Abstract
Echinacea has been viewed as an immunoenhancing herb since it became commercially available several years ago. Indeed, its medicinal significance is responsible for billions of dollars in worldwide sales annually. Unfortunately, most of the ‘evidence’ for the purported medicinal efficacy of Echinacea has been anecdotal and, moreover, to this day, there is no formal proof on how to achieve the best results—whether it should be consumed daily throughout life as a prophylactic; consumed by either young or old; or consumed after diseases, such as cancer, have taken hold. Our work over the past 5 years has led to conclusive answers to some of these questions, at least in mice. Our results have shown that daily consumption of Echinacea is indeed prophylactic, extends the life span of aging mice, significantly abates leukemia and extends the life span of leukemic mice. Given that humans are 97% genetically common with mice and that virtually all our basic physiology is identical, it is neither unjustified to extrapolate these observations to humans nor would it be an arduous task to perform many of these studies in humans, thus establishing viable scientific evidence replacing the anecdotal.
Keywords: Echinacea, hemopoiesis, immunology, leukemia, NK cells
Introduction
Natural Killer Cells and Echinacea: a Harmonious Duo
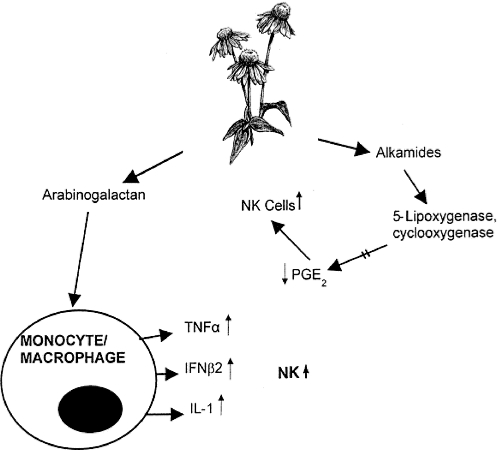
The herb, Echinacea, after making its debut on the world's commercial markets more than a decade ago, has become one of the top-selling herbs of all time. Many of its ingredients are powerful immune system stimulators. Its contents include high molecular weight polysaccharides, essential oils, alkylamides such as echinacein, isobutylamides such as pentadecadienes and hexadecadienes, polyacetylene, tannins, inulin, heteroxylan, flavonoids and vitamin C. Indeed, the biochemistry and content definition of Echinacea and most other herbs has taken place decades before the medicinal value of the phytochemicals they contain ever merited investigation. Some of the contents of Echinacea are natural killer (NK) cell stimulants while others (the alkylamides) inhibit the endogenous suppressors of NK cells, i.e. the prostaglandins. NK cells are the first line of defense in cancer immunosurveillance, and consequently any agent that will either stimulate these fundamental cells or remove any negative influence on them would be clearly of medicinal value. In spite of the manifold functions of the prostaglandins in vivo, it is clear that at least one member of the prostaglandin family is detrimental to NK cells. The alkamide family of compounds within Echinacea inhibits the production of 5-lipoxygenase and cyclooxygenase, which are enzymes needed for the production of prostaglandins (1,2). Thus, reducing or eliminating this negative influence should result in an absolute and functional increase in NK cells (Fig. 1). Indeed, this is what we found some years ago when the drug indomethacin, an inhibitor of these key enzymes in prostaglandin formation, was administered in vivo to leukemic mice. This drug in vivo resulted in statistically significant increases in NK cell numbers and function in leukemic mice (3,4). Unfortunately, indomethacin, as with most exogenously administered drugs/factors, is beset with significant undesirable side effects that necessarily restrict its long-term use.
Figure 1.
Contained within Echinacea root extract is a family of complex polysaccharides known as arabinogalactans. These sugars directly stimulate macrophages to produce three cytokines that, in turn, directly stimulate NK cells. The latter respond by means of new NK cell production/numbers and/or increased lytic functional capacity. On the other hand, contained also within Echinacea root extract are a group of molecules known as the alkamides, some of which interact with two key enzymes essential to the production of prostaglandin E2 (PGE2). Normally, PGE2 is suppressive to NK cells. Consequently, when the fundamental enzymes are blocked, PGE2 levels are negligible and NK cells, now free of their suppressors, become increased in numbers and function. Thus, via these two different avenues, i.e. stimulation indirectly through macrophages, and release from suppressor factors (PGE2), whole Echinacea is a powerful NK cell stimulant. The diagram of the Echinacea plant is reproduced with permission from The Herbal Drugstore, LB White & S Foster, Rodale Inc., 2000.
Furthermore, there is considerable evidence suggesting that other phytochemicals in Echinacea might have the capacity to reduce tumors and virus infections (5–10). Among the polysaccharides contained within Echinacea, the complex carbohydrate group known as the arabinogalactans are particularly significant (5,11,12). Macrophages upon stimulation by arabinogalactans (Fig. 1) release, in turn, a host of NK cell stimulants (11,13–16).
Consequently, any agent that contains these two valuable compounds, both so beneficial to those cells acting at the first line of defense, i.e. the NK cells, is worthy of investigation for its prophylactic potential and its therapeutic value. It was against this background, i.e. the medicinal potential of Echinacea in NK cell enhancement, that we undertook an in-depth in vivo study of this herb in (i) aged mice and (ii) mice afflicted with leukemia, under controlled laboratory conditions.
Virtually all that was known about the medicinal potential of Echinacea had been established in vitro. Our first study, in contrast, was conducted in vivo ∼5 years ago, and at that time we investigated the effect on hemopoietic and immune cells of daily dietary intake of this herb for 2 weeks (17). After 2 weeks, we analyzed quantitatively the absolute changes in all the hemopoietic and immune cells in both the spleen and the bone marrow, the latter being the organ of de novo generation of all hemopoietic and immune cells. The spleen, on the other hand, is a major repository for all these cells since this organ is on the blood circulatory highway. In the spleen there are cells that reside and function therein, i.e. the cells mediating specific (adaptive) immunity (T and B lymhocytes), as well as NK cells and monocytes—both types being responsible for non-specific, spontaneous and non-adaptive immunity. Other cells involved in the disease defense process, i.e. the mature and maturing cells of the granulocyte lineages (eosinophils, neutrophils and basophils) also either function in the spleen or pass through it en route elsewhere.
In our analysis of the effect of Echinacea on the above-mentioned cells, everything was standardized. The mice were inbred and of identical age, weight and gender (male). Moreover, housing conditions were identical between cages of mice consuming Echinacea and those consuming untreated diet. The quantity and quality of food and water were also standardized among all cages—those receiving the herb in the diet and those not given the herb (controls).
It is obviously of fundamental importance that Echinacea itself, as with any agent given either prophylactically or therapeutically, is not deleterious to the host. In the case of Echinacea, there appears to be no in vivo toxic level, i.e. overdose level, as defined by several assays and criteria (6,18,19). The immunostimulating effects of Echinacea in vivo are exclusive to cells mediating spontaneous immunity and their accessory cells, i.e. NK cells and monocytes (17). While Echinacea appears to be tailor-made for its highly positive influences on this arm of the immune system, there are instances, in vivo, where use of this herb may be contraindicated. For example, individuals demonstrating allergy to members of the Family Asteraceae, to which Echinacea belongs, would clearly be ill-advised to consume this herb for any reason (20,21). Moreover, there is very little available information concerning the potential for detrimental interactions of Echinacea with either other herbs or pharmaceuticals (22). Another problem pertains to the choice of the most effective source of Echinacea as NK stimulant—not an insignificant problem since there is extremely wide variation in the quality of Echinaceas from assorted commercial sources. For our experiments, we chose a product from a commercial supplier, which we proved was consistent in quality and NK stimulating potency, and revealed in dose–response analyses, a progressive increment in NK cell numbers up to a maximum (plateau) beyond which no further increase in NK cells occurred. It was this dosage that we have used throughout our experiments to date, including those reported in this review.
Should Echinacea be Taken When Healthy?
When healthy young adult mice consumed Echinacea daily in their diet for 7 days, we found significantly more NK cells, identified by our standard immunoperoxidase labeling methods, in their bone marrow than in the bone marrow of mice consuming untreated diet (P < 0.01), while the spleens of mice consuming Echinacea had 25% more NK cells, which is a clear elevation in number although not yet statistically significant (17). By 2 weeks, however, those mice consuming the herb had significantly more NK cells in their spleens and bone marrow (P < 0.01). The early (7 days) elevation in absolute numbers of NK cells in the bone marrow necessarily indicated that actual generation of new NK cells was underway in that organ under the influence of Echinacea. The 25% increment in the spleen simply reflected the increased new NK cell production, since it is well established that the spleen is major site to which virtually all newborn, bone marrow-derived NK cells unidirectionally migrate (via the blood). NK cells do not recirculate back to the bone marrow (23–25). However, during 2 weeks of daily Echinacea exposure, the elevating levels of new NK cell production by the bone marrow resulted in a supernormal export of these additional NK cells to the spleen, such that there was indeed a statistically significant increase in the numbers of NK cells in the spleen by 2 weeks as well.
Also of considerable interest was the observation that the ‘helper’, or accessory, cells for NK cells, i.e. the monocytes, were ∼25% more numerous in both the bone marrow and the spleen of mice consuming the herb for 7 days, and were statistically more numerous in both organs (P < 0.01) after 14 days of the dietary herb (17). To our surprise, mature granulocytes and their precursors, as well as all other lymphocytes (T and B), and the red blood cell precursors remained steadfastly at control levels (mice consuming untreated diet) in both the spleen and the bone marrow, irrespective of whether mice had consumed Echinacea for 7 days or 14 days. Moreover, we have consistently shown in all our previous studies that all mice on Echinacea-containing diets were clinically no different from littermates and cage mates consuming untreated chow, with respect to body weight, coat texture and level of activity. Our administration to mice of daily dietary doses of this herb of 0.45 mg per 25 gm body weight was indeed derived from the average recommended dose for adult humans (averaged as 125 lb), indicated on the labels of Echinacea containers provided by several different suppliers.
Since our murine studies were carried out under highly controlled conditions (above), with the only variable being the presence or absence of dietary Echinacea, then the singularly positive influence on NK cells and their accessory cells (monocytes) must have resulted only from the presence of Echinacea. A major observation of this finding is that Echinacea appears able to influence new cell generation in the NK/monocyte systems, as evidenced by the significant bone marrow increments in these cell types. Thus, the elevated numbers of these cells observed in the spleen is the direct result of the increased cell proliferation in the bone marrow with subsequent dissemination via the blood in the presence of Echinacea.
Two corollaries may extend from this study. First, the fact that these observations were made in normal, healthy adult mice indicates that the presence of Echinacea in vivo may have a prophylactic role, resulting in a sustained elevation in the available supply of NK cells/monocytes—both well-established and vitally important cell lineages in the maintenance of spontaneous, non-adaptive defenses against virus-mediated diseases and developing neoplasms. Second, since Echinacea is able to stimulate new NK cell generation in the bone marrow, could it also do so in aging and elderly animals, where these cells are in progressive, age-mediated decline and thus rejuvenate this potent disease (cancer)-defense mechanism?
Can Echinacea be Growing in the Fountain of Youth?
It has been known for some years that NK (and T) cells decline with age (26–31), and correspondingly, cancers of assorted types increase with age in both mice and humans. Believing that this inverse relationship between NK cell presence and cancer was more than coincidental, we set out to investigate some years ago, first, the mechanism whereby NK cells decline with age, and secondly, to see if there was any way that NK cells could be brought back to their levels in young adulthood. We found (27) that the decline in NK cells with age was the result of (i) reduced new NK cell production in the bone marrow and (ii) reduced efficiency of the few mature NK cells that were produced to bind to their target cells, hence preventing subsequent killing of the offensive target. With the success of our studies with Echinacea (above) in healthy, young adult mice, we conducted the same sort of experiments as in these mice, except we used healthy, elderly mice (32). We demonstrated in these healthy, elderly mice, that it is not only possible to increase the absolute numbers of NK cells in their normal bone marrow generating site by feeding daily (P < 0.004) Echinacea via the diet for 14 days but also to resurrect the functional capacity (target cell binding/lysis) of these new Echinacea-generated NK cells. Indeed, this herb in the diet returned the numbers and function of NK cells in these elderly animals to the levels of the young adult. In the spleens of these Echinacea-consuming elderly mice, NK cell numbers rose to levels 30% greater than those of their control cage mates not consuming Echinacea. The lytic capacity of this newly produced army of NK cells in these Echinacea-consuming elderly mice (32) also returned to levels equivalent to those of the young adult. These levels were statistically significantly higher (P < 0.03–0.001) than the killing capacity of identically treated elderly mice not consuming Echinacea for 14 days.
These observations appear to apply uniquely to this herb since we could never rejuvenate the NK cell-mediated component of the immune system in elderly mice by any of the other typical NK cell enhancers. For instance, we had previously found in such healthy, elderly mice that neither the cytokines IL-2 nor the drugs indomethacin was able to stimulate NK cell numbers or function in aging mice (27), in spite of the fact that both these agents are potent stimulators of NK cell numbers and function in the young adult mouse. Furthermore, in elderly humans, immunostimulating cytokines such as the NK-stimulating IL-2 are significantly impaired with respect to production levels and with respect to a decreased ability of several types of immune cells from aged humans to utilize IL-2 (31,33,34). Paralleling our observations with young adult mice consuming Echinacea for 14 days (17), we did not find in these healthy elderly mice, any influence of dietary Echinacea on the mature or precursor granulocytes, the precursors to red blood cells or the other immune cells (T and B lymphocytes) in either the spleen or bone marrow, again indicating the unique role of Echinacea in stimulating the non-adaptive limb of the immune response, i.e. NK cells and their accessory cells, the monocytes. Nevertheless, in spite of our observations of these positive influences of Echinacea consumption in aging mice, it must be borne in mind that similar controlled experiments have not been, or can they really ever be, conducted on human subjects simply because of the variability among humans even of identical age and gender. One reason for this, of course, is that precisely identical, lifelong life styles can never be achieved with the precision and control readily achievable for laboratory animals.
Are the Parts as Good as the Whole?
In a subsequent study (35), we injected arabinogalactan intraperitoneally daily for 7 days or 14 days into elderly mice. Since this complex polysaccharide is contained in whole Echinacea, it was hypothesized that this component might have as good an effect on NK cells as did the extract of whole herb. However, we found that in contrast to whole product, this component was not effective in stimulating NK cell numbers in the elderly mouse bone marrow or spleen. Arabinogalactan was not effective in altering the levels of any other hemopoietic or immune cell populations in either spleen or bone marrow. However, when we injected arabinogalactan into young adult mice the NK cell levels of the bone marrow were decreased after 7 days and returned to control (sham-injected) levels for that organ only after 14 days of daily administration of the polysaccharide. In the spleen, arabinogalactan administration for 7 days produced no change in the numbers of NK cells, and only after 14 days of daily exposure to this agent did the levels of splenic NK cells rise significantly (P < 0.004) above control levels. From these findings, it appears that at least from a prophylactic standpoint, it is more efficacious to administer whole Echinacea rather than isolated compounds contained within the herb. As discussed above (see Introduction), in the case of Echinacea at least, the whole product acts via two different mechanisms to stimulate NK cells since the whole product contains both arabinogalactans and alkamides.
With respect to other herbs of known medicinal value, the whole product may similarly contain many compounds that may act additively or even synergistically to produce, collectively, the best effects in vivo. The possibility that the collective whole may be better than any single extracted compound, is supported by already available circumstantial evidence (15,36).
Is it Possible to Get Too Much of a Good Thing?
We elected next to study the influence of daily consumption of Echinacea throughout life beginning in youth, i.e. 7 week of age (puberty), until early ‘old age’ (13 months) in inbred mice (37). There is considerable controversy concerning the duration/frequency with respect to human consumption of Echinacea. For example, the common label advice when purchasing Echinacea over the counter indicates that Echinacea should be taken for short spurts of time and then terminated for several days/weeks before resuming intake. The untested reasoning is that perhaps chronic overstimulation of the immune system via daily, long-term exposure to Echinacea could result in dependency, or worse, that immune system activity may fall to very low levels, rendering it incompetent to ward off even minor infections. Thus, in an effort to dispel or prove this theory, we fed young mice, from 7 weeks of age until 13 months, our standard, daily dose of Echinacea, previously shown to be NK-enhancing, in the chow—all other parameters strictly controlled (husbandry, gender, age, water/food intake quantity, etc.) as in our previous studies.
Our results (37), for the first time, provided concrete evidence that chronic (long-term) intake of Echinacea was not only not detrimental but also distinctly prophylactic. Mice in control cages eating untreated chow had a 79% survival by 10 months of age, while mice living under identical conditions, with the one variable being Echinacea in the daily chow, were still 100% alive by 10 months of age. By 13 months of age, control mice were 46% still alive, while those consuming Echinacea were 74% alive. Furthermore, in the Echinacea-consuming mice, NK cells were statistically significantly elevated in absolute numbers at every sampling period, in both their bone marrow generating site and their site of maximum accumulation, the spleen (37). Given that the key immune cells acting as the first line of defense against developing neoplasms in mice and humans are NK cells, it is not difficult to conclude that sustained enhancement of NK cells alone, throughout life, could readily account for the reduced frequency in deaths with advancing age. Spontaneous neoplasms, clinically undetectable, are well known to increase with advancing age in humans and mice. Thus, the logical corollary from this study indicates that chronic daily intake of Echinacea, is clearly not detrimental to the immune system, but rather prophylactic.
Will Echinacea ‘Work’ Once a Tumor is in Progress?
Since the debut of NK cells, leukemias and lymphomas have been known to be targets for NK cytolysis. Indeed, NK cells were, decades ago, established as the first line of defense against these types of neoplasms (38–40). The concept that herbal compounds can enhance NK cells has recently gained considerable attention and indeed, excellent reviews on the roles of NK cells in tumor combat and the role of such compounds in modifying antitumor responses, have been provided by Takeda and Okumura (41) and Cooper (42). Therefore, we hypothesized that significantly enhancing NK cells, even after leukemia has taken hold, may lead to actual elimination of these tumor cell ‘targets’. Thus, we induced leukemia in mice via injection of a dose of leukemia cells known to consistently result in death 3.5 weeks later, and on the same day as leukemia induction, Echinacea was added to their diet. Control leukemia-injected mice consumed regular diet. All other parameters of these experiments were identical, as usual (above). The results were most encouraging (43). NK cell numbers by 9 days after tumor onset were very significantly elevated over control (P < 0.000007). Three months after leukemia onset—long after all control (untreated chow) leukemic mice had died—NK cells were recorded at more than twice the numbers present in normal mice of identical age, strain and gender. Furthermore, all the other hemopoietic and immune cell lineages in both bone marrow and spleen in these long-term, Echinacea-consuming, originally leukemic mice were indistinguishable from the corresponding populations of cells in normal mice. Life span analysis indicated that not only had Echinacea extended life span (43) but also the survival advantage provided to leukemic mice by consuming Echinacea daily was statistically significant (P < 0.022). One-third of all Echinacea-consuming mice that survived until 3 months after leukemia onset went on to live a full-life. We believe that further manipulation of Echinacea dose/frequency/duration regimens could allow many more if not the other full two-thirds to go on to live a full life. The mechanism by which Echinacea mediates its antineoplastic activity is well known (see Introduction). It acts exclusively via the immune system and has no influence on the tumor cells themselves, the latter being highly unstable and continuously cloning out their most virulent cells to produce frank neoplasm. However, by stimulating the first line of defense, i.e. NK cells, which are so effective in detecting and lysing tumor cells immediately upon detection, the value of Echinacea can be readily seen.
Thus, the medicinal value of phytochemicals contained in Echinacea is clearly evident and indicates that these agents, as well as phytochemicals not yet discovered in other herbs, may be valuable tools to combat tumor. The therapeutic value of Echinacea can now be added to its prophylactic potential (above) and indicates that herbal therapy may soon see its debut alongside—or indeed in place of—conventional therapy, especially since virtually all chemo-‘therapy’ is so toxic to all other renewing systems in the body, that its administration (dose/frequency/duration) must necessarily be very limited, and as such are not successful in achieving complete life-long tumor eradication, i.e. cure.
Combination Therapy in Leukemia Combat—Echinacea Helps Out Again
There is another powerful NK stimulant called melatonin, which the mammalian body makes in the pineal gland, and its role is to act as a neuroimmunomodulator (44–48). When we gave the combination of Echinacea and melatonin (43), via the diet, daily to leukemic mice (leukemia induced as above), not only did we find the usual significant elevation in NK cells but also the long-term survival increased to 40%, compared with the one-third of the leukemic mice when Echinacea alone was given (above). Thus, at least for this tumor, the two NK stimulants together were indeed better than one.
In another type of combination therapy, we immunized mice 5 weeks beforehand, with killed leukemia cells (49) before injecting live cells and daily dietary Echinacea. We observed that the combination of immunization and dietary Echinacea was substantially more therapeutic than either alone. Immunization alone produced a survival rate and life span increment similar to that achieved by giving leukemic mice dietary Echinacea alone. Life span increment via combination therapy has reached 60%. Moreover, when NK cells were measured at 3 months after leukemia injection (onset), it was found that the absolute numbers of NK cells in their bone marrow birth site was three times that of immunized mice not consuming Echinacea (P < 0.003), and the numbers of NK cells in the spleens of immunized mice consuming daily dietary Echinacea rose to almost twice (P < 0.001) the numbers found in immunized mice not receiving the herb. In true ‘Echinacea style’, the stimulatory effect of this herb was directed toward NK cells. Consequently, by 3 months, the presence of this herb in the diet of leukemic mice had no influence on the lymphocytes, red blood cell precursors, mature granulocytes or their precursors in either spleen or bone marrow, again demonstrating the uniquely positive influence of this herb on non-adaptive immunity.
Therefore, it appears that combination therapy in which one agent is Echinacea and the other, a non-toxic and non-immunosuppressive agent (thus eliminating virtually all modern chemotherapeutic laboratory-derived concoctions) has great advantage at least in leukemia treatment. Given that humans and mice are ∼97% genetically common, with similar physiology in virtually every organ, it is not unjustifiable to extrapolate these collective findings to humans. Studies such as these investigations with mice warrant assessment at the clinical level especially since both Echinacea and melatonin, and a host of other herbal products, are already in the market place. Unless formal clinical studies follow, to establish regulatory guidelines, it is very conceivable that leukemia patients (and others) could begin to self-medicate—with potentially disasterous results (22).
References
- 1.Muller-Jakic B, Breu W, Probstle A, Redl K, Greger H, Bauer R. In vitro inhibition of cyclooxygenase and 5-lipoxygenase by alkamides from Echinacea and Achilles species. Planta Med. 1994;60:37–40. doi: 10.1055/s-2006-959404. [DOI] [PubMed] [Google Scholar]
- 2.Wagner H, Breu W, Willer F, Wierer M, Remilger P, Schwenker G. In vitro inhibition of arachidonic metabolism by some alkamides and prenylated phenols. Planta Med. 1989;55:566–7. doi: 10.1055/s-2006-962097. [DOI] [PubMed] [Google Scholar]
- 3.Christopher FL, Dussault I, Miller SC. Population dynamics of natural killer cells in the spleen and bone marrow of normal and leukemic mice during in vivo exposure to interleukin-2. Immunobiology. 1991;184:37–52. doi: 10.1016/S0171-2985(11)80570-X. [DOI] [PubMed] [Google Scholar]
- 4.Dussault I, Miller SC. Stimulation of natural killer cell numbers but not function in leukemic infant mice: a system primed in infancy allows survival in adulthood. Nat Immun. 1993;12:66–78. [PubMed] [Google Scholar]
- 5.Bauer R. Echinacea drugs—effects and active ingredients. Z Arztl Fortbild. 1996;90:111–5. [PubMed] [Google Scholar]
- 6.Melchart D, Linde K, Worku F, Sarkady L, Horzmann M, Jurcic K, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. J Altern Complement Med. 1995;1:145–60. doi: 10.1089/acm.1995.1.145. [DOI] [PubMed] [Google Scholar]
- 7.See DM, Broumand N, Sahl L, Tilles JG. In vitro effects of Echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients. Immunopharmacology. 1997;35:229–35. doi: 10.1016/s0162-3109(96)00125-7. [DOI] [PubMed] [Google Scholar]
- 8.Roesler J, Emmendorffer A, Steinmuller C, Leuttig B, Wagner H, Lohmann-Matthes ML. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to test subjects mediating activation of the phagocyte system. Int J Immunopharmacol. 1991;13:931–41. doi: 10.1016/0192-0561(91)90046-a. [DOI] [PubMed] [Google Scholar]
- 9.Roesler J, Steinmuller C, Kiderlen A, Emmendorffer A, Wagner H, Lohmann-Matthes ML. Application of purified polysaccharides from cell cultures of the plant Echinacea purpurea to mice mediates protection against systemic infections with Listeria monocytogenes and Candida albicans. Int J Immunopharmacol. 1991;13:27–37. doi: 10.1016/0192-0561(91)90022-y. [DOI] [PubMed] [Google Scholar]
- 10.Steinmuller C, Roesler J, Grottrup E, Franke G, Wagner H, Lohmann-Matthes ML. Polysaccharides isolated from plant cell cultures of Echinacea purpurea enhance the resistance of immunosuppressed mice against systemic infections with Candida albicans and Listeria monocytogenes. Int J Immunopharmacol. 1993;15:605–14. doi: 10.1016/0192-0561(93)90078-d. [DOI] [PubMed] [Google Scholar]
- 11.Leuttig B, Steinmuller C, Gifford GE, Wagner H, Lohmann-Matthes ML. Macrophage activation by the polysaccharide arabinogalactan isolated from plant cell cultures of Echinacea purpurea. J Natl Cancer Inst. 1989;81:669–75. doi: 10.1093/jnci/81.9.669. [DOI] [PubMed] [Google Scholar]
- 12.Stimpel M, Proksch A, Wagner H, Lohmann-Matthes ML. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect Immun. 1984;46:845–9. doi: 10.1128/iai.46.3.845-849.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauer J, Anderer FA. Mechanism of stimulation of human natural killer cytotoxicity by arabinogalactan from Larix occidentalis. Cancer Immunol Immunother. 1993;36:237–44. doi: 10.1007/BF01740905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly GS. Larch arabinogalactan: clinical relevance of a novel immune-enhancing polysaccharide. Altern Med Rev. 1999;4:95–103. [PubMed] [Google Scholar]
- 15.Rininger JA, Kickner S, Chigurupati P, McLean A, Franck Z. Immunopharmacological activity of Echinacea preparations following stimulated digestion of murine macrophages and human peripheral blood mononuclear cells. J Leukoc Biol. 2000;68:503–10. [PubMed] [Google Scholar]
- 16.Stein GM, Edlund U, Pfuller U, Bussing A, Schietzel M. Influence of polysaccharides from Viscum Album L. on human lymphocytes, monocytes and granulocytes in vitro. Anticancer Res. 1999;19:3907–14. [PubMed] [Google Scholar]
- 17.Sun LZ-Y, Currier NL, Miller SC. The American coneflower: a prophylactic role involving nonspecific immunity. J Altern Complement Med. 1999;5:437–46. doi: 10.1089/acm.1999.5.437. [DOI] [PubMed] [Google Scholar]
- 18.Lersch C, Zeuner M, Bauer A, Siements M, Hart R, Drescher M, et al. Nonspecific immunostimulation with low doses of cyclophosphamide (LDCY), thymostimulin, and Echinacea purpurea extracts (Echinacin) in patients with far advanced colorectal cancers: preliminary results. Cancer Invest. 1992;10:343–8. doi: 10.3109/07357909209024793. [DOI] [PubMed] [Google Scholar]
- 19.Mengs U, Clare CB, Poiley JA. Toxicity of Echinacea purpurea: acute, subacute and genotoxicity studies. Arzneimittelforschun. 1991;41:1075–81. [PubMed] [Google Scholar]
- 20.Mullins RJ, Heddle R. Adverse reactions associated with Echinacea: the Australian experience. Ann Allergy Asthma Immunol. 2002;88:42–51. doi: 10.1016/S1081-1206(10)63591-0. [DOI] [PubMed] [Google Scholar]
- 21.Soon SL, Crawford RI. Recurrent erythema nodosum associated with Echinacea herbal therapy. J Am Acad Dermatol. 2001;44:298–9. doi: 10.1067/mjd.2001.112219. [DOI] [PubMed] [Google Scholar]
- 22.Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: potential adverse interactions with anticancer agents. J Clin Oncol. 2004;22:2489–503. doi: 10.1200/JCO.2004.08.182. [DOI] [PubMed] [Google Scholar]
- 23.Miller SC. Production and renewal of murine natural killer cells in the spleen and bone marrow. J Immunol. 1982;129:2282–6. [PubMed] [Google Scholar]
- 24.Seaman WE, Blackman MA, Gindhart TD, Roubinian JR, Loeb JM, Talal N. Estradiol reduces natural killer cells in mice. J Immunol. 1978;121:2193–8. [PubMed] [Google Scholar]
- 25.Zoller M, Bellgrau D, Axbert I, Wigzell H. Natural killer cells do not belong to the recirculating lymphocyte population. Scand J Immunol. 1982;15:159–67. doi: 10.1111/j.1365-3083.1982.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 26.Albright JW, Albright JF. Age-associated impairment of murine natural killer activity. Proc Natl Acad Sci USA. 1983;80:6371–5. doi: 10.1073/pnas.80.20.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dussault I, Miller SC. Decline in natural killer cell-mediated imunosurveillance in aged mice—a consequence of reduced cell production and tumor binding capacity. Mech Ageing Dev. 1994;75:115–29. doi: 10.1016/0047-6374(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 28.Ghoneum M, Suzuki K, Gollapud S. Phorbol myristate acetate corrects impaired NK function of old mice. Scand J Immunol. 1991;34:391–8. doi: 10.1111/j.1365-3083.1991.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 29.Hanna N. The role of natural killer cells in the control of tumor growth and metastasis. Biochim Biophys Acta. 1985;780:213–26. doi: 10.1016/0304-419x(85)90004-6. [DOI] [PubMed] [Google Scholar]
- 30.Krishnaraj R. Immunosenescence of human NK cells: effects on tumor target recognition, lethal hit and interferon sensitivity. Immunol Lett. 1992;34:79–84. doi: 10.1016/0165-2478(92)90030-r. [DOI] [PubMed] [Google Scholar]
- 31.Cheung TH, Twu S, Jr, Richardson A. Mechanism of age-related decline in lymphocyte proliferation: role of IL-2 production and protein synthesis. Exp Gerontol. 1983;18:451–60. doi: 10.1016/0531-5565(83)90024-4. [DOI] [PubMed] [Google Scholar]
- 32.Currier NL, Miller SC. Natural killer cells from aging mice treated with extracts from Echinacea purpurea are quantitatively and functionally rejuvenated. Exp Gerontol. 2000;35:627–39. doi: 10.1016/s0531-5565(00)00106-6. [DOI] [PubMed] [Google Scholar]
- 33.Chouaib S, Chatenoud L, Klatzman D, Frandelizzi D. The mechanisms of inhibition of human IL-2 production. II. PGE2 induction of suppressor T lymphocytes. J Immunol. 1984;132:1851–7. [PubMed] [Google Scholar]
- 34.Gillis S, Kozak R, Durante M, Weksler ME. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981;67:837–42. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Currier NL, Lejtenyi D, Miller SC. Effect over time of in-vivo administration of the polysaccharide arabinogalactan on immune and hemopoietic cell lineages in murine spleen and bone marrow. Phytomedicine. 2003;10:145–53. doi: 10.1078/094471103321659852. [DOI] [PubMed] [Google Scholar]
- 36.Voaden DJ, Jocobson M. Tumor inhibitors. 3. Identification and synthesis of an oncolytic hydrocarbon from American coneflower roots. J Med Chem. 1972;15:619–23. doi: 10.1021/jm00276a013. [DOI] [PubMed] [Google Scholar]
- 37.Brousseau M, Miller SC. Enhancement of natural killer cells and increased survival of aging mice fed daily Echinacea root extract from youth. Biogerontology. 2005;6:157–63. doi: 10.1007/s10522-005-7951-8. [DOI] [PubMed] [Google Scholar]
- 38.Kasai M, Yoneda T, Habu S, Maruyama Y, Okumura K, Tokunaga T. In vivo effect of anti-asialo GM1 antibody on natural killer activity. Nature. 1981;291:334–5. doi: 10.1038/291334a0. [DOI] [PubMed] [Google Scholar]
- 39.Keissling R, Klein E, Pross H, Wigzell H. Natural killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells: characteristics of the killer cell. Eur J Immunol. 1975;5:117–21. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 40.Lotzova E, Savary CA, Lowlachi M, Murasko DM. Cytotoxic and morphologic profile of endogenous and pyrimidinone-activated murine NK cells. J Immunol. 1986;136:732–40. [PubMed] [Google Scholar]
- 41.Takeda K, Okumura K. CAM and NK cells. eCAM. 2004;1:17–27. doi: 10.1093/ecam/neh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper EL. Commentary on CAM and NK cells by K. Takeda and K. Okumura. eCAM. 2004;1:29–34. doi: 10.1093/ecam/neh011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Currier NL, Miller SC. Echinacea purpurea and melatonin augment natural killer cells in leukemic mice and prolong life span. J Altern Complement Med. 2001;7:241–51. doi: 10.1089/107555301300328115. [DOI] [PubMed] [Google Scholar]
- 44.Demas GE, Nelson RJ. Exogenous melatonin enhances cell-mediated, but not humoral, immune function in adult male deer mice (Peromyscus maniculatus) J Biol Rhythms. 1998;13:245–52. doi: 10.1177/074873098129000084. [DOI] [PubMed] [Google Scholar]
- 45.Liebmann PM, Wolfler A, Felsner P, Hofer D, Schauenstein K. Melatonin and the immune system. Int Arch Allergy Immunol. 1997;112:203–11. doi: 10.1159/000237455. [DOI] [PubMed] [Google Scholar]
- 46.Maestroni GJ, Hertens E, Galli P, Conti A, Pedrinis E. Melatonin-induced T-helper cell hematopoietic cytokines resembling both interleukin-4 and dynorphin. J Pineal Res. 1996;21:131–9. doi: 10.1111/j.1600-079x.1996.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 47.Poon AM, Liu ZM, Pang CS, Brown GM, Pang SF. Evidence for a direct action of melatonin on the immune system. Biol Signals. 1994;3:107–17. doi: 10.1159/000109532. [DOI] [PubMed] [Google Scholar]
- 48.Yu Q, Miller SC, Osmond DG. Melatonin inhibits apoptosis during early B-cell development in mouse bone marrow. J Pineal Res. 2000;2:86–93. doi: 10.1034/j.1600-079x.2000.290204.x. [DOI] [PubMed] [Google Scholar]
- 49.Currier NL, Miller SC. The effect of immunization with killer tumor cells, with/without feeding of Echinacea purpurea in an erythroleukemic mouse model. J Altern Complement Med. 2002;8:49–58. doi: 10.1089/107555302753507177. [DOI] [PubMed] [Google Scholar]