Abstract
Alcoholic extract of the stems of Coscinium fenestratum, a medicinal plant indigenous to India and Sri Lanka used in ayurveda and siddha medicine for treating diabetes, was studied for its carbohydrate metabolism effect and antioxidant status in streptozotocin–nicotinamide induced type 2 diabetic rats. Oral administration of C. fenestratum stem extract in graded doses caused a significant increase in enzymatic antioxidants such as catalase, superoxide dismutase, glutathione synthetase, peroxidase, and glutathione peroxidase and in the nonenzymatic antioxidants ascorbic acid, ceruloplasmin and tocopherol. Effects of alcoholic extract on glycolytic enzymes such as glucose-6-phosphate dehydrogenase, lactate dehydrogenase and hexokinase showed a significant increase in their levels, whereas a significant decrease was observed in the levels of gluconeogenic enzyme, glucose-6-phosphatase and alanine aminotransferase in treated diabetic rats. Serum creatinine and urea levels also declined significantly. This investigation demonstrates significant antidiabetic activity of C. fenestratum.
Keywords: carbohydrate metabolism, Coscinium fenestratum, enzymatic antioxidants, nonenzymatic antioxidants, streptozotocin–nicotinamide induced diabetes
Introduction
Free radicals may play an important role in the causation and complication of diabetes mellitus. The increased oxidative stress and accompanying decrease in antioxidants may be related to the causation of diabetes (1). In diabetes mellitus, alterations in the endogenous free radical scavenging defense mechanisms may lead to ineffective scavenging of reactive oxygen species, resulting in oxidative damage and tissue injury (2). It has been proposed that streptozotocin acts as a diabetogenic owing to its ability to destroy pancreatic b-islet cells, possibly by a free radical mechanism. The level of lipid peroxidation in cells is controlled by various cellular defense mechanisms consisting of enzymatic and nonenzymatic scavenger systems (3,4), the levels of which are altered in diabetes (5).
Coscinium fenestratum Colebr. (Menispermaceae), commonly known as tree turmeric, grows widely in the Western Ghats (India) and Sri Lanka. The plant has been mainly used for treating diabetes mellitus in the traditional Ayurvedic and Siddha systems of medicine (6). The stem contains berberine, ceryl alcohol, hentriacontane, sitosterol, palmitic acid, oleic acid and saponin, together with some resinous material (7). Isolation of tertiary alkaloids, berlambine, dihydroberlambine and noroxyhydrastinine from the roots has been reported (8). The stem is used for dyspepsia, and as a febrifuge. Its hypotensive (9) and hepatoprotective (10) actions have also been reported. An earlier study carried out by the authors established the significant antidiabetic activity of C. fenestratum (11). In the present investigation we attempted to investigate further the alcoholic stem extract of C. fenestratum for its antioxidant status and its effect on key enzymes of carbohydrate metabolism in streptozotocin and nicotinamide induced type 2 diabetic rats.
Methods
Chemicals and Instruments Used
The following chemicals were used for the study: glucose-6-phosphate dehydrogenase, glucose-6-phosphate, lactate dehydrogenase, streptozotocin (Sigma Aldrich Co., Germany); ascorbic acid, meta phosphoric acid, O-phosphoric acid, magnesium chloride, EDTA, sodium citrate (NICE Chemicals Pvt. Ltd, Cochin, India); phenazine methosulfate, nitroblue tetrazolium chloride, NADH, NADPH, ATP, glutathione, 5, 5′ dithio nitro bis benzoic acid, tocopherol (Himedia Laboratories Ltd, Mumbai, India); disodium hydrogen phosphate, potassium hydrogen phosphate (E. Merck India Ltd, Mumbai, India); 2,4 dinitro phenylhydrazine (Sarabhai M. Chemicals, Baroda, India); sodium pyruvate (S.D. fine-chem Ltd, Mumbai, India); Tris buffer (SISCO Research Laboratories Ltd, Mumbai, India); sodium pyro phosphate (Thomas Baker Chemicals Ltd, Mumbai, India); nicotinamide (Qualigens Fine Chemicals, a division of Glaxo, Mumbai, India).
A UV spectrophotometer (Shimadazu 160 IPC), homogenizer, centrifuge and pH meter were the instruments used for the study.
Albino Rats
Healthy adult male Wistar albino rats weighing ∼250–300 g were used. Rats were housed in polypropylene cages, maintained under standard conditions (12 h light/12 h dark cycle; 25 ± 3°C; 35–60% humidity), and were fed with a standard rat pellet diet (Hindustan Lever Ltd, Mumbai, India) and water ad libitum. The study was approved by the Institutional Animal Ethical Committee of Kasturba Medical College, Manipal, India (IAEC/KMC/03/2003-04).
Plant Material
The C. fenestratum plant material, purchased from Jogappa Shanbag Ayurvedic Store, Udupi, Karnataka, India during August 2003, was authenticated by Dr Gopalakrishna Bhat, Professor, Department of Botany, Poorna Prajna College, Udupi, Karnataka, India. A voucher specimen (PP 526) has been deposited at the Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal, India.
Preparation of Alcoholic Stem Extract
Approximately 160 g of the stem powder was placed in a soxhlet extractor and extracted with ethanol for 72 h. The solvent was recovered by distillation in vacuo, and the residue (yield 18 g), stored in the desiccator, was used for subsequent experiments (12,13).
Induction of Experimental Diabetes
An rat model of type 2 diabetes mellitus (non-insulin dependent diabetes mellitus, NIDDM) was induced (14) in overnight-fasted rats by a single intraperitoneal injection of 60 mg kg−1 streptozotocin 15 min after the intraperitoneal administration of 120 mg kg−1 nicotinamide. Hyperglycemia was confirmed by elevated blood glucose levels determined at 72 h and then on day 7 after injection. Only rats confirmed to have permanent NIDDM were used for the antidiabetic study (15).
Experimental Design
Rats were divided into four groups (n = 6): normal rats administered with 2% gum acacia solution, diabetic rats administered with 2% gum acacia solution, diabetic rats administered with C. fenestratum alcoholic extract 250 mg kg−1 and diabetic rats administered with C. fenestratum alcoholic extract 500 mg kg−1, respectively, for 12 days orally (16).
Sample Collection
Blood Sample
At the end of day 12, blood samples were collected under light ether anesthesia retro-orbitally from the inner canthus of the eye using capillary tubes (Micro Hematocrit Capillaries, Mucaps). Blood was collected in fresh vials containing anticoagulant, and serum was separated in a centrifuge at 2000 r.p.m. for 2 min.
Collection of Organs
Rats were euthanized using an overdose of intraperitoneal anesthesia, and tissue samples collected for assessing the following parameters.
Estimation of Enzymes in Carbohydrate Metabolism
The following parameters were evaluated.
Hexokinase (EC 2.7.1.1)
The hexokinase assay is based on the reduction of NAD+ through a coupled reaction with glucose-6-phosphate dehydrogenase. The excised liver tissue homogenate was prepared in saline. To 0.1 ml of homogenate were added 2.28 ml of Tris (200 mmol l−1)–MgCl2 buffer (20 mol l−1), pH 8, 0.5 ml of 0.67 M glucose, 0.1 ml of 16 mM ATP, 0.1 ml of 6.8 mM NAD and 0.01 ml of 300 U ml−1 glucose-6-phospate dehydrogenase. The solution was mixed thoroughly, and the absorbance was measured at 340 nm (17).
Glucose-6-Phosphate Dehydrogenase (EC 1.1.1.49)
The measure of glucose-6-phosphate dehydrogenase activity is the rate of increase in absorbance. Addition of maleimide inhibits oxidation of reaction products by 6-phospho gluconolactone. Liver tissue was excised and rinsed with saline solution and the homogenate prepared in saline solution. To 0.02 ml of homogenate were added 0.6 ml of distilled water, 0.1 ml of 3.8 mmol l−1 NADP, 0.1 ml of 0.5 mol l−1 Tris buffer (pH 7.5), 0.1 ml of 0.63 mol l−1 MgCl2 and 0.1 ml of 33 mol l−1 glucose-6-phosphate. Then ∼0.5 mg of maleimide was added, with gentle mixing to dissolve the maleimide. The absorbance was measured at 339 nm. One unit of enzyme activity is defined as that quantity which catalyses the reduction of 1 mM of NADP per minute (18).
Lactate Dehydrogenase (EC 1.1.1.27)
Lactate dehydrogenase catalyzes the conversion of l-lactate to pyruvate with simultaneous reduction and oxidation of NAD to NADH. Change in absorbance with time as a result of converting NAD to NADH is directly proportional to LDH activity. The liver and kidney homogenate was prepared in saline. To 0.05 ml of tissue homogenate was added 2.5 ml of Tris (81.3 mmol, pH 7.2)/NaCl (203.3 mmol)/NADH 0.244 mmol l−1), and the solution was mixed thoroughly. To this solution was added 0.5 ml of Tris (81.3 mmol, pH 7.2)/NaCl (203.3 mmol)/pyruvate (9.76 mmol l−1). The solution was mixed well and the absorbance measured at 339 nm (19).
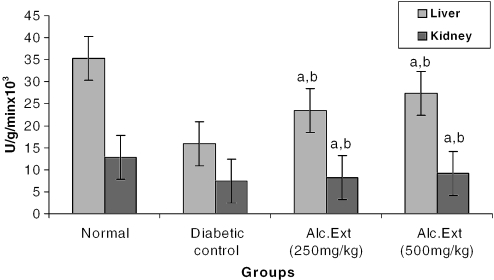
Glucose-6-Phosphatase (EC 3.1.3.9)
Glucose-6-phosphatase catalyzes the conversion of glucose-6-phosphate to glucose. The liver was homogenized in ice-cold sucrose (250 mM) solution. To 0.1 ml of sucrose/EDTA buffer were added 0.1 ml of glucose-6-phosphate (100 mM), 0.1 ml of imidazole buffer (100 mM, pH 6.5) and 0.1 ml of homogenate, with thorough mixing. The tubes were incubated at 37°C for 15 min. The enzymatic activity was terminated by the addition of 2 ml of TCA/ascorbate (10%/2%, w/v), and the solution was centrifuged at 3000 r.p.m. for 10 min. To 1 ml of clear supernatant were added 0.5 ml of ammonium molybdate (1%, w/v) and 1 ml of sodium citrate (2%, w/v). The absorbance was measured at 700 nm. The enzyme activity was expressed as unit per gram per minute in tissue (20).
Alanine Amino Transferase (EC 2.6.1.2)
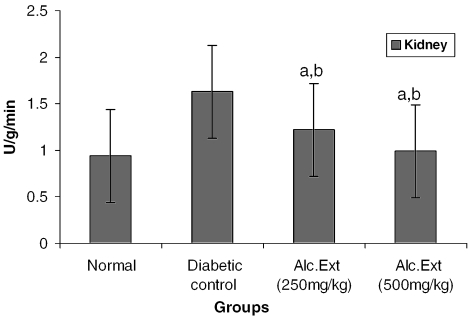
Alanine amino transferase (ALT) catalyzes the transamination of l-alanine to 2-oxo glutarate, forming a glutamate and a pyruvate. The pyruvate formed is reduced to lactate by lactate dehydrogenase with simultaneous oxidation of reduced NADH to NAD. The change in absorbance with time as a result of the conversion of NADH to NAD is directly proportional to the ALT activity. The kidney homogenate was prepared in liver homogenate. To 0.2 ml homogenate were added 2.3 ml l-alanine (610 mmol) in Tris buffer (0.10 mol l−1, pH 7.15), 0.1 ml of NADH (4.2 mmol l−1), 0.1 ml of pyridoxal 6 phosphate (3.4 mmol l−1) and 0.1 ml of lactate dehydrogenase 72 000 U l−1. The reaction was initiated by the addition of 0.2 ml of 2-oxo glutarate solution. The absorbance was measured at 339 nm (21).
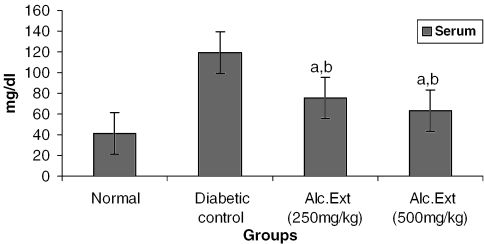
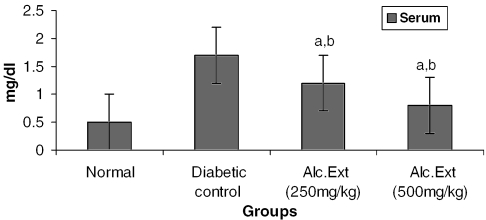
Urea and Creatinine
Serum urea level was determined using a Urease enzyme kit modified Berthlot method (Agappe Diagnostics, Thane, India). The creatinine level in serum was estimated using the alkaline picrate-modified Jaffe's method with a creatinine kit (Agappe Diagnostics).
Estimation of Antioxidant Parameters
Enzymatic antioxidants [glutathione synthetase and glutathione peroxidase (22), catalase (EC 1.11.1.6) (23,24), peroxidase (EC 1.11.1.7) (25), superoxide dismutase (EC 1.15.1.1) (26)] and nonenzymatic antioxidants [ceruloplasmin (27), tocopherol (28) and ascorbic acid (29)] were determined. The protein content in the tissue homogenate was estimated following the standard methodology (30).
Statistical Analysis
Data were statistically evaluated using one-way ANOVA, followed by a post hoc Scheffe's test using the SPSS computer software, version 7.5. The values were considered significant when P < 0.05.
Results and Discussion
Carbohydrate Metabolism
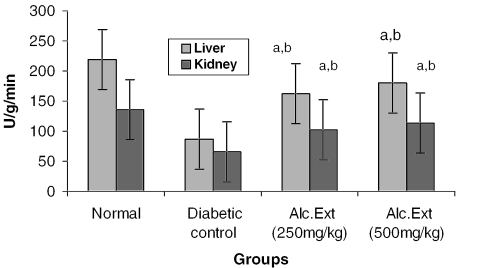
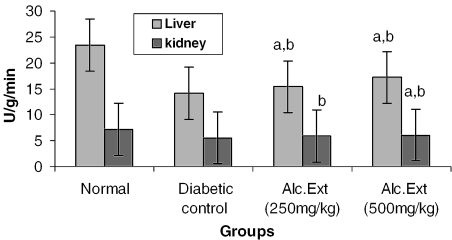
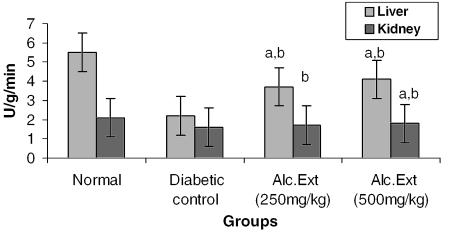
The activities of different key enzymes (hexokinase, glucose-6-phosphate dehydrogenase and lactate dehydrogenase) are represented in Figs 1, 2 and 3. Diabetic animals treated with 500 mg kg−1 of the alcoholic extract of C. fenestratum showed better enzyme activity than those treated with the lower (250 mg kg−1) dose of the alcoholic extract. The significant increase in the levels of hexokinase, a key glycolytic enzyme known to decrease in the diabetic state (31), may be due to the direct stimulation of glycolysis in tissues with increased glucose removal from the blood. The significant reversal of diabetes induced decreased levels of glucose-6-phosphate dehydrogenase and lactate dehydrogenase may be attributed to an increase in glucose utilization through the pentose phosphate pathway (32), interfering with the mitochondrial respiratory chain and promoting the peripheral glucose utilization by enhancing anaerobic glycolysis (33).
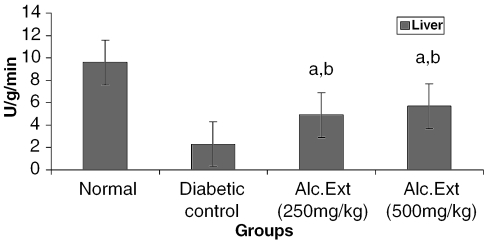
Figure 1.
Alcoholic extract of C. fenestratum significantly increases hexokinase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol reduction of NAD+ per minute.
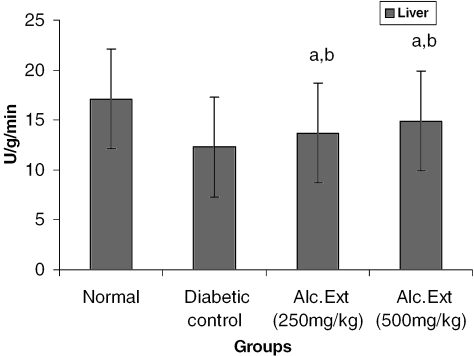
Figure 2.
Alcoholic extract of C. fenestratum significantly increases glucose-6-phosphate dehydrogenase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol conversion of NAD to NADH per minute.
Figure 3.
Alcoholic extract of C. fenestratum significantly increases lactate dehydrogenase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol conversion of NAD to NADH per minute.
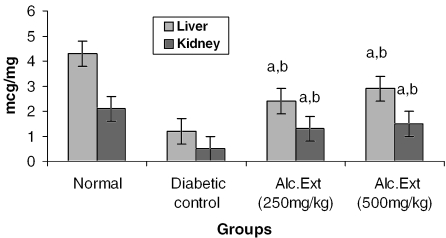
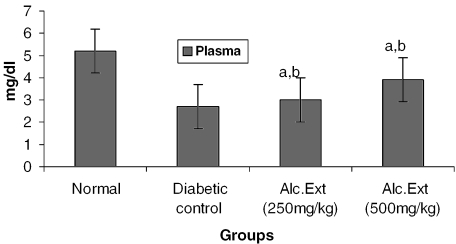
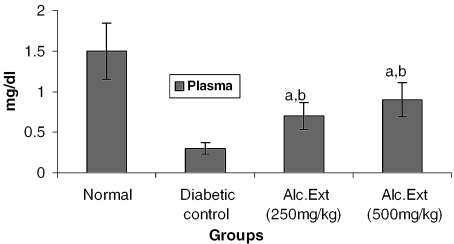
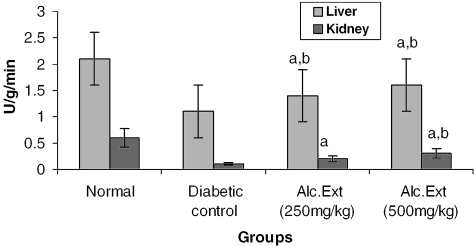
Treated groups exhibited a significant decrease in the levels of glucose-6-phosphatase (Fig. 4) and alanine amino transferase (Fig. 5). Glucose-6-phosphatase, a key enzyme in gluconeogenesis, plays an important role in glucose homeostasis in the liver and kidney (34). The decreased levels observed in treated diabetic animals may be because of the suppression of hepatic gluconeogenesis and glucose output from liver. The elevation in alanine amino transferase in liver and kidney observed in streptozotocin induced diabetic rats corroborates earlier findings (35,36) which attribute the increased gluconeogenesis and ketogenesis observed in diabetes to the high activity of transaminases. In our study, supplementation with the alcoholic extract decreased the enhanced transaminase activity significantly. Serum urea (Fig. 6) and creatinine levels (Fig. 7) were also decreased significantly compared with the diabetic control.
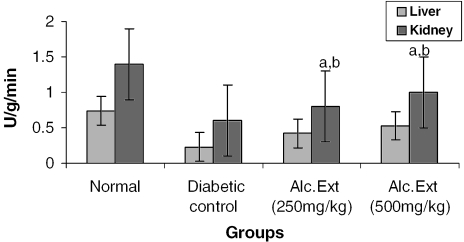
Figure 4.
Alcoholic extract of C. fenestratum significantly decreases glucose-6-phosphatase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol conversion of glucose-6-phosphate to glucose.
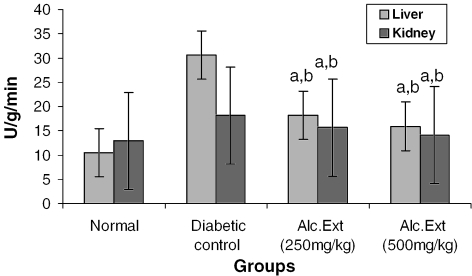
Figure 5.
Alcoholic extract of C. fenestratum significantly decreases alanine amino transferase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol conversion of NADH to NAD per minute.
Figure 6.
Alcoholic extract of C. fenestratum significantly decreases urea levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05).
Figure 7.
Alcoholic extract of C. fenestratum significantly decreases creatinine levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05).
Antioxidant Status
Enzymatic Antioxidants
Antioxidants are of two types: enzymatic and nonenzymatic antioxidants. Catalase, superoxide dismutase, peroxidase, glutathione synthetase and glutathione peroxidase are examples of enzymatic antioxidants. Superoxide dismutase and catalase are considered primary enzymes since they are involved in the direct elimination of reactive oxygen species (37). Superoxide dismutase is an important defense enzyme which catalyzes the dismutation of superoxide radicals (38), and catalase is a hemoprotein which catalyzes the reduction of hydrogen peroxides and protects tissues from highly reactive hydroxyl radicals (39). The reduced activity of superoxide dismutase and catalase in the liver and kidney observed during diabetes may result in deleterious effects as a result of the accumulation of superoxide anion radicals and hydrogen peroxide (40). Glutathione synthetase, the most important biomolecule protecting against chemical induced toxicity, participates in the elimination of reactive intermediates by reduction of hydroperoxide in the presence of glutathione peroxidase (41,42). The decreased level of glutathione synthetase observed in the diabetic animals represents increased utilization resulting from oxidative stress (43). Glutathione peroxidase, a selenium-containing enzyme present in significant concentrations, detoxifies H2O2 to H2O through the oxidation of reduced glutathione (44). Depression of glutathione peroxidase activity, observed in diabetic liver and kidney, has been shown to be an important adaptive response to increased peroxidative stress (45). The activity of enzymatic antioxidants [catalase, glutathione peroxidase, glutathione synthetase, peroxidase and superoxide dismutase (Figs 8–12)] increased significantly in extract-treated animals (P < 0.05).
Figure 8.
Alcoholic extract of C. fenestratum significantly increases catalase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol of hydrogen peroxide consumed per minute.
Figure 12.
Alcoholic extract of C. fenestratum significantly increases superoxide dismutase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol inhibition of Nitro blue tetrazolium chloride reduction per minute.
Nonenzymatic Antioxidants
Tocopherol, ceruloplasmin and ascorbic acid are nonenzymatic antioxidants. Whereas α-tocopherol reduces lipid hydroperoxides generated during the process of peroxidation and protects cell structures against damage (46), the powerful nonenzymatic antioxidant ceruloplasmin inhibits lipid peroxidation by binding to copper (47). Vitamin C or ascorbic acid is an excellent hydrophilic antioxidant in plasma and disappears faster than other antioxidants on exposure to reactive oxygen species (48). The decreased level of ascorbic acid in diabetic rats may be due either to increased utilization as an antioxidant defense against increased reactive oxygen species or to a decrease in glutathione level, since glutathione is required for the recycling of ascorbic acid (49,50). The effect of treatment with C. fenestratum on the nonenzymatic antioxidants tocopherol, ceruloplasmin and ascorbic acid is presented in Figs 13, 14 and 15.
Figure 13.
Alcoholic extract of C. fenestratum significantly increases tocopherol levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05).
Figure 14.
Alcoholic extract of C. fenestratum significantly increases ceruloplasmin levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05).
Figure 15.
Alcoholic extract of C. fenestratum significantly increases ascorbic acid levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05).
In our investigations, the levels of both enzymatic and nonenzymatic antioxidants, which declined in the diabetic animals, were significantly restored on treatment with the alcoholic extract. The overexpression of these antioxidant parameters in diabetic rats treated with C. fenestratum implies that this potential oxidant defense is reactivated by the active principles of C. fenestratum, with an increase in the capacity for detoxification through enhanced scavenging of oxy radicals. The tetrahydro protoberberine analogue of berberine has been reported to inhibit lipid peroxidation and to scavenge hydroxyl free radicals (51). Preliminary phytochemical screening of C. fenestratum showed the presence of phenolic compounds (11), which have been reported to have antioxidant activity (52). Hence in our study the antioxidant activity of C. fenestratum may be due to the presence of berberine and phenolic compounds.
The present investigation draws out a sequential metabolic correlation between increased glycolysis and decreased gluconeogenesis stimulated by C. fenestratum, which may have been the biochemical mechanism through which glucose homeostasis was regulated. The enhanced effect and the protective action on cellular antioxidant defense of C. fenestratum suggest protection against oxidative damage in streptozotocin–nicotinamide induced diabetes.
Figure 9.
Alcoholic extract of C. fenestratum significantly increases glutathione peroxidase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol of glutathione consumed per minute.
Figure 10.
Alcoholic extract of C. fenestratum significantly increases glutathione synthetase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol of 1-Chloro-2, 4-dinitro benzene (CDNB)–glutathione synthetase conjugate formed per minute.
Figure 11.
Alcoholic extract of C. fenestratum significantly increases peroxidase levels in diabetic rats. Each value represents mean ± SE, n = 6; a, statistical significance versus control (P < 0.05); b, statistical significance versus normal (P < 0.05); U, μmol of hydrogen peroxide consumed per minute.
References
- 1.Mohamed AK, Bierhaus A, Schiekofer S, Tritschlet H, Ziegler H, Nawroth PP. The role of oxidative stress and NF (B) activation in late diabetic complications. Biofactors. 1999;10:175–9. doi: 10.1002/biof.5520100211. [DOI] [PubMed] [Google Scholar]
- 2.Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–24. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JMC. Lipid peroxidation, oxygen radicals, cell damage and antioxidant therapy. Lancet. 1994;344:1396–7. doi: 10.1016/s0140-6736(84)91886-5. [DOI] [PubMed] [Google Scholar]
- 4.Simmons KJ. Defense against free radicals has therapeutic implications. J Am Med Assoc. 1984;251:2187–92. doi: 10.1001/jama.251.17.2187. [DOI] [PubMed] [Google Scholar]
- 5.Wohaieb SA, Godin DV. Alterations in free radical tissue defense mechanism in STZ induced diabetes in rat, effects of insulin treatment. Diabetes. 1987;36:1014–18. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 6.Varier PS. Coscinium fenestratum; Indian Medicinal Plants, Compendium of 500 species; Hyderabad, India: Orient Longman Ltd; 1994. pp. 191–193. [Google Scholar]
- 7.Coscinium fenestratum; The Wealth of India, Publication Information and Directorate; India: CSIR; 1950. p. 360. [Google Scholar]
- 8.Datta SC, Mathur RK, Baruah JN. Minor alkaloids of Coscinium fenestratum root. Indian Drugs. 1988;25:350. [Google Scholar]
- 9.Singh GB, Singh S, Bani S, Malhotra S. Hypotensive action of a Coscinium fenestratum stem extract. J Ethnopharmacol. 1990;38:151–5. doi: 10.1016/0378-8741(90)90004-d. [DOI] [PubMed] [Google Scholar]
- 10.Venukumar MR, Latha MS. Effect of Coscinium fenestratum on hepatotoxicity in rats. Indian J Exp Biol. 2004;42:792–7. [PubMed] [Google Scholar]
- 11.Shirwaikar A, Rajendran K, Punitha ISR. Antidiabetic activity of alcoholic stem extract of C. fenestratum in streptozotocin nicotinamide induced type 2 diabetic rats. J Ethnopharmacol. 2005;97:369–74. doi: 10.1016/j.jep.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Kokate CK, Purohit AP, Gokhale SB. Practical Pharmacognosy. New Delhi: Vallabh Prakashan; 1994. Phytochemical Screening; pp. 107–13. [Google Scholar]
- 13.Harborne JB. Phytochemical Methods. London: Chapman & Hall; 1998. Methods of Extraction and Isolation; pp. 60–6. [Google Scholar]
- 14.Masiello P, Broca C, Gross R, Roye M, Manteghetti M, Hillaire-Buys D, et al. Development of a new model of type 2 diabetes in adult rats administered with streptozotocin and nicotinamide. Diabetes. 1998;47:224. doi: 10.2337/diab.47.2.224. [DOI] [PubMed] [Google Scholar]
- 15.Shirwaikar A, Rajendran K, Dinesh Kumar C. Oral antidiabetic activity of Annona squamosa leaf alcohol extract in NIDDM rats. Pharmaceut Biol. 2004;42:30–5. [Google Scholar]
- 16.Shirwaikar A, Rajendran K, Dinesh Kumar C, Ram Gopal Bodla. Antidiabetic activity of aqueous leaf extract of Annona squamosa in streptozotocin nicotinamide type 2 diabetic rats. J Ethnopharmacol. 2004;91:171–5. doi: 10.1016/j.jep.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Brandstrup N, Kirk JE, Bruni C. Determination of hexokinase in tissues. J Gerontol. 1957;12:166–71. doi: 10.1093/geronj/12.2.166. [DOI] [PubMed] [Google Scholar]
- 18.Deutsch J. Bergmeyer Methods of Enzymatic Analysis. 3rd edition. Vol. III. Deerfield Beach, FL: Verlag Chemie; 1983. Glucose 6 Phosphate dehydrogenase; p. 190. [Google Scholar]
- 19.Vassault A. Bergmeyer Methods of Enzymatic Analysis. 3rd edition. Vol III. Deerfield Beach, FL: Verlag Chemie; 1983. Lactate dehydrogenase; p. 118. [Google Scholar]
- 20.Baginsky ES, Foa PP, Zad B. Bergmeyer's Methods in Enzymatic Analysis. Vol. 2. New York: Academic Press; 1974. Glucose 6-phosphatase; pp. 788–92. [Google Scholar]
- 21.Horder M, Rej R. Bergmeyer Methods of Enzymatic analysis. 3rd edition. Vol. III. Deerfield Beach, FL: Verlag Chemie; 1983. Alanine amino transferase; p. 190. [Google Scholar]
- 22.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium biochemical role as a component of glutathione peroxide purification and assay. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 23.Aebi EH. Bergmeyer Methods of Enzymatic analysis. 3rd edition. Vol. III. Deerfield Beach, FL: Verlag Chemie; 1983. Catalase; p. 273. [Google Scholar]
- 24.Sinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 25.Putter J, Becker R. Bergmeyer Methods of Enzymatic analysis. 3rd Edition. Vol. III. Deerfield Beach, Florida: Verlag Chemie; 1983. Peroxidase; p. 286. [Google Scholar]
- 26.Kakkar P, Dos B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 27.Ravin HA. An improved colorimetric assay of ceruloplasmin. J Labor Clin Med. 1961;589:161–8. [PubMed] [Google Scholar]
- 28.Desai LD. Vitamin E analysis methods for animal tissues. Methods Enzymol. 1984;105:138–42. doi: 10.1016/s0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- 29.Omaye ST, Turnball JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. In: McCormick DB, Wright CD, editors. Methods in Enzymology. Vol. 62. New York: Academic Press; 1979. pp. 1–11. [DOI] [PubMed] [Google Scholar]
- 30.Lowry OH, Roesborough MJ, Farr AL, Randall RJ. Protein measurement with folin-phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 31.Baquer NZ, Gupta D, Raju J. Regulation of metabolic pathways in liver and kidney during experimetal diabetes: effects of antidiabetic compounds. Ind J Clin Biochem. 1998;13:63–80. doi: 10.1007/BF02867866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ugochukwu NH, Babady NE. Antihyperglycaemic effect of aqueous and ethanolic extract of Gongronema latifolium levels of glucose and glycogen metabolism in liver of normal and STZ induced diabetic rats. Life Science. 2003;73:1924–38. doi: 10.1016/s0024-3205(03)00543-5. [DOI] [PubMed] [Google Scholar]
- 33.Burtis CA, Ashwood ER. Tietz Textbook Of Clinical Chemistry. 3rd edition. Medina, USA: W.B. Saunder's Company; 1998. Carbohydrate metabolism. Methods of Extraction and Isolation; pp. 594–655. [Google Scholar]
- 34.Berg JM, Tymoczko JL, Stryer L. Glycolysis and gluconeogenesis. In: Berg JM, Tymoczko JL, Stryer L, editors. Biochemistry. New York: W.H. Freeman and Company; 2001. pp. 425–64. [Google Scholar]
- 35.Ghosh S, Suryawanshi SA. Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. Ind J Expt Biol. 2001;39:748–59. [PubMed] [Google Scholar]
- 36.Felig P, Marliss E, Ohman J, Cahill JF. Plasma aminoacid levels in diabetic ketoacidosis. Diabetes. 1970;19:727–30. doi: 10.2337/diab.19.10.727. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Clarendon Press; 1985. Free Radicals and Toxicology; pp. 1–27. [Google Scholar]
- 38.Mccord JM, Keele BB, Fridovich I. An enzyme based theory of obligate anaerobiosis, the physiological functions of superoxide dismutase. Proc Natl Acad Sci USA. 1976;68:1024–7. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chance B, Greenstein DS, Roughton RJW. The mechanism of catalase action—steady state analysis. Arch Biochem Biophys. 1952;37:301–39. doi: 10.1016/0003-9861(52)90194-x. [DOI] [PubMed] [Google Scholar]
- 40.Searle AJ, Wilson R. Glutathione perioxide effect of superoxide, hydroxyl and bromine free radicals on enzyme activity. Int J Radiat Biol. 1980;37:213–17. doi: 10.1080/09553008014550261. [DOI] [PubMed] [Google Scholar]
- 41.Meister A. New aspects of glutathione biochemistry and transport selective alterations of glutathione metabolism. Nutr Rev. 1984;42:397–410. doi: 10.1111/j.1753-4887.1984.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 42.Nicotera P, Orrenius S. Role of thiols in protection against biological reactive intermediates. Adv Exp Med Biol. 1986;197:41–9. doi: 10.1007/978-1-4684-5134-4_4. [DOI] [PubMed] [Google Scholar]
- 43.Anuradha CV, Selvam R. Effect of oral methionine on tissue lipid peroxidation and antioxidants in alloxan induced diabetic rats. J Nutr Biochem. 1993;4:212–17. doi: 10.1016/0955-2863(90)90027-i. [DOI] [PubMed] [Google Scholar]
- 44.Bruce A, Freeman D, James C. Biology of disease-free radicals and tissue injury. Lab Invest. 1982;47:412–26. [PubMed] [Google Scholar]
- 45.Kinalski M, Sledziewski A, Telejko B, Zarzycki W, Kinalska T. Lipid peroxidation and scavenging enzyme activity in streptozotocin induced diabetes. Acta Diabetologia. 2000;37:179–83. doi: 10.1007/s005920070002. [DOI] [PubMed] [Google Scholar]
- 46.Halliwell B, Gutteridge JMC. The antioxidant of human extra cellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 47.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA. 1986;86:6377–81. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inefers H, Sies H. The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur J Biochem. 1988;174:353–7. doi: 10.1111/j.1432-1033.1988.tb14105.x. [DOI] [PubMed] [Google Scholar]
- 49.Sajithlal GB, Chitra P, Chandrakasan G. Effect of curcumin on the advanced glycation and cross linking of collagen in diabetic rats. Biochem and Pharmacol. 1998;56:1607–14. doi: 10.1016/s0006-2952(98)00237-8. [DOI] [PubMed] [Google Scholar]
- 50.Jin XL, Shao Y, Wang MJ, Chen LJ, Jin GZ. Tetrahydro protoberberines inhibit lipid peroxidation and scavenge hydroxyl free radicals. Acta Pharmacol Sin. 2000;21:477–80. [PubMed] [Google Scholar]
- 51.Auddy B, Ferreiru M, Blasina F, Lafon L, Arredondo F, Dajas F, et al. Screening of antioxidant activity of three Indian medicinal plant traditionally used for the management of neurodegerative diseases. J Ethnopharmacol. 2003;84:131–8. doi: 10.1016/s0378-8741(02)00322-7. [DOI] [PubMed] [Google Scholar]
- 52.Hung CY, Yen GC. Antioxidant activity of phenolic compounds isolated from Mesona procumbens Hemsl. J Agric Food Chem. 2002;50:2993–7. doi: 10.1021/jf011454y. [DOI] [PubMed] [Google Scholar]