Abstract
Wild waterfowl are the natural reservoir of all influenza A viruses, and these viruses are usually nonpathogenic in these birds. However, since late 2002, H5N1 outbreaks in Asia have resulted in mortality among waterfowl in recreational parks, domestic flocks, and wild migratory birds. The evolutionary stasis between influenza virus and its natural host may have been disrupted, prompting us to ask whether waterfowl are resistant to H5N1 influenza virus disease and whether they can still act as a reservoir for these viruses. To better understand the biology of H5N1 viruses in ducks and attempt to answer this question, we inoculated juvenile mallards with 23 different H5N1 influenza viruses isolated in Asia between 2003 and 2004. All virus isolates replicated efficiently in inoculated ducks, and 22 were transmitted to susceptible contacts. Viruses replicated to higher levels in the trachea than in the cloaca of both inoculated and contact birds, suggesting that the digestive tract is not the main site of H5N1 influenza virus replication in ducks and that the fecal-oral route may no longer be the main transmission path. The virus isolates' pathogenicities varied from completely nonpathogenic to highly lethal and were positively correlated with tracheal virus titers. Nevertheless, the eight virus isolates that were nonpathogenic in ducks replicated and transmitted efficiently to naïve contacts, suggesting that highly pathogenic H5N1 viruses causing minimal signs of disease in ducks can propagate silently and efficiently among domestic and wild ducks in Asia and that they represent a serious threat to human and veterinary public health.
Wild aquatic birds, including ducks, are the natural reservoir of influenza type A viruses and play an important role in the viruses' ecology and propagation. Virus representatives of all 16 hemagglutinin (HA) and all 9 neuraminidase (NA) subtypes have been isolated from waterfowl. From this reservoir, influenza A viruses can occasionally be transmitted to other avian and mammalian hosts, including humans, and can cause outbreaks of severe disease. Influenza viruses in wild aquatic birds have long been in a state of evolutionary equilibrium (stasis), and infected hosts usually show no signs of disease (27). Most avian influenza viruses replicate preferentially in the gastrointestinal tract of wild ducks, are excreted at high levels in feces, and are transmitted through the fecal-oral route (15, 29).
Since their emergence in Hong Kong in 1997, highly pathogenic H5N1 influenza viruses have repeatedly caused serious outbreaks among poultry farms and markets in the territory (10, 22, 23). These outbreaks resulted in the repeated slaughter of poultry in Hong Kong to contain the spread of these viruses. These zoonotic agents also pose a significant threat to human health, because they have repeatedly shown their potential to transmit directly from birds to humans (5, 12, 18, 25, 26). Between late 2003 and early 2005 H5N1 avian influenza spread in an unprecedented fashion across 10 Asian countries, resulting in 53 human fatalities in Thailand, Vietnam, and Cambodia, and the death and slaughter of more than 150 million birds, as reported by the World Health Organization (http://www.who.int/csr/disease/avian_influenza/en/). Despite numerous efforts at containment, H5N1 influenza viruses and their precursors still circulate among poultry and wild birds in Asia (3, 4, 11, 13, 14, 28, 31) and remain a threat to both veterinary and human public health.
Previous research had consistently demonstrated that ducks infected with highly pathogenic avian influenza viruses of the H5 and H7 subtypes (either naturally or experimentally inoculated) developed very mild or no disease signs (1, 6, 11, 16, 19, 22). In fact, highly pathogenic avian influenza (HPAI) viruses in waterfowl tended to behave like low-pathogenic avian influenza viruses in other avian species, despite having multiple basic amino acids at the HA cleavage site, a universal trait of HPAI viruses. However, recent events indicate that the long-standing equilibrium between influenza viruses and aquatic birds may have been disrupted. During the 1999-2000 H7 avian influenza outbreak in Italy, mortality was observed in two domestic geese and two Muscovy ducks (2). In December of 2002, new H5N1 outbreaks in two Hong Kong parks caused the deaths of many resident avian species, including waterfowl (7, 24). These were the first reported incidents of influenza virus-caused mortality among aquatic birds since 1961. During the Asian H5N1 outbreak of 2003-2005, domestic ducks were among the species affected by the epidemic, although they were not as dramatically affected as the more susceptible host, the chicken (http://www.fao.org/ag/againfo/subjects/en/health/diseases-cards/avian_update.html). These recent developments prompted us to ask whether waterfowl such as ducks can still act as asymptomatic carriers of influenza virus when infected with highly pathogenic H5N1.
Here we report the characterization of 23 avian and human H5N1 virus isolates collected in Hong Kong, mainland China, Indonesia, Thailand, and Vietnam during outbreak investigations performed in 2003 and 2004. These viruses were compared by antigenic analysis and then assessed for their potential to replicate, transmit, and be pathogenic in mallards. Our findings showed that the H5N1 virus isolates tested replicated and transmitted efficiently in ducks. Additionally, the virus isolates exhibited different pathogenic potentials in ducks, ranging from the complete absence of clinical disease to severe neurological dysfunction and death. Tracheal virus titers were found to be consistently higher than cloacal virus titers in mallards, and they were positively correlated with virus pathogenicity in this avian model.
MATERIALS AND METHODS
Viruses.
All influenza viruses used in this study were isolates sent to the St. Jude Children's Research Hospital Influenza Repository by collaborators at the University of Hong Kong as part of the World Health Organization Global Influenza Program and by scientists in China, Indonesia, Vietnam, and Thailand (Table 1). Stock viruses were grown in 10-day-old embryonated chicken eggs for 36 to 48 h at 35°C. The allantoic fluid was then harvested, and aliquots were stored at −80°C until use. Virus titer was determined by calculating the 50% egg infectious dose (EID50) per ml of virus stock, using the method of Reed and Muench (21). The lower limit of virus titer quantitation was 101 EID50 per ml. All experimental work with the H5N1 viruses, including animal studies, was performed in a biosafety level 3+ laboratory approved for use by the U.S. Department of Agriculture and the U.S. Centers for Disease Control and Prevention.
TABLE 1.
H5N1 influenza viruses characterized in this study
| Virus | Abbreviationa | Date isolated | Source |
|---|---|---|---|
| A/Pheasant/Hong Kong/NT123/03 | A/Ph/HK/NT123/03 | 3/03 | Retail poultry market, Hong Kong |
| A/Silky chicken/Hong Kong/YU17/03 | A/S.Ck/HK/YU17/03 | 1/03 | Retail poultry market, Hong Kong |
| A/Chicken/Hong Kong/AP111/03 | A/Ck/HK/AP111/03 | 4/03 | Retail poultry market, Hong Kong |
| A/Chicken/Hong Kong/SSP94/03 | A/Ck/HK/SSP94/03 | 2/03 | Retail poultry market, Hong Kong |
| A/Chicken/Hong Kong/WF27/03 | A/Ck/HK/WF27/03 | 1/03 | Retail poultry market, Hong Kong |
| A/Pigeon/Hong Kong/WF32/03 | A/Pigeon/HK/WF32/03 | 1/03 | Retail poultry market, Hong Kong |
| A/Chicken/Hong Kong/YU250/03 | A/Ck/HK/YU250/03 | 3/03 | Retail poultry market, Hong Kong |
| A/Chicken/Hong Kong/YU46/03 | A/Ck/HK/YU46/03 | 1/03 | Retail poultry market, Hong Kong |
| A/Chicken/Hong Kong/SSP171/03 | A/Ck/HK/SSP171/03 | 3/03 | Retail poultry market, Hong Kong |
| A/Silky chicken/Hong Kong/SSP7/03 | A/S.Ck/HK/SSP7/03 | 1/03 | Retail poultry market, Hong Kong |
| A/Chicken/Hong Kong/NT71/03 | A/Ck/HK/NT71/03 | 2/03 | Retail poultry market, Hong Kong |
| A/Mallard/Vietnam/16D/03 | A/Mal/VN/16D/03 | 1/03 | Mallard duck, Vietnam |
| A/Chicken/Vietnam/C58/04 | A/Ck/VN/C58/04 | 1/04 | Chicken, Vietnam |
| A/Chicken/Vietnam/48C/04 | A/Ck/VN/48C/04 | 1/04 | Chicken, Vietnam |
| A/Duck/Vietnam/40D/04 | A/Dk/VN/40D/04 | 1/04 | Duck, Vietnam |
| A/Vietnam/1203/04 | A/VN/1203/04 | 1/04 | Human patient, Vietnam |
| A/Vietnam/3046/04 | A/VN/3046/04 | 1/04 | Human patient, Vietnam |
| A/Chicken/Vietnam/133/04 | A/Ck/133/04 | 7/04 | Chicken, Vietnam |
| A/Peregrine Falcon/Hong Kong/D0028L/04 | A/Falcon/HK/D0028L/04 | 1/04 | Dead wild bird, Hong Kong |
| A/Duck/Thailand/71.1/04 | A/Dk/Thai/71.1/04 | 2/04 | Duck, Thailand |
| A/Thailand/1(Kan-1)/04 | A/Thai/1(Kan-1)/04 | 2/04 | Human patient, Thailand |
| A/Chicken/Anhui-Chaohu/85/04 | A/Ck/AH/85/04 | 7/04 | Chicken, China |
| A/Chicken/Pangkal Pinang/BPPV3/04 | A/Ck/PP/BPPV3/04 | 1/04 | Chicken, Indonesia |
Abbreviations: Ph, pheasant; HK, Hong Kong; S.Ck, silky chicken; Dk, duck; VN, Vietnam; Thai, Thailand.
Antigenic analysis.
The antigenic characteristics of the H5 influenza viruses were compared by the hemagglutination inhibition (HI) assay with a panel of polyclonal antisera and monoclonal antibodies to the H5 HA, as previously described (24).
Animal studies.
All animal experiments were approved by the Animal Care and Use Committee of St. Jude Children's Research Hospital and performed in compliance with relevant institutional policies, the Association for the Accreditation of Laboratory Animal Care guidelines, the National Institutes of Health regulations, and local, state, and federal laws.
Duck infection and transmission studies.
Two 4- to 6-week-old mallards (Anas platyrhynchos) were each inoculated with 106 to 107 EID50 of virus in a 1-ml volume via the natural route, as described previously (24). Briefly, a 0.5-ml volume of the inoculum was applied via the cloaca, 0.2 ml was applied via the trachea, and the remaining 0.3 ml was dripped into the throat, nares, and eyes (0.1 ml in each). One or two uninfected ducks were placed in the cage with the inoculated birds within 1 hour of inoculation and shared food and drinking water with them. Birds were weighed and observed daily for signs of morbidity or mortality over a period of 14 days. Birds that could not eat or drink on their own due to severe disease signs were euthanized, and their deaths were recorded on the following day of observation. Tracheal and cloacal swabs were collected from all ducks 3 and 5 days after inoculation, and influenza virus was detected by virus isolation in 10-day-old chicken embryos as previously described (14, 24). Positive samples were subjected to virus titer determination by calculating the EID50, using the method of Reed and Muench (21), which had a lower limit of quantitation of 101 EID50/ml. Swab samples with detectable influenza virus but whose titers were below the limit of quantitation were reported as having a virus titer of <101 EID50/ml.
Assessing virulence of H5N1 viruses in ducks.
The intravenous pathogenicity index (IVPI)—the mean score per bird per observation over a 10-day period—is routinely used to determine the virulence of influenza virus isolates in domestic poultry such as chickens. Isolates are considered to be highly pathogenic if they cause more than 75% mortality within 10 days. The IVPI test procedures were followed (30) with a couple of modifications: juvenile ducks were used instead of chickens, and the infectious dose was normalized. Groups of 8 to 10 4-week-old ducks were inoculated intravenously with 107 EID50 of infectious virus in a 0.1-ml volume. The birds were observed for clinical signs of disease every 24 h over a 10-day period. During each observation, each duck was scored as follows: 0 if healthy, 1 if mildly sick, 2 if severely sick, and 3 if dead. An IVPI can range from 0.0 for nonpathogenic isolates to 3.0 for the most highly pathogenic isolates (causing the death of all infected birds within 24 h).
Statistical analysis of virus titers.
To maximize statistical power, we combined data presented in a previous paper (24) with the data we collected during our recent transmission studies on cloacal and tracheal virus shedding in mallards. We were able to combine these data because both studies had been performed in closely matched study populations (the ducks were all 4- to 6-week-old mallards raised in isolation) and used identical experimental procedures (inoculation, housing of birds, and virus titer quantitation). Every duck infected, either by direct inoculation or by contact with an infected bird, was considered an individual study subject. Analyses were conducted separately for virus titers in inoculated ducks and contact ducks, because the route of infection was biologically different (inoculation or transmission) and directly affected the virus titers. For example, virus titers in contact ducks peaked later than they did in inoculated ducks. Virus titers were tested for normality using the Shapiro-Wilk W test and graphic depiction. The frequency distributions of cloacal and tracheal virus titers were not distributed normally on the natural or the logarithmic (log10) scale; therefore, nonparametric statistical methods were used for the analyses. The statistical analysis of virus titers was performed using the logarithmic scale, and for this purpose, swab samples positive for influenza virus but with a titer of <101 EID50/ml were assigned a value of 100.5 EID50/ml (the halfway point between zero and the lower limit of quantitation), while swab samples negative for influenza virus were assigned a value of 100 EID50/ml (the smallest possible positive value on a log10 scale). A univariate analysis of association with virus titers in inoculated or contact ducks was performed on factors such as pathogenicity group classification, development of neurological symptoms, and year of isolation by using the Kruskal-Wallis equality-of-population test and the Mann-Whitney U test. We compared paired samples such as cloacal and tracheal virus titers in ducks by using the Wilcoxon matched-pairs signed-rank test. We analyzed all data by using Small Stata statistical software (version 9.0; StataCorp, College Station, TX).
RESULTS
Antigenic analysis.
The antigenic characteristics of virus samples isolated in 2003 and 2004 from Hong Kong, Vietnam, and Thailand were compared using HI testing against a panel of polyclonal postinfection antisera and monoclonal antibodies specific to the H5 HA (see Table S1 in the supplemental material). All the virus isolates tested cross-reacted poorly with the reference antiserum raised against the HA of A/Tern/SA/61 (H5N3) and with the antiserum raised against the HA of a recent human isolate, A/HK/213/03. The virus isolates had diverse reactivity patterns; no specific reactivity pattern correlated with pathogenicity in mallards. Most of the Hong Kong virus isolates failed to react against the A/HK/156(483)/97 (H5N1) antiserum, a result similar to what we observed when we tested isolates from 2002 (24). Some of the Hong Kong poultry market isolates from 2003 (A/Ck/HK/NT71/03, A/Ck/HK/WF27/03, and A/Ck/HK/SSP171/03) had very low or nonexistent titers against all the polyclonal antiserum and the monoclonal antibodies tested. Interestingly, the three virus isolates obtained in 2004 from Vietnam and Thailand (A/VN/1203/04, A/Ck/VN/C58/04, and A/Dk/Thai/71.1/04) and one isolate obtained from a wild bird in Hong Kong (A/Falcon/HK/D0028L/04) had very similar antigenic reactivity patterns, a finding clearly different from all the virus isolates originating in Hong Kong poultry markets. The differences in reactivity patterns observed with these virus isolates indicate that these viruses are antigenically distinguishable from each other. This fact is evidence of considerable antigenic drift in the HA of recent H5N1 viruses.
Pathogenicity of H5N1 viruses in mallards.
To determine whether waterfowl are resistant to H5N1 influenza virus disease and can act as asymptomatic carriers of the virus, we compared the pathogenicity in mallards following infection by the natural route with 23 H5N1 virus isolates obtained in 2003 and 2004 from Hong Kong (12 samples), Vietnam (7 samples), Thailand (2 samples), China (1 sample), and Indonesia (1 sample). Ducks were susceptible to infection with all the H5N1 isolates tested, and virus was isolated from all experimentally infected ducks at 3 days postinoculation (dpi) (Table 2). Ducks were observed for 14 days to compare the morbidity and mortality caused by the different virus isolates. The H5N1-infected ducks showed a continuum of disease signs (none to acute neurological symptoms and death). On the basis of these observations, the virus isolates were classified into two groups, according to their pathogenic potential in mallards after infection via the natural route. Infection with the virus isolates in the low-pathogenicity group did not cause mortality in either inoculated or contact birds. In contrast, infection with virus isolates in the high-pathogenicity group caused death to at least one duck (Table 3). This classification of virus isolates into low- or high-pathogenicity groups was used for the subsequent descriptions and analyses.
TABLE 2.
Replication of H5N1/03 and -04 influenza viruses in mallards
| Pathogenicity in ducks and strain | Detection of H5N1 virus at different times after inoculationa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 3 postinoculation
|
Day 5 postinoculation
|
|||||||
| Inoculated ducks
|
Contact ducks
|
Inoculated ducks
|
Contact ducks
|
|||||
| Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | |
| Low-pathogenicity group | ||||||||
| A/Ck/HK/NT71/03 | 2/2 (2.6) | 2/2 (<1) | 2/2 (<1) | 1/2 (<1) | 0/2 | 0/2 | 2/2 (<1) | 0/2 |
| A/Ck/HK/AP111/03 | 2/2 (5.9) | 2/2 (<1) | 0/2 | 0/2 | 1/2 (1.0) | 0/2 | 2/2 (4.2) | 1/2 (1.0) |
| A/Ck/HK/YU250/03 | 2/2 (3.2) | 2/2 (<1) | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| A/Falcon/HK/D0028L/04 | 2/2 (5.2) | 2/2 (<1) | 0/2 | 0/2 | 2/2 (<1) | 0/2 | 1/2 (<1) | 0/2 |
| A/Ck/AH/85/04 | 2/2 (4.0) | 2/2 (2.8) | 2/2 (1.0) | 1/2 (1.0) | 2/2 (1.0) | 2/2 (<1) | 2/2 (2.3) | 0/2 |
| A/Ck/PP/BPPV3/04 | 2/2 (3.5) | 2/2 (2.5) | 2/2 (1.0) | 1/2 (1.0) | 1/2 (<1) | 1/2 (1.0) | 2/2 (3.5) | 2/2 (2.3) |
| A/VN/3046/04 | 2/2 (2.5) | 2/2 (1.0) | 2/2 (1.0) | 0/2 | 2/2 (1.0) | 2/2 (<1) | 2/2 (3.3) | 2/2 (<1) |
| A/Thai/1(Kan-1)/04 | 2/2 (5.5) | 2/2 (2.3) | 2/2 (1.0) | 2/2 (<1) | 2/2 (<1) | 1/2 (<1) | 1/2 (2.5) | 2/2 (<1) |
| High-pathogenicity group | ||||||||
| A/Ph/HK/NT123/03 | 2/2 (<1) | 0/2 | 2/2 (<1) | 1/2 (<1) | 2/2 (<1) | 0/2 | 2/2 (4.0) | 1/2 (<1) |
| A/Ck/HK/SSP94/03 | 2/2 (3.2) | 0/2 | 1/2 (<1) | 0/2 | 2/2 (2.5) | 0/2 | 2/2 (<1) | 1/2 (<1) |
| A/Ck/HK/WF27/03 | 2/2 (5.0) | 1/2 (3.0) | 2/2 (1.0) | 1/2 (<1) | 2/2 (3.4) | 1/2 (1.0) | 2/2 (3.8) | 1/2 (<1) |
| A/Ck/VN/133/04 | 2/2 (4.8) | 2/2 (2.5) | 1/1 (3.8) | 1/1 (2.3) | 1/2 (<1) | 1/2 (<1) | 1/1 (3.8) | 1/1 (1.0) |
| A/Ck/VN/48C/04 | 2/2 (3.3) | 2/2 (3.5) | 2/2 (3.5) | 2/2 (2.3) | 1/2 (1.0) | 1/2 (1.0) | 2/2 (3.5) | 2/2 (1.0) |
| A/Dk/VN/40D/04 | 2/2 (4.3) | 2/2 (1.0) | 0/2 | 0/2 | 1/1b (2.8) | 0/1b | 2/2 (3.3) | 1/2 (<1) |
| A/Pigeon/HK/WF32/03 | 2/2 (4.6) | 2/2 (<1) | 2/2 (3.0) | 2/2 (<1) | 1/1b (3.25) | 0/1b | 1/1b (1.0) | 1/1b (<1) |
| A/S.Ck/HK/YU17/03 | 2/2 (4.6) | 1/2 (<1) | 2/2 (5.0) | 2/2 (2.5) | 1/1b (3.25) | 0/1b | —b | —b |
| A/Ck/HK/YU46/03 | 2/2 (4.4) | 2/2 (<1) | 2/2 (<1) | 2/2 (<1) | 1/1b (2.0) | 1/1b (<1) | 1/1b (2.8) | 1/1b (<1) |
| A/Ck/HK/SSP171/03 | 2/2 (3.0) | 2/2 (<1) | 2/2 (<1) | 2/2 (<1) | —b | —b | 2/2 (<1) | 2/2 (1.7) |
| A/S.Ck/HK/SSP7/03 | 2/2 (5.4) | 2/2 (<1) | 2/2 (4.2) | 2/2 (2.9) | —b | —b | 2/2 (4.6) | 2/2 (2.7) |
| A/Mal/VN/16D/03 | 2/2 (4.3) | 2/2 (1.0) | 1/1 (3.5) | 1/1 (2.5) | 1/1b (<1) | 1/1b (2.5) | —b | —b |
| A/Ck/VN/C58/04 | 2/2 (<1) | 1/2 (<1) | 0/2 | 0/2 | 1/2 (2.25) | 1/2 (<1) | 2/2 (4.0) | 1/2 (<1) |
| A/VN/1203/04 | 2/2 (3.8) | 2/2 (<1) | 2/2 (<1) | 2/2 (<1) | 2/2 (4.9) | 2/2 (<1) | 2/2 (2.9) | 2/2 (<1) |
| A/Dk/Thai/71.1/04 | 1/1b (1.0) | 1/1b (2.0) | 1/1b (2.5) | 0/1b | —b | —b | 1/1b (4.3) | 0/1b |
Number shedding/number sampled (virus titer, log10 EID50/ml). Virus titer is the average of positive samples
Ducks died.
TABLE 3.
Pathogenic potential of H5N1/03 and -04 influenza viruses in mallards
| Pathogenicity in ducksa and strain | IVPI | Infection route | No. dead/ total no. | Clinical signs of infection
|
|
|---|---|---|---|---|---|
| Cloudy eyes | Neurological signsb | ||||
| Low-Pathogenicity Group | |||||
| A/Ck/HK/NT71/03 | NDc | Inoculated | 0/2 | 2/2 | 0/2 |
| Contact | 0/2 | 1/2 | 0/2 | ||
| A/Ck/HK/AP111/03 | 1.96 | Inoculated | 0/2 | 1/2 | 0/2 |
| Contact | 0/2 | 0/2 | 0/2 | ||
| A/Ck/HK/YU250/03 | ND | Inoculated | 0/2 | 0/2 | 0/2 |
| Contact | 0/2 | 0/2 | 0/2 | ||
| A/Falcon/HK/D0028L/04 | 0.73 | Inoculated | 0/2 | 2/2 | 0/2 |
| Contact | 0/2 | 1/2 | 0/2 | ||
| A/Ck/AH/85/04 | 1.76 | Inoculated | 0/2 | 0/2 | 0/2 |
| Contact | 0/2 | 0/2 | 0/2 | ||
| A/Ck/PP/BPPV3/04 | 1.83 | Inoculated | 0/2 | 0/2 | 0/2 |
| Contact | 0/2 | 0/2 | 0/2 | ||
| A/VN/3046/04 | 2.19 | Inoculated | 0/2 | 1/2 | 0/2 |
| Contact | 0/2 | 1/2 | 0/2 | ||
| A/Thai/1(Kan-1)/04 | 0.39 | Inoculated | 0/2 | 0/2 | 0/2 |
| Contact | 0/2 | 1/2 | 0/2 | ||
| High-Pathogenicity Group | |||||
| A/Ph/HK/NT123/03 | ND | Inoculated | 1/2 | 1/2 | 1/2 |
| Contact | 0/2 | 1/2 | 0/2 | ||
| A/Ck/HK/SSP94/03 | ND | Inoculated | 1/2 | 2/2 | 0/2 |
| Contact | 0/2 | 2/2 | 0/2 | ||
| A/Ck/HK/WF27/03 | ND | Inoculated | 1/2 | 1/2 | 1/2 |
| Contact | 0/2 | 2/2 | 0/2 | ||
| A/Ck/VN/133/04 | ND | Inoculated | 0/2 | 2/2 | 1/2 |
| Contact | 1/1 | 1/1 | 0/1 | ||
| A/Ck/VN/48C/04 | ND | Inoculated | 1/2 | 1/2 | 0/2 |
| Contact | 0/2 | 1/2 | 1/2 | ||
| A/Dk/VN/40D/04 | ND | Inoculated | 1/2 | 0/2 | 0/2 |
| Contact | 0/2 | 2/2 | 0/2 | ||
| A/Pigeon/HK/WF32/03 | ND | Inoculated | 2/2 | 2/2 | 2/2 |
| Contact | 2/2 | 2/2 | 2/2 | ||
| A/S.Ck/HK/YU17/03 | ND | Inoculated | 2/2 | 1/2 | 1/2 |
| Contact | 2/2 | 0/2 | 0/2 | ||
| A/Ck/HK/YU46/03 | ND | Inoculated | 2/2 | 1/2 | 2/2 |
| Contact | 2/2 | 1/2 | 1/2 | ||
| A/Ck/HK/SSP171/03 | ND | Inoculated | 2/2 | 0/2 | 0/2 |
| Contact | 2/2 | 1/2 | 1/2 | ||
| A/S.Ck/HK/SSP7/03 | ND | Inoculated | 2/2 | 0/2 | 0/2 |
| Contact | 2/2 | 1/2 | 2/2 | ||
| A/Mal/VN/16D/03 | 2.96 | Inoculated | 1/2 | 1/2 | 2/2 |
| Contact | 1/1 | 0/2 | 0/1 | ||
| A/Ck/VN/C58/04 | 2.36 | Inoculated | 1/2 | 2/2 | 1/2 |
| Contact | 1/2 | 0/2 | 0/2 | ||
| A/VN/1203/04 | 2.32 | Inoculated | 1/2 | 2/2 | 1/2 |
| Contact | 1/2 | 2/2 | 1/2 | ||
| A/Dk/Thai/71.1/04 | 2.87 | Inoculated | 2/2 | 1/2 | 1/2 |
| Contact | 2/2 | 0/2 | 1/2 | ||
Pathogenicity groups were determined on the basis of lethality in both inoculated and contact ducks over a 14-day period following infection by the natural route. Low, no lethality; high, lethality in either inoculated or contact ducks.
Neurological signs included twitching head, ataxia, violent tremors, severe torticolitis, and loss of balance.
ND, not determined.
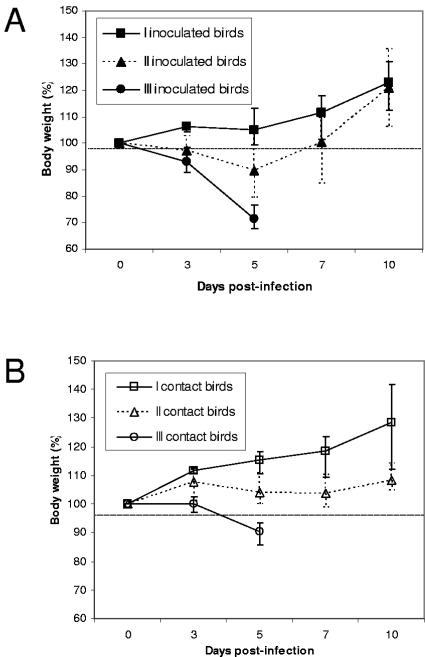
Eight of 23 (34.8%) H5N1 virus isolates were classified as having low pathogenic potential in mallards. Infection with the eight virus isolates induced very limited morbidity in both inoculated and contact birds. The ducks were active, eating and drinking normally, and continued to gain weight during the course of the study (Fig. 1). One contact bird exposed to A/Ck/PP/BPPV3/04 developed some mild signs of disease, such as ruffled feathers and mild depression for about 3 days, but it continued to gain weight, and the duck recovered completely by the end of the experiment. The most commonly observed sign of infection, if any, was the development of cloudy eyes in some ducks at 4 to 5 dpi (Table 3). No mortality was observed in ducks exposed to these virus isolates (Fig. 2).
FIG. 1.
Weight loss of mallards infected with H5N1 influenza viruses isolates. Inoculated ducks were infected with 106 to 107 EID50 of virus and housed with contact ducks within 1 hour of inoculation, sharing food and water. Ducks were weighed daily for 10 days. Virus isolates were grouped based on their pathogenic potential in ducks as causing no mortality among either inoculated or contact ducks (I), causing death among inoculated ducks only (II), and causing death in both inoculated and contact ducks (III). Data points and error bars represent the averages and ranges of weight loss caused by virus isolates in each pathogenic group in inoculated ducks (A) and contact ducks (B).
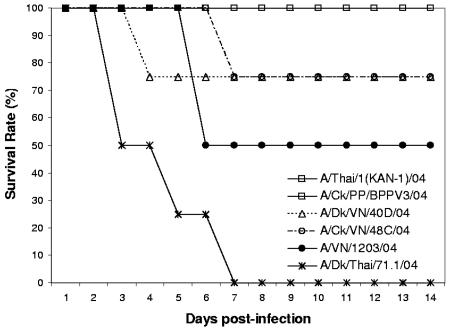
FIG. 2.
Survival curve of mallards infected with different H5N1 influenza virus isolates. Ducks were observed for 14 days after inoculation with 106 to 107 EID50 of virus via the natural route. Ducks that exhibited severe neurological signs were euthanized, and their deaths were recorded on the following day of observation. Although survival curves were generated for all 23 virus isolates, for clarity only representative curves of a few isolates per pathogenicity group were included on the graph. Classification is as follows: low pathogenicity, A/Thai/1(Kan-1)/04 and A/Ck/PP/BPPV3/04; high pathogenicity, A/Dk/VN/40D/04, A/Ck/VN/48C/04, A/Dk/Thai/71.1/04, and A/VN/1203/04.
Fifteen of 23 (65.2%) H5N1 virus isolates were classified as having high pathogenic potential in ducks. However, even among these 15 isolates, there were considerable differences in pathogenic potential. Five of these isolates caused mortality only in experimentally inoculated ducks and not in contact ducks (Table 3). Ducks inoculated with any of these five isolates showed initial signs of disease, such as weight loss, cloudy eyes, and depression starting at 3 dpi (Fig. 1 and Table 3). For three virus isolates, one of two inoculated birds developed more severe neurological dysfunction (such as ataxia and torticollis) and ultimately died at 6 to 10 dpi (Fig. 2 and Table 3). However, the surviving inoculated ducks regained energy and started gaining weight at approximately 7 dpi. Cloudy eyes were the most widespread sign of disease in contact ducks exposed to ducks infected with these five pathogenic isolates. Ducks inoculated with A/Ck/VN/133/04 developed disease signs but ultimately recovered. In contrast, a contact duck placed in the same cage died from the exposure, without developing severe neurological symptoms. Nine of the 15 H5N1 virus isolates classified as having high pathogenic potential in mallards were able to induce mortality not only among inoculated ducks but also in contact birds. Both inoculated and contact ducks showed rapid and persistent weight loss, as well as cloudy eyes (Fig. 1 and Table 3). Neurological dysfunction, such as head twitching, ataxia, tremors, severe torticollis, and loss of balance, was common at 4 to 5 dpi, and the ducks died soon after. Ducks that were unable to eat or drink on their own due to the severity of their neurological symptoms were euthanized. Infection with virus isolates A/Ck/HK/YU46/03, A/S.Ck/HK/YU17/03, and A/Dk/Thai/71.1/04 resulted in the most dramatic disease progression, as both inoculated and contact ducks died as early as 3 dpi (Fig. 2). Both A/Ck/HK/YU46/03 and A/Dk/Thai/71.1/04 were also able to induce neurological dysfunction in ducks starting at 2 dpi. Infection with six of the virus isolates in the high-pathogenicity group caused the deaths of the two inoculated and the two contact ducks.
In addition to determining the pathogenicity of H5N1 virus isolates in mallards after infection by the natural route, IVPI scores were determined for six virus isolates from the low-pathogenicity group and for four isolates from the high-pathogenicity group. The IVPI scores are listed in Table 3. The IVPI scores ranged from 0.39 to 2.96. Interestingly, only two of six virus isolates from the low-pathogenicity group had IVPI scores below 1.0, which would classify them as being nonpathogenic in mallards by this procedure. Of the other four virus isolates from the low-pathogenicity group tested, three had IVPI scores between 1.7 and 2.0 and one isolate (A/VN/3046/04) had a score over 2.0. Although these four isolates had IVPI scores that would classify them as intermediate or highly pathogenic, they did not cause any severe morbidity or mortality in mallards following infection by the natural route. The four virus isolates from the high pathogenicity group that were tested had IVPI scores above 2.3. These four isolates all caused severe morbidity and mortality in mallards following infection by the natural route.
Transmission and replication of H5N1 viruses in mallards.
Table 2 summarizes the transmission and replication potential of all 23 different H5N1 isolates newly characterized in this study. All isolates tested were found to replicate in inoculated ducks. Additionally, only A/Ck/HK/YU250/03 was not transmitted from the experimentally infected ducks to the susceptible contact ducks, and A/Falcon/HK/D0028L/04 was transmitted to only one of two susceptible birds. All the other virus isolates transmitted efficiently, resulting in the infection of both contact ducks within a 5-day period. Both A/Ck/HK/YU250/03 and A/Falcon/HK/D0028L/04 were virus isolates classified in the low-pathogenicity group.
Most of the viruses had replicated to high titers by 3 dpi in the inoculated ducks, and titers were consistently higher in the tracheal swab, except for A/Dk/Thai/71.1/04 and A/Ck/VN/C58/04, which replicated to low titers both in the trachea and the cloaca (≤102 EID50/ml). At 5 dpi, many experimentally infected ducks had no detectable virus or very low virus titers (≤101 EID50/ml) in their cloacal swabs, except for those infected with A/Mal/VN/16D/03 (102.5 EID50/ml). Mallards inoculated with virus isolates from the low-pathogenicity group had very low virus titers in their tracheal swabs at 5 dpi (≤101 EID50/ml). In contrast, virus isolates from the high-pathogenicity group still replicated to high titers in the trachea of surviving ducks at 5 dpi.
Virus titers in contact ducks infected by 3 dpi were low; most of the tracheal and cloacal swabs harbored titers of <101 EID50/ml. Virus replication increased over time in contact birds, and virus titers were much higher in tracheal swabs at 5 dpi. However, certain virus isolates from the high-pathogenicity group replicated to high levels (>103 EID50/ml) in the tracheas of contact birds as early as 3 dpi. As for inoculated birds, the virus titers in cloacal swabs remained low at both 3 and 5 dpi.
Comparison of H5N1 infection in mallards and Pekin ducks.
Most of our experimental work in ducks was performed in mallards. However, to establish the comparability of the mallard model to Pekin ducks, a waterfowl species heavily used in agriculture in Asia, we performed some infection, transmission, and pathogenicity studies in this species as well. These studies were performed following the same experimental procedures used for mallards and matching the birds for age (4 to 6 weeks old). Four isolates from the low-pathogenicity group [A/Ck/AH/85/04, A/Ck/PP/BPPV3/04, A/VN/3046/04, and A/Thai/1(Kan-1)/04] and five isolates from the high-pathogenicity group (A/Ck/VN/133/04, A/Ck/VN/48C/04, A/S.Ck/HK/SSP7/03, A/Mal/VN/16D/03, and A/Dk/Thai/71.1/04) were used to inoculate Pekin ducks via the natural route.
All tested isolates replicated efficiently in the inoculated ducks, and all isolates, except A/Thai/1(Kan-1)/04, were transmitted to susceptible contacts. As in mallards, tracheal virus titers were higher than cloacal virus titers, in both inoculated and contact ducks (see Table S2 in the supplemental material). At 3 dpi, tracheal titers in contact Pekin ducks exposed to low-pathogenicity isolates were higher than in mallards exposed to the same isolates: the range of cloacal titers of contact Pekin ducks was 101.8 to 105.2 EID50/ml, compared to 101 EID50/ml in contact mallards.
The classification of the nine virus isolates based on pathogenic potential following infection by the natural route was the same in both duck species. The four virus isolates found to be of low pathogenicity in mallards did not cause any severe morbidity or mortality in inoculated or contact Pekin ducks. In fact, most inoculated and contact ducks exhibited no disease signs at all. The only exception was one contact Pekin duck exposed to ducks infected with A/Ck/PP/BPPV3/04, which developed cloudy eyes after 8 days of observation. The five virus isolates found to be of high pathogenicity in mallards caused severe disease and death in Pekin ducks as well. Disease progression in Pekin ducks infected with A/Ck/VN/133/04 and A/Ck/VN48C/04 was somewhat more rapid than for mallards. One Pekin duck inoculated with A/Ck/VN/48C/04 died as early as 3 dpi, and all the contact ducks were dead by 5 dpi. As with mallards, Pekin ducks exposed to Ck/HK/YU17/03, A/Mal/VN/16D/03, and A/Dk/Thai/71.1/04 exhibited severe disease and rapid mortality. Only one contact Pekin duck exposed to Ck/HK/YU17/03 remained alive at 5 dpi.
We calculated the IVPI scores for two viruses, A/Ck/VN/C58/04 and A/VN/1203/04, in Pekin ducks. The IVPI scores for A/Ck/VN/C58/04 and A/VN/1203/04 in Pekin ducks were 2.95 and 2.85, respectively, compared to 2.36 and 2.32, respectively, in mallards. Although the IVPI scores of these virus isolates were higher in Pekin ducks than in mallards, the two isolates were classified as being highly pathogenic in both duck species.
Analysis of H5N1 virus shedding in mallard ducks.
Previous research has shown that most avian influenza viruses replicate preferentially in the gastrointestinal tract of ducks and are excreted at high levels in feces (15, 29). As a result, viruses were characteristically transmitted among susceptible birds via the fecal-oral route. However, our recent laboratory studies suggest that ducks infected with the latest H5N1 virus isolates from Asia shed virus more persistently from the trachea than from the cloaca. As described in Materials and Methods, we performed a statistical analysis of cloacal and tracheal shedding in experimentally infected ducks and contact ducks by combining shedding data from a previous study (24) and the current study. By combining these data, we obtained a study population of 135 mallards—70 experimentally inoculated ducks and 65 contact ducks infected with 35 different H5N1 virus isolates obtained from 1997 to 2004. The virus titer data were markedly non-normally distributed, as most were skewed toward low values. The measures of central tendency (median) and dispersion (range) for both tracheal and cloacal shedding data in the larger combined data set were as follows: at 3 dpi, the median value for tracheal shedding was 102.5 EID50/ml (range, 100 to 106.5 EID50/ml; 126 observations), and the median value for cloacal shedding was 100.5 EID50/ml (range, 100 to 104.75 EID50/ml; 126 observations). At 5 dpi, the median value for tracheal shedding was 100.5 EID50/ml (range, 100 to 105.5 EID50/ml; 109 observations), and the median value for cloacal shedding was 100 EID50/ml (range, 100 to 103.75 EID50/ml; 111 observations).
Our analysis of the combined data set showed that virus titers shed from the trachea were significantly higher than those shed from the cloaca for inoculated and contact ducks at both 3 and 5 dpi (P < 0.05, Wilcoxon matched-pairs signed-rank test). At 3 dpi, experimentally inoculated ducks had a median tracheal virus titer of 103.5 EID50/ml (range, 100 to 106.25 EID50/ml; 64 observations) compared with a median cloacal virus titer of 100.5 EID50/ml (range, 100 to 104.75 EID50/ml; 63 observations). Similarly, contact ducks infected with H5N1 viruses by exposure to inoculated ducks had a median tracheal virus titer of 102.25 EID50/ml (range, 100 to 105.5 EID50/ml; 54 observations) compared with a median cloacal virus titer of 100.5 EID50/ml (range, 100 to 103.75 EID50/ml; 55 observations) at 5 dpi.
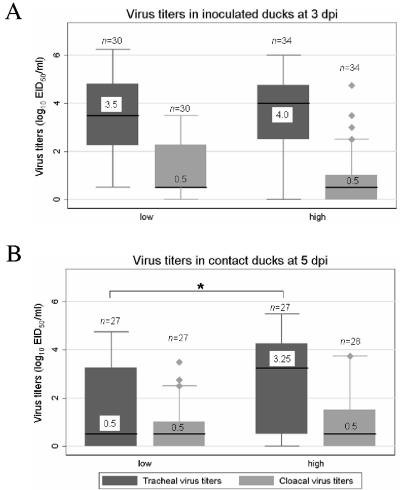
Due to the differences in pathogenic potential exhibited by the various virus isolates, we investigated whether there was an association between pathogenic potential and viral shedding in mallards. At 3 dpi, the experimentally inoculated ducks had similar tracheal and cloacal virus titers, regardless of the virus isolate's pathogenic potential. In contrast, the contact ducks had higher tracheal and cloacal virus titers when they were exposed to highly pathogenic viruses (P < 0.05, Mann-Whitney U test). This finding indicates that highly pathogenic viruses replicated more efficiently in contact ducks following transmission than did low pathogenic viruses, despite the fact that all the ducks had been exposed to similarly high titers of viruses shed by the experimentally inoculated ducks (Fig. 3A). By 5 dpi, both the experimentally inoculated ducks and the contact ducks had higher tracheal virus titers if they were infected with highly pathogenic viruses (P < 0.05, Mann-Whitney U test and K-sample test on equality of median). The difference in tracheal virus titers was particularly large for contact ducks. In contrast, both experimentally infected ducks and contact ducks had low levels of cloacal virus titers at 5 dpi, regardless of the virus isolate's pathogenic potential (Fig. 3B). These results indicate that, in addition to replicating more efficiently in contact ducks following transmission, the highly pathogenic viruses replicated to higher titers in the trachea of both infected and contact ducks than did the low pathogenic viruses. One of the novel characteristics of the H5N1 virus found to be highly pathogenic in ducks is their ability to induce neurological symptoms in the afflicted birds. We analyzed our data to determine if there was any significant association between the development of neurological symptoms and virus shedding in ducks. Ducks that developed neurological symptoms within the 14 days of observation were found to have significantly higher tracheal virus titers than ducks that did not develop these symptoms (P < 0.05, Mann-Whitney U test and K-sample test on equality of median). This was observed among both inoculated and contact ducks. At 5 dpi, inoculated ducks that developed neurological symptoms had a median tracheal titer value of 102.0 EID50/ml (range, 100.5 to 103.75 EID50/ml; 11 observations) compared to a median value of 100.5 EID50/ml (range, 100 to 105.25 EID50/ml; 44 observations) for inoculated birds that did not develop such symptoms. Similarly, contact ducks that developed neurological symptoms had a median tracheal titer value of 103.4 EID50/ml (range, 100.5 to 104.75 EID50/ml; 12 observations) compared to a median value of 100.75 EID50/ml (range, 100 to 105.5 EID50/ml; 42 observations) for contact birds that did not develop such symptoms. Taken together, all these results indicate a significant association between tracheal virus titers and pathogenic potential of H5N1 viruses in ducks that was not observed with cloacal virus titers.
FIG. 3.
Tracheal and cloacal virus titers in mallards infected with H5N1 influenza viruses. Inoculated ducks were infected with 106 to 108.5 EID50 of virus and then housed with contact ducks within 1 hour of inoculation, sharing food and water. Tracheal and cloacal swabs were collected at 3 and 5 days postinoculation and tested for the presence of influenza virus, and positive samples were subjected to titer determinations for infectivity by calculating the EID50. The data represented in this figure comprise virus titer data from this study and data from previously published work (24). The study population comprised 135 mallards: 70 experimentally inoculated ducks and 65 contact ducks infected with 35 different H5N1 virus isolates obtained in Asia from 1997 to 2004. Virus isolates were classified in two groups based on their pathogenic potential in ducks: low pathogenicity and high pathogenicity. Box plots represent the distribution of tracheal and cloacal virus titers in each pathogenic group (low and high) in inoculated ducks on day 3 postinfection (A) and in contact ducks on day 5 postinfection (B). Each box shows the median value of the data set (black line and associated numerical value), the interquartile range (upper and lower boundaries of the box), the 10th percentile and 90th percentile (lower and upper error bars, respectively), and the size of each group (n). *, P < 0.05 (Kruskal-Wallis equality-of-population test).
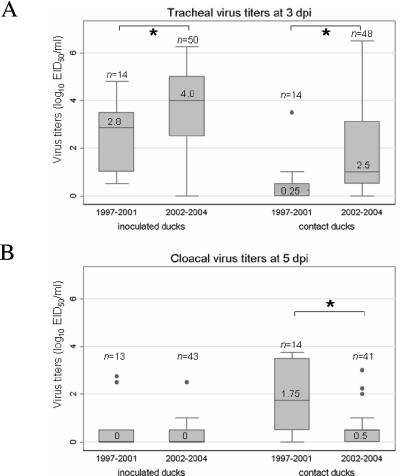
At 3 dpi, titers of virus in the trachea were higher in both inoculated and contact ducks infected with virus isolates from 2002 to 2004 than they were in those infected with virus isolates from 1997 to 2001 (P < 0.05, Mann-Whitney U test and K-sample test on equality of median). Additionally, at 5 dpi, cloacal virus titers were significantly lower in contact ducks infected with viruses from 2002 to 2004 than they were in contact ducks infected with virus isolates from 1997 to 2001 (P < 0.05, Mann-Whitney U test and K-sample test on equality of median). These results, illustrated in Fig. 4, suggest that tracheal shedding may be the main route by which the more recent H5N1 viruses are now transmitted. However, the association between the date of isolation and levels of virus titers may be due to the fact that most viruses that have any pathogenic potential in ducks emerged in 2002 and later.
FIG. 4.
Tracheal and cloacal virus titers in mallards infected with H5N1 influenza viruses. Inoculated ducks were infected with 106 to 108.5 EID50 of virus and then housed with contact ducks within 1 hour of inoculation, sharing food and water. Tracheal and cloacal swabs were collected at 3 and 5 days postinoculation and tested for the presence of influenza virus, and positive samples were subjected to titer determinations for infectivity by calculating the EID50. The data represented in this figure comprise virus titer data from this study and data from previously published work (24). The study population comprised 135 mallards: 70 experimentally inoculated ducks and 65 contact ducks infected with 35 different H5N1 virus isolates obtained in Asia from 1997 to 2004. Virus isolates were classified in two groups based on their date of isolation (1997 to 2001 and 2002 to 2004). Box plots represent the distribution of tracheal (A) and cloacal (B) virus titers in inoculated and contact ducks at 3 and 5 dpi, respectively. Each box shows the median value of the data set (black line and associated numerical value), the interquartile range (upper and lower boundaries of the box), the 10th percentile and 90th percentile (lower and upper error bars, respectively), and the size of each group (n). *, significant difference in median value (P < 0.05; K-sample test on equality of median).
DISCUSSION
Waterfowl, the natural reservoir of all influenza A viruses, usually carry the infection with no sign of disease. We recently reported the characterization of H5N1 virus isolates that were pathogenic to ducks (7, 24), but published reports of pathogenic influenza infection in ducks remain few. Most published work emphasizes the ducks' resistance to disease development after they are experimentally infected with H5N1 influenza virus (4, 19, 22). Until recently, we did not have enough data to determine whether the recent appearance of virus isolates pathogenic to ducks was an aberration of the evolutionary stasis that exists between influenza viruses and their reservoir or rather whether pathogenicity for ducks is a new characteristic of the H5N1 viruses currently arising in Asia. In this study, we characterized 23 H5N1 viruses isolated in Hong Kong poultry markets in early 2003 or isolates that were obtained during the 2004 outbreaks in China, Indonesia, Vietnam, and Thailand. We showed that these recent isolates are antigenically diverse, infect and transmit efficiently in ducks, and elicit a continuum of disease signs in these birds. Additionally, the data presented here show that recent H5N1 avian influenza viruses replicate more efficiently in the upper respiratory tract than they do in the gastrointestinal tract of ducks and that this characteristic directly affects these viruses' natural histories and transmission patterns.
Our antigenic analysis of the 23 isolates characterized in this study showed evidence of antigenic drift. Indeed, virus isolates had markedly different antigenic patterns. Interestingly, virus isolates from Vietnam and Thailand and one Hong Kong isolate obtained from a wild falcon had a similar antigenic pattern, which was distinct from the pattern of viruses isolated in poultry markets in Hong Kong. However, there was no obvious correlation between reactivity pattern and pathogenic potential in ducks.
Our findings indicate that mallards are highly susceptible to infection with H5N1 viruses currently circulating in Asia. All the isolates we tested replicated in inoculated mallards, and only 1 of the 23 viruses tested did not transmit to susceptible ducks. In the past, most avian influenza viruses were found to preferentially replicate in the gastrointestinal tract of wild ducks, to be excreted at high levels in the feces, and to be transmitted primarily via the oral-fecal route (15). However, in the current study, virus was excreted at high levels in the trachea (upper respiratory tract), rather than in the cloaca. We observed similar results in experimentally inoculated ducks and ducks infected by contact with birds inoculated with H5N1 viruses isolated in late 2002 from Hong Kong (24). Analysis of the combined data from our studies shows that there is a significant difference in levels of tracheal virus shedding and cloacal virus shedding in ducks, particularly in those infected by recent H5N1 viruses (post-2002). Although route of inoculation may affect the viral shedding pattern or organ tropism in an infected host, the same observation was made among inoculated ducks and ducks infected by contact, confirming that this was not an experimental artifact. Additionally, similar results were observed among Pekin ducks exposed to nine different H5N1 virus isolates from 2003-2004 (see Table S2 in the supplemental material). This is in contrast to previous studies, which reported that experimental infection via the natural route in domestic ducks (Pekin and Sheldrake ducks) with H5N1 viruses isolated in Hong Kong in 1997 and from the coastal province of southern China in 1999 to 2002 resulted in similar titers of virus shedding in the trachea and in the cloaca (4, 22). Thus, the increased level of tracheal virus shedding is a particularity of the recent viruses emerging in Asia, from late 2002 onwards, and so far has been observed in both a wild and a domestic duck species. These results indicate that the digestive tract may no longer be the main site of replication in ducks infected by recent H5N1 viruses, and this will most likely influence the natural history of the virus. Indeed, the main path of transmission may have shifted from an oral-fecal route to a more oral-oral route or even an airborne route—or a combination of all of these. This putative change in transmission route could affect the epidemiology of H5N1 viruses and may result in an increase in transmissibility. If this is in fact the case, the implications for H5N1 surveillance and control are important. As part of surveillance programs, cloacal swabs or feces from beneath poultry cages are often collected. In fact, the Food and Agriculture Organization (FAO) of the United Nations' recently released Guiding Principles for Highly Pathogenic Avian Influenza Surveillance and Diagnostic Networks in Asia recommends the use of serology to assess exposure to H5 viruses in domestic waterfowl and then virus isolation from cloacal swabs to determine the current presence of virus (8). Cloacal swabs were chosen for surveillance work partly due to the scientific evidence that influenza viruses replicated preferentially in the gastrointestinal tract of aquatic birds. In light of the data presented in this study, H5N1 surveillance sampling of ducks may need to include cloacal/fecal swabbing plus tracheal/oro-pharyngeal swabbing when possible.
A recent study by Li et al. showed that despite the observed genetic diversity of H5N1 virus isolates, the recent Asia-wide outbreak of H5N1 influenza was caused by a dominant H5N1 Z genotype (17). All the virus isolates investigated in the present study were of the Z genotype, except for one isolate that belonged to genotype Z+ (Y. Guan and G. Smith, personal communication). This homogeneity in virus genotype contrasts with the virus isolates' pathogenic potentials, which ranged from nonpathogenic to lethal (IVPI scores of 0.39 to 2.96). Therefore, H5N1 pathogenicity in ducks does not correlate with genotypes and may be due to genetic traits more subtle than genotypes, such as an individual allogene or even just a few specific amino acids. Future molecular studies will define the detailed genetic basis of pathogenicity in ducks. The mallard is the duck model routinely used in our research laboratory and is a good model for wild ducks, but other species of ducks, such as Pekin and Muscovy, are most frequently used in agriculture in Asia. Our findings indicate that there is no significant difference in the outcome of H5N1 infection in mallards and Pekin ducks. The slight increase in disease progression and higher IVPI scores for highly pathogenic viruses observed in Pekin ducks may indicate a small increase in susceptibility to H5N1 disease in this species, but the classification based on pathogenic potential remained constant in both duck species. The isolates that were classified as have low pathogenicity in mallard ducks also were classified as having low pathogenicity in Pekin ducks. On the other hand, H5N1 isolates that were classified as highly pathogenic in mallards were also classified as highly pathogenic in Pekin ducks. This finding may not be surprising, considering that Pekin ducks were originally bred from mallards and are very closely related as species. Future studies may be warranted to determine if other domestic duck species that are more distant from mallards are as susceptible to H5N1 infection and subsequent disease.
Results from routine surveillance among healthy poultry in southern China show that H5N1 viruses have been circulating among domestic ducks since 1999 (4). However, the first reported cases of H5N1-induced pathogenicity in waterfowl were in late 2002 in Hong Kong (7), despite the presence of H5N1 viruses in the region for the previous 3 years. All experimental infections of ducks with H5N1 virus isolates indicate that earlier viruses (pre-2002) caused only subclinical or mild disease in ducks (4, 19, 22). Increased pathogenicity in ducks therefore is a phenomenon observed with recent H5N1 isolates (late 2002 on) as well. In our current study, only 8 of the 23 isolates characterized had low pathogenicity potential following infection by the natural route. All the virus isolates characterized were isolated during identified H5N1 outbreaks; therefore, they may not be representative of the H5N1 virus population endemic in the region. It is, however, disconcerting to see that such a high proportion of the H5N1 virus isolates tested were lethal to ducks. There is considerable scientific interest in understanding the molecular basis of pathogenicity of H5N1 influenza viruses in ducks. Indeed, historically, influenza virus had been shown to asymptomatically infect ducks, including virus isolates of the H5 and H7 HA subtypes that were shown to be highly pathogenic in other avian hosts. But in the past 5 years, there have been more frequent reports of ducks developing severe disease, such as neurological signs and mortality after infection with HPAI viruses during outbreaks (2, 7). For this reason, the relatively new phenomenon of viruses being pathogenic in their natural reservoir is intriguing, but the data gathered so far have not allowed us to identify a clear viral factor associated with lethality in ducks. In this study we showed a significant positive correlation between tracheal virus titers and pathogenicity. However, the analysis did not allow us to determine whether these high tracheal virus levels are a factor in the increased pathogenicity of the virus or are just a consequence of increased levels of virus replication in the duck. The recent H5N1 isolates' neutrotropic behaviors in ducks may also be an important factor in their pathogenicity, and future studies will explore this possibility.
The existence of H5N1 viruses that present as having low pathogenicity in ducks following natural infection is of great concern to human and veterinary public health. Recent reports from Asia show that H5N1 viruses are still actively circulating in the area and causing outbreaks among poultry flocks (http://www.fao.org/ag/againfo/subjects/en/health/diseases-cards/avian_update.html). While veterinary health experts and government officials are analyzing the situation and formulating region-wide plans to fight avian influenza, it is crucial to determine what risk is posed to humans and other (more-susceptible) poultry by H5N1 viruses that cause little or no disease in ducks. Even if H5N1 viruses that are nonpathogenic in ducks replicate to lower titers than viruses that do cause disease in ducks, they still replicate very efficiently in their hosts, they readily transmit to susceptible ducks, and they may therefore be a source of infection to other birds. Early observations from the Asian H5N1 outbreaks clearly showed evidence of duck pathogenicity, and subsequent surveillance of duck flocks initially concentrated on clinical outbreak detection. However, current field observations, the data reported here, and other recently published studies have highlighted the importance of conducting surveillance for H5N1 viruses from healthy ducks. Four out of six virus isolates that presented no mortality in mallards following infection by the natural route had IVPI scores above 1.7. The study by Chen et al. demonstrated that H5N1 viruses isolated from healthy ducks between 1999 and 2002 were pathogenic to chicken and to mice, despite not being pathogenic in ducks (4). More recently, A/Thai/1(Kan-1)/04, which was isolated from a fatal human case, was found to be highly pathogenic in ferrets (9), while the data presented in this study show that the same isolate was nonpathogenic in ducks (IVPI score of 0.39). These findings clearly indicate that viruses that cause no obvious disease in ducks are still a potential threat not only to ducks but also to the health of other hosts. Due to the plastic nature of influenza viruses, the more recent H5N1 viruses arising in Asia need to be similarly studied. If a significant proportion of H5N1 viruses do not cause any signs of disease in ducks, it is reasonable to postulate that domestic and/or wild duck populations may play a crucial new role as reservoirs and effective carriers of H5N1 viruses by maintaining and further propagating the virus to other bird species, and potentially to mammals such as pigs or humans. A recent study of the distribution of HPAI outbreaks in Thailand performed by the Thai Ministry of Agriculture and the FAO reinforced this theory (8a). Even though 64% of the HPAI outbreaks in Thailand were recorded in chickens and only 28% were recorded in ducks, there was a very strong association between HPAI outbreaks and the spatial distribution of ducks (domestic and free grazing) across the country. Additionally, the chronology of the different outbreaks across Thailand indicates that ducks may have played a role in the genesis of HPAI outbreaks among chickens. Indeed, the first wave of outbreaks (early 2004) was concentrated in areas of the country with a high density of free-grazing ducks. A similar pattern was observed during the second wave of HPAI outbreaks (July to November 2004), but in addition there were subsequent outbreaks in areas with a high density of chickens. Solid active surveillance studies are needed to determine the role played by ducks in the origin and propagation of H5 HPAI viruses in Asia. Clearly, one cannot rely on passive surveillance or outbreak reports to estimate the prevalence of H5N1 viruses in duck populations, because they will be underestimated and so may the role played by ducks in highly pathogenic avian influenza outbreaks.
Supplementary Material
Acknowledgments
We thank Gavin Smith (Department of Microbiology, University of Hong Kong) for H5N1 influenza virus genotype classifications, Scott Krauss for excellent technical assistance, and Margaret Carbaugh for scientific editing of the manuscript.
These studies were supported by Public Health Service research grant AI95357 from the National Institute of Allergy and Infectious Diseases, by the Ellison Foundation, and by the American Lebanese Syrian Associated Charities.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alexander, D. J., G. Parsons, and R. J. Manvell. 1986. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol. 15:647-662. [DOI] [PubMed] [Google Scholar]
- 2.Capua, I., and F. Mutinelli. 2001. Mortality in Muscovy ducks (Cairina moschata) and domestic geese (Anser anser var. domestica) associated with natural infection with a highly pathogenic avian influenza virus of H7N1 subtype. Avian Pathol. 30:179-183. [DOI] [PubMed] [Google Scholar]
- 3.Cauthen, A. N., D. E. Swayne, S. Schultz-Cherry, M. L. Perdue, and D. L. Suarez. 2000. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J. Virol. 74:6592-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, H., G. Deng, Z. Li, G. Tian, Y. Li, P. Jiao, L. Zhang, Z. Liu, R. G. Webster, and K. Yu. 2004. The evolution of H5N1 influenza viruses in ducks in southern China. Proc. Natl. Acad. Sci. USA 101:10452-10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. de Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. [DOI] [PubMed] [Google Scholar]
- 6.Cooley, A. J., H. Van Campen, M. S. Philpott, B. C. Easterday, and V. S. Hinshaw. 1989. Pathological lesions in the lungs of ducks infected with influenza A viruses. Vet. Pathol. 26:1-5. [DOI] [PubMed] [Google Scholar]
- 7.Ellis, T. M., B. Bousfield, L. Bissett, K. Dyrting, G. S. M. Luk, S. T. Tsim, K. Sturm-Ramirez, R. G. Webster, Y. Guan, and M. J. S. Peiris. 2004. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 33:492-505. [DOI] [PubMed] [Google Scholar]
- 8.Food and Agriculture Organization of the United Nations. 2004. Guiding principles for highly pathogenic avian influenza surveillance and diagnostic networks in Asia. [Online.] http://www.fao.org/ag/againfo/subjects/en/health/diseases-cards/Guiding%20principles.pdf.
- 8a.Gilbert, M., and J. Slingenbergh. 2004. Highly pathogenic avian influenza in Thailand: an analysis of the distribution of outbreaks in the 2nd wave, identification of risk factors, and prospects for real-time monitoring. Food and Agriculture Organization of the United Nations and the Department of Livestock Development, Ministry of Agriculture and Cooperatives, Bangkok, Thailand.
- 9.Govorkova, E. A., J. E. Rehg, S. Krauss, H. L. Yen, Y. Guan, M. Peiris, T. D. Nguyen, T. H. Hanh, P. Puthavathana, H. T. Long, C. Buranathai, W. Lim, R. G. Webster, and E. Hoffmann. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 79:2191-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan, Y., J. S. Peiris, A. S. Lipatov, T. M. Ellis, K. C. Dyrting, S. Krauss, L. J. Zhang, R. G. Webster, and K. F. Shortridge. 2002. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc. Natl. Acad. Sci. USA 99:8950-8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan, Y., M. Peiris, K. F. Kong, K. C. Dyrting, T. M. Ellis, T. Sit, L. J. Zhang, and K. F. Shortridge. 2002. H5N1 influenza viruses isolated from geese in southeastern China: evidence for genetic reassortment and interspecies transmission to ducks. Virology 292:16-23. [DOI] [PubMed] [Google Scholar]
- 12.Guan, Y., L. L. Poon, C. Y. Cheung, T. M. Ellis, W. Lim, A. S. Lipatov, K. H. Chan, K. M. Sturm-Ramirez, C. L. Cheung, Y. H. Leung, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. USA 101:8156-8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan, Y., K. F. Shortridge, S. Krauss, P. S. Chin, K. C. Dyrting, T. M. Ellis, R. G. Webster, and M. Peiris. 2000. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 74:9372-9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan, Y., K. F. Shortridge, S. Krauss, and R. G. Webster. 1999. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 96:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinshaw, V. S., R. G. Webster, and B. Turner. 1980. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can. J. Microbiol. 26:622-629. [DOI] [PubMed] [Google Scholar]
- 16.Laudert, E. A., V. Sivanandan, and D. A. Halvorson. 1993. Effect of intravenous inoculation of avian influenza virus on reproduction and growth in mallard ducks. J. Wildl. Dis. 29:523-526. [DOI] [PubMed] [Google Scholar]
- 17.Li, K. S., Y. Guan, J. Wang, G. J. Smith, K. M. Xu, L. Duan, A. P. Rahardjo, P. Puthavathana, C. Buranathai, T. D. Nguyen, A. T. Estoepangestie, A. Chaisingh, P. Auewarakul, H. T. Long, N. T. Hanh, R. J. Webby, L. L. Poon, H. Chen, K. F. Shortridge, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209-213. [DOI] [PubMed] [Google Scholar]
- 18.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.Perkins, L. E., and D. E. Swayne. 2002. Susceptibility of laughing gulls (Larus atricilla) to H5N1 and H5N3 highly pathogenic avian influenza viruses. Avian Dis. 46:877-885. [DOI] [PubMed] [Google Scholar]
- 21.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 22.Shortridge, K. F., N. N. Zhou, Y. Guan, P. Gao, T. Ito, Y. Kawaoka, S. Kodihalli, S. Krauss, D. Markwell, K. G. Murti, M. Norwood, D. Senne, L. Sims, A. Takada, and R. G. Webster. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331-342. [DOI] [PubMed] [Google Scholar]
- 23.Sims, L., T. M. Ellis, K. K. Liu, K. Dyrting, H. Wong, M. Peiris, Y. Guan, and K. F. Shortridge. 2003. Avian influenza in Hong Kong 1997-2002. Avian Dis. 47:832-838. [DOI] [PubMed] [Google Scholar]
- 24.Sturm-Ramirez, K. M., T. Ellis, B. Bousfield, L. Bissett, K. Dyrting, J. E. Rehg, L. Poon, Y. Guan, M. Peiris, and R. G. Webster. 2004. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J. Virol. 78:4892-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subbarao, K., A. Klimov, J. Katz, H. Regnery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393-396. [DOI] [PubMed] [Google Scholar]
- 26.Tran, T. H., T. L. Nguyen, T. D. Nguyen, T. S. Luong, P. M. Pham, V. C. Nguyen, T. S. Pham, C. D. Vo, T. Q. Le, T. T. Ngo, B. K. Dao, P. P. Le, T. T. Nguyen, T. L. Hoang, V. T. Cao, T. G. Le, D. T. Nguyen, H. N. Le, K. T. Nguyen, H. S. Le, V. T. Le, D. Christiane, T. T. Tran, d. J. Menno, C. Schultsz, P. Cheng, W. Lim, P. Horby, and J. Farrar. 2004. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 350:1179-1188. [DOI] [PubMed] [Google Scholar]
- 27.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster, R. G., Y. Guan, M. Peiris, D. Walker, S. Krauss, N. N. Zhou, E. A. Govorkova, T. M. Ellis, K. C. Dyrting, T. Sit, D. R. Perez, and K. F. Shortridge. 2002. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J. Virol. 76:118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webster, R. G., M. Yakhno, V. S. Hinshaw, W. J. Bean, and K. G. Murti. 1978. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology 84:268-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization Global Influenza Programme. 2004. WHO manual on animal influenza diagnosis and surveillance, p. 62-63. [Online.] http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_NCS_2002_5/en/print.html.
- 31.Xu, X., Subbarao, N. J. Cox, and Y. Guo. 1999. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261:15-19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.