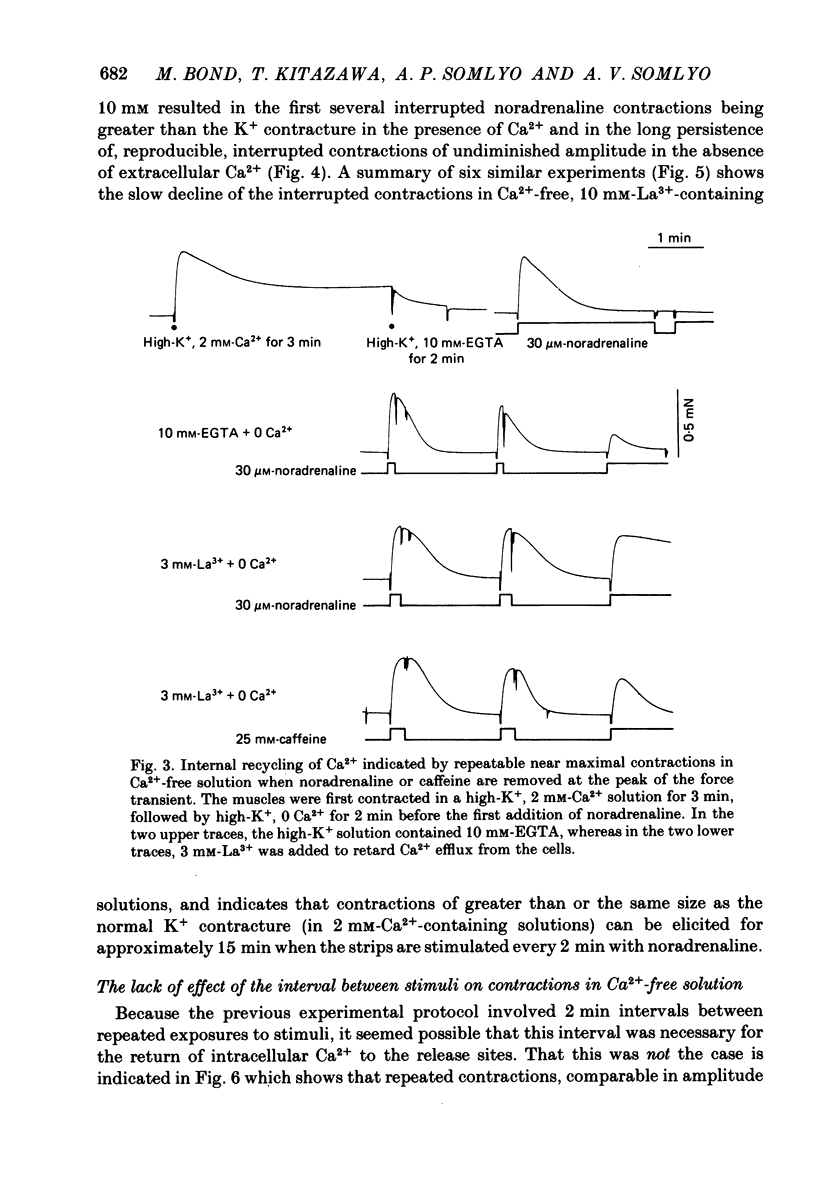
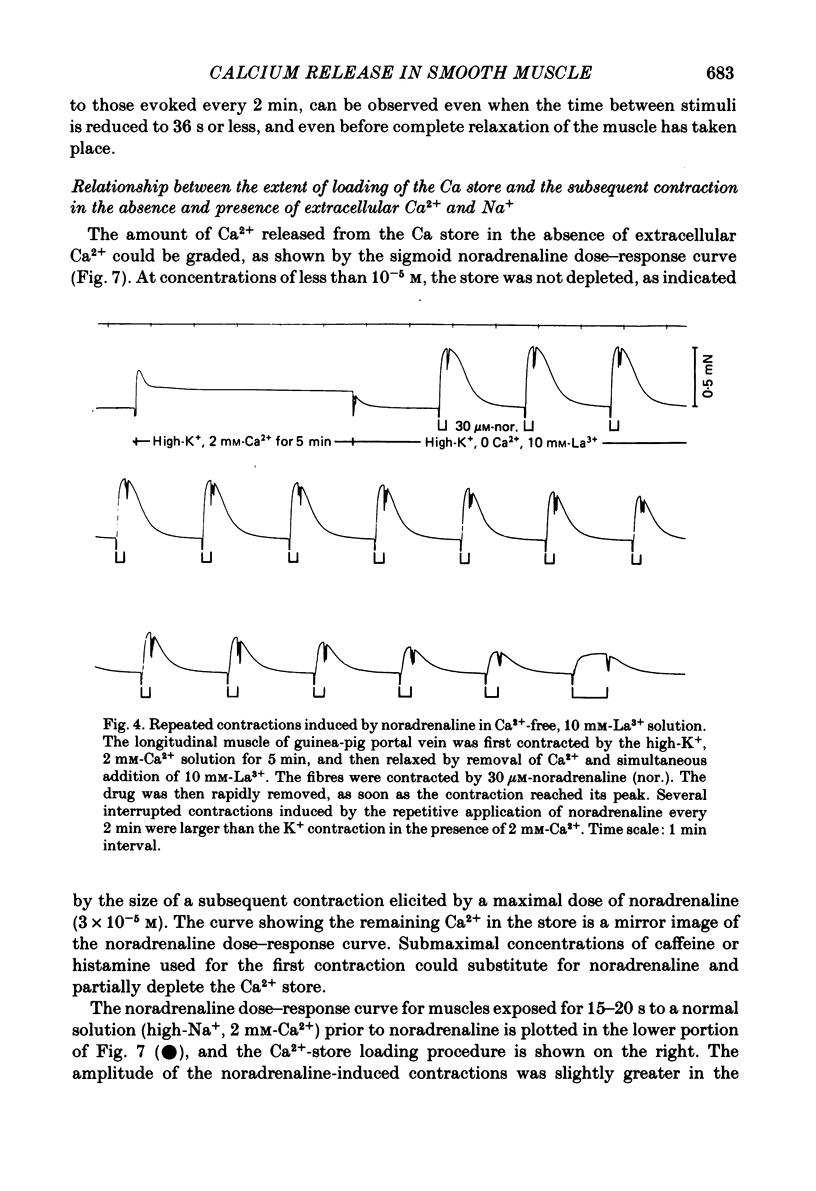
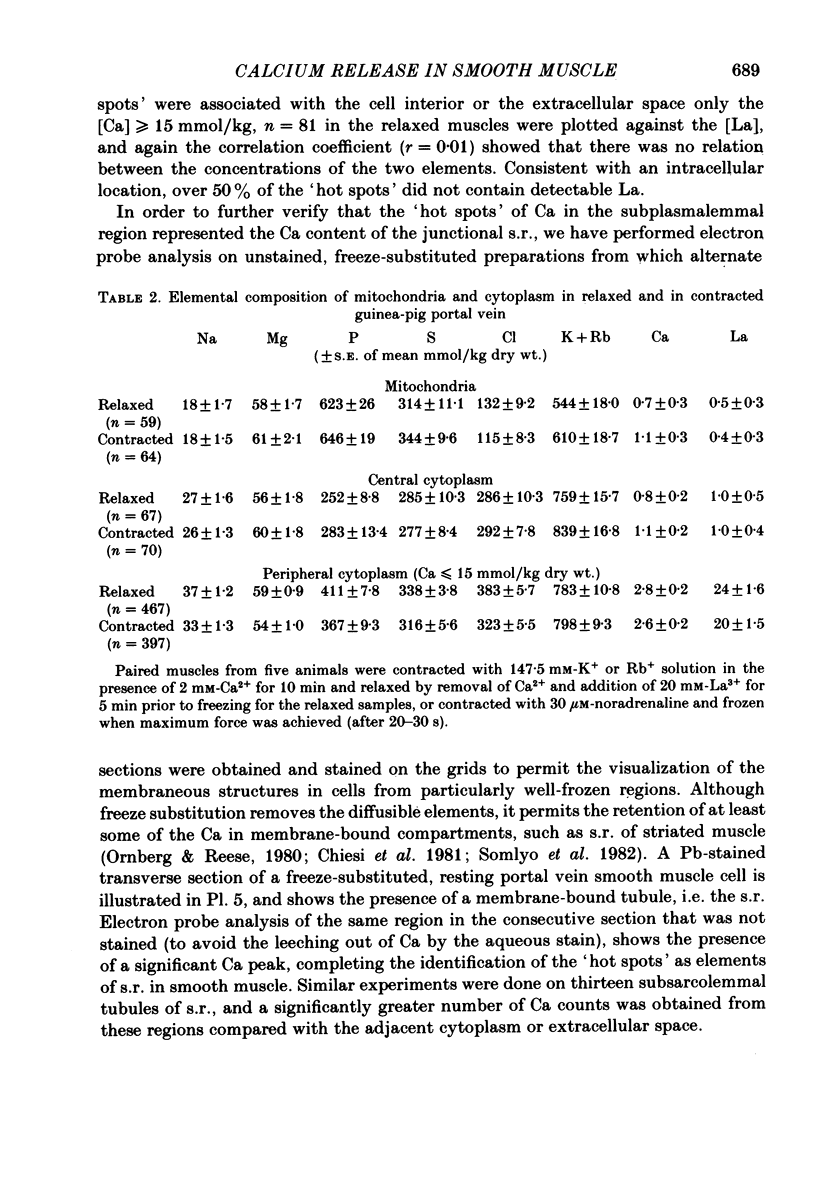
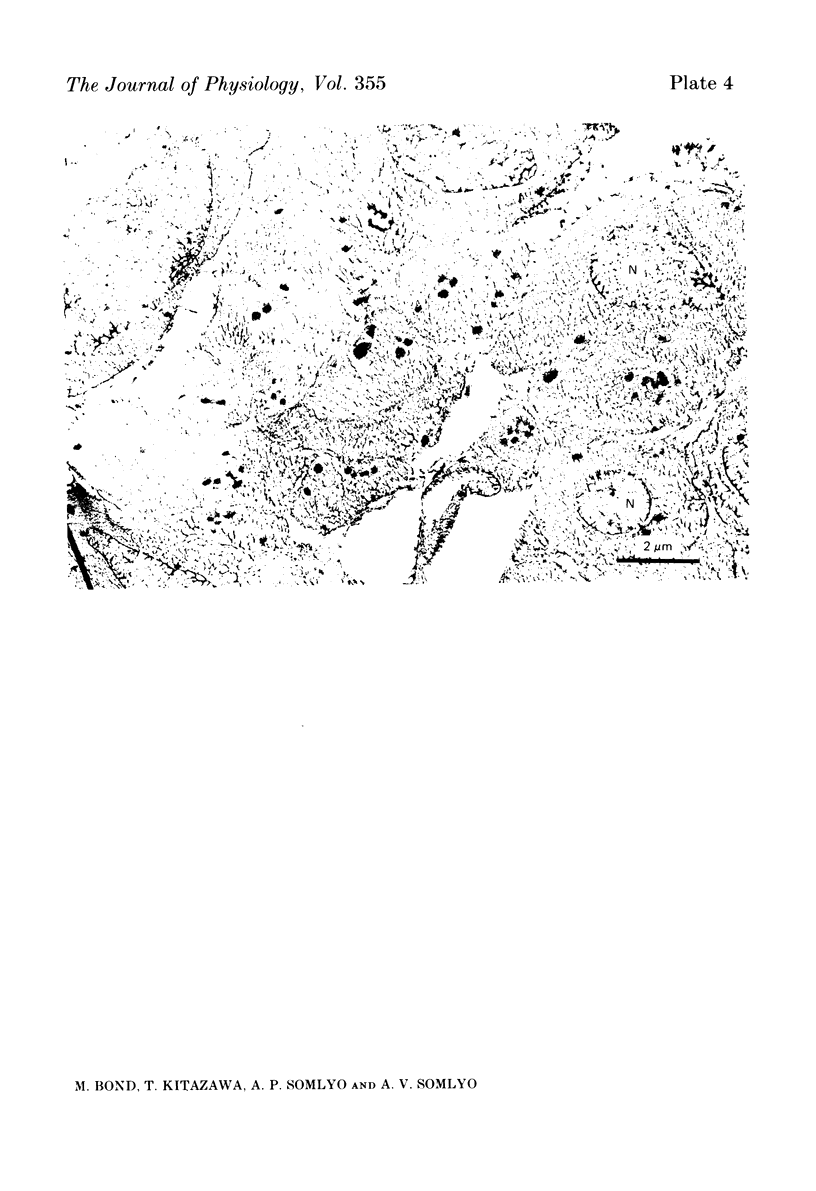
The amplitude of interrupted contractions evoked by noradrenaline or caffeine in Ca2+-free, high-K+ solutions containing EGTA or La3+ was determined in small (40-60 micron thick) bundles of guinea-pig portal anterior mesenteric vein. Interrupted contractions were produced by removing the stimulating agent as soon as the amplitude of the tension record reached its peak. The distribution of intracellular Ca2+ was determined, with electron probe X-ray microanalysis, in cryosections of preparations frozen in the relaxed state and at the peak of noradrenaline-induced contractions. Interrupted contractions of maximal or near-maximal amplitudes could be evoked every 2 min for up to 15 min in the virtual absence of extracellular Ca2+. If noradrenaline was allowed to remain in the solution throughout the period of spontaneous relaxation, a subsequent contraction could no longer be evoked in the absence of extracellular Ca2+. Interrupted contractions, similar to those evoked by noradrenaline, could also be stimulated by caffeine. The amplitude of reproducible interrupted contractions in Ca2+-free, high-K+ solution was graded with noradrenaline concentration. The ability of these smooth muscles to contract repeatedly and maximally in Ca2+-free solutions indicates the recycling of Ca2+ released from an intracellular store. The occurrence of these contractions in high-K+ (depolarizing) solutions supports the conclusion (Devine, Somlyo & Somlyo, 1972) that the release of intracellular Ca2+ is one of the mechanisms of pharmacomechanical coupling. The number of subplasmalemmal regions in which high Ca concentrations (greater than 10 mmol/kg dry wt.) were detected, with approximately 75 nm diameter electron probes, was reduced in muscles frozen at the peak of contraction, from 4.7/cell periphery in the relaxed to 1.4/cell periphery in the contracted preparations. In freeze-substituted smooth muscles, in which the membranes of the junctional sarcoplasmic reticulum could be visualized, the regions containing high Ca were identified as part of the sarcoplasmic reticulum (s.r.), indicating that the s.r. is the store from which noradrenaline and caffeine release Ca2+.
Full text
PDF



















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bozler E. Role of calcium in initiation of activity of smooth muscle. Am J Physiol. 1969 Mar;216(3):671–674. doi: 10.1152/ajplegacy.1969.216.3.671. [DOI] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L., Suzuki H., Van Eldere J. Tension response and 45Ca release in vascular smooth muscle incubated in Ca-free solution. Pflugers Arch. 1981 Dec;392(2):139–145. doi: 10.1007/BF00581262. [DOI] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesi M., Ho M. M., Inesi G., Somlyo A. V., Somlyo A. P. Primary role of sarcoplasmic reticulum in phasic contractile activation of cardiac myocytes with shunted myolemma. J Cell Biol. 1981 Dec;91(3 Pt 1):728–742. doi: 10.1083/jcb.91.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deth R., Casteels R. A study of releasable Ca fractions in smooth muscle cells of the rabbit aorta. J Gen Physiol. 1977 Apr;69(4):401–416. doi: 10.1085/jgp.69.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Yagi S., Iino M. Tension-pCa relation and sarcoplasmic reticulum responses in chemically skinned smooth muscle fibers. Fed Proc. 1982 May;41(7):2245–2250. [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Calcium uptake and force development by skinned muscle fibres in EGTA buffered solutions. J Physiol. 1972 May;223(1):1–19. doi: 10.1113/jphysiol.1972.sp009830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Heuser J. E., Reese T. S., Somlyo A. P., Somlyo A. V. T-tubule swelling in hypertonic solutions: a freeze substitution study. J Physiol. 1978 Oct;283:133–140. doi: 10.1113/jphysiol.1978.sp012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler G., Richards J. G., Thorens S. Noradrenaline contractions in rabbit mesenteric arteries skinned with saponin. J Physiol. 1981 Dec;321:537–556. doi: 10.1113/jphysiol.1981.sp014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. A., Gupta B. L. The localization and assay of chemical elements by microprobe methods. Q Rev Biophys. 1983 Aug;16(3):279–339. doi: 10.1017/s0033583500005114. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. W., Somlyo A. P., Somlyo A. V. Potassium accumulation in smooth muscle and associated ultrastructural changes. J Physiol. 1973 Jul;232(2):247–273. doi: 10.1113/jphysiol.1973.sp010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp R. D., Silcox J. C., Somlyo A. V. Cryoultramicrotomy: evidence against melting and the use of a low temperature cement for specimen orientation. J Microsc. 1982 Feb;125(Pt 2):157–165. doi: 10.1111/j.1365-2818.1982.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Kato S., Ogasawara T., Osa T. Calcium diffusion in uterine smooth muscle sheets. J Gen Physiol. 1982 Aug;80(2):257–277. doi: 10.1085/jgp.80.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Ca concentration and flux in Ca-deprived arteries. J Physiol. 1972 Jul;224(1):35–59. doi: 10.1113/jphysiol.1972.sp009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T., Somlyo A. P., Somlyo A. V. The effects of valinomycin on ion movements across the sarcoplasmic reticulum in frog muscle. J Physiol. 1984 May;350:253–268. doi: 10.1113/jphysiol.1984.sp015199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangel A. W., Nelson D. O., Connor J. A., Prosser C. L. Contractions of cat small intestinal smooth muscle in calcium-free solution. Nature. 1979 Oct 18;281(5732):582–583. doi: 10.1038/281582a0. [DOI] [PubMed] [Google Scholar]
- Ornberg R. L., Reese T. S. A freeze-substitution method for localizing divalent cations: examples from secretory systems. Fed Proc. 1980 Aug;39(10):2802–2808. [PubMed] [Google Scholar]
- Popescu L. M., Diculescu I. Calcium in smooth muscle sarcoplasmic reticulum in situ. Conventional and X-ray analytical electron microscopy. J Cell Biol. 1975 Dec;67(3):911–918. doi: 10.1083/jcb.67.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeymaekers L., Hasselbach W. Ca2+ uptake, Ca2+-ATPase activity, phosphoprotein formation and phosphate turnover in a microsomal fraction of smooth muscle. Eur J Biochem. 1981 May 15;116(2):373–378. doi: 10.1111/j.1432-1033.1981.tb05345.x. [DOI] [PubMed] [Google Scholar]
- Shuman H., Somlyo A. V., Somlyo A. P. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy. 1976 Sep-Oct;1(4):317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Devine C. E., Somlyo A. V., North S. R. Sarcoplasmic reticulum and the temperature-dependent contraction of smooth muscle in calcium-free solutions. J Cell Biol. 1971 Dec;51(3):722–741. doi: 10.1083/jcb.51.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Shuman H. Electron probe and electron energy loss analysis in biology. Ultramicroscopy. 1982;8(1-2):219–233. doi: 10.1016/0304-3991(82)90290-x. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H. Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol. 1979 May;81(2):316–335. doi: 10.1083/jcb.81.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H., Endo M. Calcium and monovalent ions in smooth muscle. Fed Proc. 1982 Oct;41(12):2883–2890. [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Elemental distribution in striated muscle and the effects of hypertonicity. Electron probe analysis of cryo sections. J Cell Biol. 1977 Sep;74(3):828–857. doi: 10.1083/jcb.74.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exp Ther. 1968 Jan;159(1):129–145. [PubMed] [Google Scholar]
- Somlyo A. V., Somlyo A. P. Strontium accumulation by sarcoplasmic reticulum and mitochondria in vascular smooth muscle. Science. 1971 Nov 26;174(4012):955–958. doi: 10.1126/science.174.4012.955. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., Simmonds W. J. The relationship of the calcium content of smooth muscle to its contractility in response to different modes of stimulation. Biochim Biophys Acta. 1965 Nov 29;109(2):503–511. doi: 10.1016/0926-6585(65)90175-5. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]