Abstract
The protective effect of methanolic extract of milk thistle seeds and silymarin against cisplatin-induced renal toxicity in male rats after a single intraperitoneal injection of 3 mg kg−1 cisplatin were studied. Over 5 days, cisplatin-treated rats showed tubular necrosis and elevation in blood urea nitrogen (BUN) and serum creatinine (Scr). Pretreatment of animals with silymarin (50 mg kg−1) or extract (0.6 g kg−1) 2 h before cisplatin prevented the tubular damage. Rats treated with silymarin or extract 2 h after cisplatin had BUN and Scr significantly lower than those receiving cisplatin, but mild to moderate necrosis was observed. These results suggested that milk thistle may protect against cisplatin-induced renal toxicity and might serve as a novel combination agent with cisplatin to limit renal injury.
Keywords: cisplatin, milk thistle, renal toxicity, silymarin
Introduction
Cisplatin is a potent anticancer agent against solid tumors of the testes, ovaries, breasts, lungs, bladder, etc. However, in practice, the use of cisplatin is limited by its marked renal toxicity (1–4). It has been suggested that the generation of reactive oxygen species and lipid peroxidation is responsible for the cisplatin-induced renal tubular injury (5–7).
Flavonoids are naturally occurring substances that possess various pharmacological actions and therapeutic applications. Some due to their phenolic structures have antioxidant effect and inhibit free radical-mediated processes (8–11). Silymarin, a mixture of three isomeric flavonolignans, is isolated from milk thistle (Silybum marianum) seeds. Silymarin is used clinically to treat chronic inflammatory liver disease and hepatic cirrhosis. Hepatoprotection can be attributed to its antioxidant properties by scavenging free radicals and increasing intracellular concentration of glutathione (12,13).
The aim of this study was to evaluate the protective effect of milk thistle methanolic extract and silymarin against cisplatin-induced acute renal failure in rats.
Materials and Methods
Animals
The study was performed on male wistar rats, 8–10 weeks, 200–240 g that were bred and kept at the animal center of Mashhad School of Pharmacy. They were housed in ventilated rooms at temperature of 24 ± 2°C with a 12 h light/dark cycle and 60 ± 5% humidity. They were provided with food and water ad libitum.
Plant Material
The seeds of milk thistle plant were purchased from local market of Mashhad and authenticated by matching with the specimen available in pharmacognosy section of pharmacy school. A voucher specimen (S/A/102) was also deposited in the school.
Preparation of Extract
The dried seeds of milk thistle were powdered and 5 g of the powder was extracted with 95% methanol (100 ml) thrice, overnight at 28°C with continuous stirring. The pooled extracts were evaporated under reduced pressure to a known volume. The residual solvent was evaporated to dryness at 40°C on a water bath.
Experimental Procedure
The method is based on the modification of Baliga et al. (14). Four groups of rats (n = 5) were used to study the effect of silymarin and methanolic extract on cisplatin-induced renal toxicity and changes in renal function. Group 1 animals received cisplatin (3 mg kg−1) intraperitoneally. Groups 2 and 3 received intraperitoneal methanolic extract (0.6 g kg−1) or silymarin (50 mg kg−1; Sigma, UK) 2 h before cisplatin injection. Groups 4 and 5 received the same dose of extract or silymarin 2 h after cisplatin administration. Group 6 acted as vehicle (normal saline + 1% w/v methylcellulose) control. Rats were sacrificed 5 days after cisplatin administration. The protocols used conformed with guidelines of the conduct of animal experiments issued by pharmacy school and were approved by the committee on the ethics of animal experiments in Mashhad University. Blood samples were collected and analyzed for blood urea nitrogen (BUN) and serum creatinine (Scr) by using the commercial kits (15). After bleeding, the kidneys were removed and fixed in 10% neutral buffered formalin for at least 24 h. Tissues were processed for microscopical examination using a standard protocol and paraffin sections were stained with hematoxylin and eosin (16). The changes seen were limited to the tubulointerstitial areas and graded as follows: grade 0, normal; grade I, areas of tubular epithelial cell swelling, necrosis and desquamation involving <25% of cortical tubules; grade II, similar changes involving >25% but <50% of cortical tubules; grade III, similar changes involving <50% but <75% of cortical tubules; and grade IV, similar changes involving >75% of cortical tubules.
Statistical Analysis
The results are expressed as mean ± SEM. Data were analyzed by one-way analysis of variance. Sequential difference among mean were calculated at the level of P < 0.05, using Tukey contrast analysis as needed.
Results and Discussion
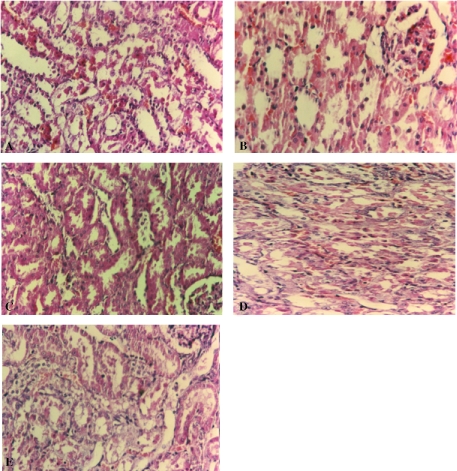
The injection of cisplatin produced proximal and distal tubular necrosis, mainly in the corticomedullary region and intratubular casts in the outer stripe of the outer medulla (Fig. 1A; grade IV). Functional nephrotoxicity indices such as BUN and Scr were elevated in cisplatin-treated rats compared with saline. Treatment with silymarin or extract 2 h before cisplatin prevented the increases in BUN and Scr as well as tubular damage (Fig. 1B and C; grades 0 and I). Rats treated with silymarin or extract 2 h after cisplatin had BUN and Scr levels significantly lower than those receiving cisplatin alone, but mild to moderate cell injury was observed (Fig. 1D and E; grades II and III). The results of renal functional determinations are summarized in Table 1. Our results are in agreement with previous study that showed silibinin (200 mg kg−1, intravenously), one of the component of silymarin, 1 h before the cisplatin (5 mg kg−1, intraperitoneally) prevented the effects of cisplatin on creatinine clearance and proteinuria, and diminished morphological alteration in proximal tubules (17).
Figure 1.
Histological examination. (A) Areas of sever necrosis of proximal tubules, 5 days after injection of 3 mg kg−1 cisplatin. (B) Minimal tubular necrosis after treatment with extract (0.6 g kg−1) 2 h before cisplatin. (C) Minimal tubular necrosis after treatment with silymarin (50 mg kg−1) 2 h before cisplatin. (D) Minimal to moderate tubular necrosis after treatment with extract (0.6 g kg−1) 2 h after cisplatin. (E) Minimal to moderate tubular necrosis after treatment with silymarin (50 mg kg−1) 2 h after cisplatin.
Table 1.
Protective effect of silymarin (50 mg kg−1) and methanolic extract of milk thistle (0.6 g kg−1) against cisplatin (3 mg kg−1) induced elevation of BUN and Scr
| BUN (mg dl−1) | Scr (mg dl−1) | |
|---|---|---|
| Vehicle | 17.48 ± 1.80*** | 0.75 ± 0.05** |
| Cisplatin | 56.80 ± 2.40 | 1.50 ± 0.20 |
| + Silymarin (2 h before cisplatin) | 31.20 ± 2.60** | 1.15 ± 0.05* |
| + Silymarin (2 h after cisplatin) | 30.10 ± 1.50** | 1.12 ± 0.09* |
| + Extract (2 h before cisplatin) | 28.00 ± 2.80** | 1.12 ± 0.15* |
| + Extract (2 h after cisplatin) | 32.26 ± 1.70** | 1.05 ± 0.10* |
n = 5, mean ± SEM;
*P < 0.5,
**P < 0.01,
***P < 0.001 significantly different compared with cisplatin-treated group.
Induction of nephrotoxicity by cisplatin is assumed to be a rapid process involving reaction with proteins in the renal tubules (18,19). Because this renal damage occurs within 1 h after administration (20), it is important that the protective agent is present in renal tissue before damage occurs. This might explain why complete protection did not result when silymarin or extract were given after administration of cisplatin. The acute renal failure indicated by increased Scr and BUN occurred before the development of tubular necrosis. These parameters are markers of glomerular filtration rate. However, it cannot be excluded that the enhancement of these parameters may be the result of tubular obstruction or tubular backleak (21). Our finding has been interpreted as a support of the hypothesis that tubular obstruction and/or tubular fluid backleak are not involved in the initiation of acute renal failure in this model of nephrotoxicity.
The mechanisms by which silymarin and extract ameliorate cisplatin toxicity remains to be elucidated. We supposed they may inhibit lipid peroxidation by scavenging free radicals and increasing intracellular concentration of glutathione. Previous studies have indicated that superoxide anions inactivate nitric oxide (NO) and that NO-dependent vascular relaxation is enhanced by superoxide dismutase (22,23). There is a possibility that they could have maintained RBF as a result of preserved NO through scavenging of the superoxide anions. Further studies are needed to determine the exact mechanism of silymarin and extract.
In conclusion, the present finding suggest that S. marianum protects against acute cisplatin nephrotoxicity and may be considered as a potentially useful candidate in the combination chemotherapy with cisplatin.
Acknowledgments
We thank Dr Omidi and Dr Kalantari for helping in histological studies and Vice Chancellor of research for financial support.
References
- 1.Rozeneweing M, Van Hoff D, Slavil M, Maggia FM. cis-Diaminedichloroplatinum, a new anticancer agent. Ann Int Med. 1977;86:803–9. doi: 10.7326/0003-4819-86-6-803. [DOI] [PubMed] [Google Scholar]
- 2.Winston JA, Safirstein R. Reduced renal flow in early cisplatin-induced acute renal failure in the rat. Am Physiol. 1985;249:F490–6. doi: 10.1152/ajprenal.1985.249.4.F490. [DOI] [PubMed] [Google Scholar]
- 3.Greggi Antunes LM, Darin JD, Bianchi M. Protective effects of vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats. Pharmacol Res. 2000;41:405–11. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 4.Chirino YI, Hernandez-pando R, Pedraza-Chaveri J. Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol. 2004;4:20–9. doi: 10.1186/1471-2210-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugihara K, Nakano S, Koda M, Tanaka K, Fukuishi N, Gemba M. Stimulatory effect of cisplatin on production of lipid peroxidation in renal tissues. Jpn J Pharmacol. 1987;43:247–52. doi: 10.1254/jjp.43.247. [DOI] [PubMed] [Google Scholar]
- 6.Mora L, Antunes LM, Francescato HD, Bianchi M. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2003;47:517–22. doi: 10.1016/s1043-6618(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 7.Xiao T, Choudhary S, Zhang W, Ansari NH, Salahudeen A. Possible involvement of oxidative stress in cisplatin-induced apoptosis in LLC-PK1 cells. J Toxicol Environ Health. 2003;66:469–79. doi: 10.1080/15287390306449. [DOI] [PubMed] [Google Scholar]
- 8.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–8. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 9.Mora A, Paya M, Rios J, Alcaraz M. Structure activity relationships of polymetoxyflavones and other flavonoids as inhibitors of non-enzymatic lipid peroxidation. Biochem Pharmacol. 1990;40:793–4. doi: 10.1016/0006-2952(90)90317-e. [DOI] [PubMed] [Google Scholar]
- 10.Singh D, Chander V, Chopra K. Protective effect of catechin on ischemia-reperfusion-induced renal injury in rats. Pharmacol Rep. 2005;57:70–6. [PubMed] [Google Scholar]
- 11.Zhao B. Natural antioxidants for neurodegenerative diseases. Mol Neurobiol. 2005;31:283–94. doi: 10.1385/MN:31:1-3:283. [DOI] [PubMed] [Google Scholar]
- 12.DerMarderosian A. The Review of Natural Product. St Louis: Fact and Comparison; 2001. pp. 405–9. [Google Scholar]
- 13.Fleming T. PDR for Herbal Medicines. New Jersy: Medical Economics Company; 2000. pp. 516–8. [Google Scholar]
- 14.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998;53:394–401. doi: 10.1046/j.1523-1755.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 15.McClatchey KD. Clinical Laboratory Medicine. London: Williams & Wilkins; 1994. pp. 376–8. [Google Scholar]
- 16.Hammersen F. Histology. Munich: Urban and Schwarzenberg; 1985. pp. 1–4. [Google Scholar]
- 17.Gaedeke J, Fels LM, Bokemeyer C, Mengs U, Stolte H, Lentzen H. Cisplatin nephrotoxicity and protection by silibinin. Nephrol Dial Transplant. 1996;11:55–62. [PubMed] [Google Scholar]
- 18.Heidman HT, Gerkens JF, Jackson EK, Branch RA. Attenuation of cisplatin induced nephrotoxicity in the rat by high salt diet, furosemide and acetazolamide. Arch Pharmacol. 1985;329:201–5. doi: 10.1007/BF00501213. [DOI] [PubMed] [Google Scholar]
- 19.Montine TJ, Borch RF. Role of endogenous sulfur containing nucleophiles in an in vitro model of cis-diamminedichloroplatinium-induced nephrotoxicity. Biochem Pharmacol. 1990;39:1751–7. doi: 10.1016/0006-2952(90)90121-z. [DOI] [PubMed] [Google Scholar]
- 20.Rao M, Rao MM. Protective effects of selenomethionine against cisplatin-induced renal toxicity in mice and rats. J Pharm Pharmacol. 1992;50:687–91. doi: 10.1111/j.2042-7158.1998.tb06906.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones TW, Chopra S, Kaufman JS, Flamenbaum W, Trump B. Cis-diammine-dichloroplatinium-induced acute renal failure in the rat. Lab Invest. 1985;52:363–74. [PubMed] [Google Scholar]
- 22.Rubanyi GM, Vanhoutte PM. Superoxide anions and hypoxia inactivate endothelium derived relaxing factor. Am J Physiol. 1986;250:H822–7. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 23.Modlinger PS, Wilcox CS, Aslam S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin Nephrol. 2004;24:354–65. doi: 10.1016/j.semnephrol.2004.04.007. [DOI] [PubMed] [Google Scholar]