Abstract
In the course of human immunodeficiency virus (HIV) disease, CCR5-utilizing HIV type 1 (HIV-1) variants (R5), which typically transmit infection and dominate its early stages, persist in approximately half of the infected individuals (nonswitch virus patients), while in the other half (switch virus patients), viruses using CXCR4 (X4 or R5X4) emerge, leading to rapid disease progression. Here, we used a system of ex vivo tonsillar tissue to compare the pathogeneses of sequential primary R5 HIV-1 isolates from patients in these two categories. The absolute replicative capacities of HIV-1 isolates seemed to be controlled by tissue factors. In contrast, the replication level hierarchy among sequential isolates and the levels of CCR5+ CD4+ T-cell depletion caused by the R5 isolates seemed to be controlled by viral factors. R5 viruses isolated from nonswitch virus patients depleted more target cells than R5 viruses isolated from switch virus patients. The high depletion of CCR5+ cells by HIV-1 isolates from nonswitch virus patients may explain the steady decline of CD4+ T cells in patients with continuous dominance of R5 HIV-1. The level of R5 pathogenicity, as measured in ex vivo lymphoid tissue, may have a predictive value reflecting whether, in an infected individual, X4 HIV-1 will eventually dominate.
Transmitted human immunodeficiency virus type 1 (HIV-1) variants almost exclusively use CCR5 for viral entry, and these viruses (R5 variants) also predominate in early stages of HIV-1 infection (2, 3, 58, 61). Later in the course of HIV-1 infection, viruses that use CXCR4 in addition to CCR5 (R5X4 variants) or CXCR4 alone (X4 variants) emerge (2, 3, 49, 55) in many patients (switch virus patients). The switch to use of CXCR4 has been linked to increased virulence. Thus, switch virus patients often show accelerated rates of CD4+ lymphocyte loss and more rapid progression to AIDS after the switch from R5 to R5X4 or to X4 virus (10, 28, 33, 55). This acceleration is partially due to the expression of CXCR4 by the majority CD4+ T cells in tissue and blood (5, 22). However, about one-half of infected individuals progress to AIDS without such an R5-to-X4 (or R5-to-R5X4) switch (nonswitch virus patients) (12, 28), and the cause of the differential cytopathicity of R5 viruses remains unknown.
The classification of patients into switch and nonswitch, as well as the monitoring of HIV-1 disease progression, is largely based on analysis of peripheral blood. However, about 98% of CD4+ T cells are located in lymphoid tissues, including gut-associated tissues, where the proportion of HIV-1-infected cells is much higher than in blood (38, 43). Therefore, lymphoid tissue is a major site of HIV-1 infection and is where the critical events of HIV disease occur (39).
In this study, we compared the pathogeneses of sequential primary R5 HIV-1 isolates from switch and nonswitch virus patients in ex vivo lymphoid tissue, which supports productive HIV-1 infection without exogenous stimulation (16). We found that, in human lymphoid tissue, the absolute replicative capacities of isolates are determined by host (tissue) factors, whereas the relative replicative capacities are an intrinsic property of these HIV isolates. Also, the ability of the R5 patients' isolates to deplete CCR5-expressing CD4+ T cells in ex vivo-infected human lymphoid tissue does not depend on the tissue donor but rather is an intrinsic viral property. This ability is more pronounced for R5 HIV-1 isolated from nonswitch virus patients than for virus from switch virus patients and may be relevant to the differential patterns of disease progression in these two groups of infected individuals.
MATERIALS AND METHODS
Patients and virus isolates.
The five patients studied here were selected from a cohort of 23 HIV-1-infected individuals described earlier (28-30). The patients were adult homosexual or bisexual men living in Sweden with a median follow-up of 103 months. This follow-up included CD4 counts, viral isolations, and (from 1996) measurement of plasma viral RNA load at South Hospital in Stockholm, Sweden. For the present study, patients and sequential isolates (those taken from the same patient at several time points throughout the course of disease) were selected on the basis of differences in the virus biological phenotypes as assayed from coreceptor use on U87.CD4 and GHOST (3) cells (29). In this work, two groups of patients were studied. From the first group, nonswitch virus patients, only CCR5-using viruses (R5 phenotype) could be isolated throughout the observation period. From the other group, switch virus patients, R5 viruses were isolated initially, but later isolations yielded viruses that could use CXCR4 in addition to CCR5 (R5X4 phenotype).
The nonswitch virus group contained three patients (435, 1047, and 1838), and two R5 isolates from each patient were studied. These patients all showed signs of disease progression, with declining CD4+ T-cell counts (−5.7 × 106, −4.6 × 106, and −2.9 × 106 cells per liter of blood per month, respectively) during the follow-up period. The first clinical symptom appeared in these patients at 99 months, 71 months, and 131 months after infection, respectively (Table 1). The isolates studied were obtained between 41 and 124 months after infection.
TABLE 1.
Characteristics of patients and isolates
| Patient category according to viral phenotypea | Patient no. | Isolate no. | Time of isolation (mo from infection)b | CD4+ T-cell count (106 cells/liter) at time of isolation | Antiretroviral therapy at time of isolationc | Loss of CD4+ T cells (106 cells/liter/mo) | p24 in serum
|
First appearance of clinical symptome(mo from infection)c | |
|---|---|---|---|---|---|---|---|---|---|
| Follow-up (mo from infection)b | Results/no. of testsd | ||||||||
| Nonswitch | 435 | 1577 | 67 | 510 | −5.7 | 62-89 | Neg/4 | 99 | |
| 3415 | 87 | 290 | |||||||
| 1047 | 314 | 41 | 630 | −4.6 | 48-62 | Neg/3 | 71 | ||
| 4223 | 89 | 574 | Pos/1 | ||||||
| 1838 | 5379 | 85 | 410 | −2.9 | 35-140 | Neg/6 | 131 | ||
| 8590 | 121 | 290 | |||||||
| Switch | 2112 | 171 | 15 | 340 | −4.8 | 31-68 | Pos/10 | 23 | |
| 1156 | 31 | 370 | |||||||
| 3502 | 57 | 338 | AZT | ||||||
| 2242 | 1886 | 45 | 180 | −5.2 | 33-84 | Pos/11 | 18 | ||
| 3700 | 64 | 213 | AZT | ||||||
Nonswitch, patients with virus that used only CCR5 throughout the study; Switch, patients with a detected switch to R5X4 virus.
The infection date is calculated as the midpoint between the last negative and the first positive samples.
AZT, zidovudine.
HIV-1 antigen enzyme-linked immunosorbent assay (Abbott, Stockholm, Sweden). Neg, negative; Pos, positive.
The first clinical symptom to appear in patient 435 was perianal herpes infection; in 1838 it was septic arthritis; in 1047 it was oral candidiasis; and in 2242 and 2112 it was persistent generalized lymphadenopathy.
In the switch virus group, two patients, 2112 and 2242, with three and two R5 isolates, respectively, were studied. These patients had declining CD4+ T-cell counts (−4.8 × 106 and −5.2 × 106 cells per liter of blood per month, respectively) during the follow-up period, and the first clinical symptom appeared early, at 23 and 18 months after infection, respectively (Table 1). The studied isolates from patients 2112 and 2242 were obtained between 15 and 64 months postinfection. CXCR4-using virus (R5X4 phenotype) appeared at 76 months after infection in both switch virus patients.
Viruses were isolated from peripheral blood mononuclear cells (PBMC) according to a standard procedure (46) and were passaged only twice in donor PBMC before coreceptor use of sequential isolates was determined (29). We studied the evolutionary relationship between virus isolates from the same patients using phylogenetic analysis of V3 sequences, as previously described (34). We prepared virus stocks by infecting 6 × 106 to 8 × 106 PBMC, which had been obtained from two healthy donors and activated for 3 days with phytohemagglutinin (2.5 μg/ml; Boule, Stockholm, Sweden), with 1.5 ml of supernatant from patients' infected PBMC in the presence of 2 μg/ml Polybrene (Sigma, Stockholm, Sweden). The cultures were maintained in RPMI (Invitrogen, Lidingö, Sweden) containing 10% fetal bovine serum (Invitrogen, Lidingö, Sweden), 50 U/ml penicillin (Invitrogen, Lidingö, Sweden), 50 U/ml streptomycin (Invitrogen, Lidingö, Sweden), and 10 U/ml interleukin-2 (IL-2; Sigma, Stockholm, Sweden). Supernatants were harvested on day 7 and on day 10 or 11 after infection and stored at −80°C.
HIV infection of human lymphoid tissue ex vivo.
Human tonsil tissue removed during routine tonsillectomy and not required for clinical purposes was received within 5 h of excision and was sectioned into 2- to 3-mm blocks. These tissue blocks were placed onto collagen sponge gels in culture medium at the air-liquid interface and infected the next day, as described earlier (16). Five microliters of virus, containing at least 10 ng/ml p24, were applied to the top of each tissue block. We assessed productive HIV infection by measuring p24 in the culture medium using an HIV-1 p24 antigen enzyme-linked immunosorbent assay (Beckman-Coulter, Miami, FL); we used the concentration of p24 accumulated in culture medium bathing 27 or 54 tissue blocks in three or six wells during the 3 days between successive medium changes as a measure of virus replication. We terminated the experiments at day 12 to avoid tissue deterioration, which typically starts after 2 weeks and which may affect viral replication, as well as the quality of flow cytometry analysis.
Flow cytometry.
Flow cytometry was performed on cells mechanically isolated from control and infected tissue blocks. Lymphocytes were first identified according to their light-scattering properties and then analyzed for expression of lymphocyte markers. For identification of CD3+, CD4+, CD8+, CD25+, CD69+, HLA-DR+, CCR5+, and CXCR4+ cells, cells were stained for surface markers with anti-CD3 fluorescein isothiocyanate or phycoerythrin (PE)-Cy7, anti-CD4 allophycocyanin (APC), anti-CD8 TriColor or APC-Cy7, anti-CD25 PE, or anti-CD69 biotin, followed by neutravidin cascade blue (Molecular Probes, Eugene, Oregon), anti-HLA-DR fluorescein isothiocyanate (Caltag Laboratories, Burlingame, CA), or anti-CD25 APC, anti-CCR5 APC and PECy5, or anti-CXCR4 PE (BD Pharmingen, San Diego, CA), respectively. To identify productively infected cells, we stained cells for surface markers, fixed and permeabilized them with Fix&Perm (Caltag Laboratories), and then stained them with an anti-p24 PE-labeled antibody (KC57; Beckman-Coulter). Data were acquired on a BD LSRII instrument using DIVA software version 3.0 and analyzed with FlowJo software (Tree Star).
Multiplexed fluorescent microsphere immunoassay of human cytokines.
The levels of cytokines (MIP-1α, MIP-1β, RANTES, MIG, IP-10, tumor necrosis factor alpha, SDF-1, gamma interferon [IFN-γ], granulocyte-macrophage colony-stimulating factor, IL-1α, IL-1β, IL-2, IL-4, IL-12, IL-15, and IL-16) were evaluated in culture medium by means of a multiplexed fluorescent microsphere immunoassay using the Luminex 100 system (Luminex). Cytokines, capture antibodies, and biotinylated detection antibodies were obtained from R&D Systems. Cytokine capture antibodies were coupled covalently to carboxylate-modified microspheres in a two-step carbodiimide coupling procedure. Binding of biotinylated detection antibodies was ascertained with streptavidin-phycoerythrin (Molecular Probes). Microsphere sets coupled with capture antibodies (1,250 of each specificity) were mixed with 50 μl of standards or culture medium and were incubated overnight at 4°C. Bound cytokines were detected with biotinylated antibodies and streptavidin-phycoerythrin. Data were analyzed with Delta Soft 3 (BioMetallics) using a four-parameter fitting algorithm.
Statistical analysis.
We used the Wilcoxon signed rank test to compare the replication capacities of different isolates, the Mann-Whitney test to compare the distributions of activation markers and CD25 in different cell populations, and mixed-model analysis to compare CCR5+ T-cell depletion by R5 isolates from switch and nonswitch patients. For these analyses, we used SPSS 12.0.
RESULTS
Fourteen R5 HIV-1 isolates derived from five patients, including two or three sequential isolates from each patient, were individually used to infect blocks of human tonsillar tissue obtained from multiple donors. All of these isolates were of R5 phenotype, as evaluated with U87.CD4 and GHOST (3) cell assays (29). The R5 phenotype was confirmed here from inhibition of their replication by the CCR5 ligand RANTES (100 nM) and from lack of inhibition by the CXCR4 ligand AMD3100 (1 μg/ml) (48; also data not shown). In two of the five patients, CXCR4-using HIV-1 evolved after the isolates used for the current study had been collected, whereas later isolates from the other patients remained R5.
In this study, we individually infected human lymphoid tissues ex vivo with all the isolates and monitored viral replication, cell loss, and the activation status of productively infected T cells. Out of the above-mentioned 14 isolates tested, 3 caused an unexplainable loss of tissue lymphocytes of various subsets, in sharp contrast with findings reported earlier (21, 41) regarding selective loss of CD4+ T cells in this ex vivo tissue system. We excluded these 3 isolates from further studies, and we report below on the behavior of 11 isolates tested in lymphoid tissue obtained from five donors.
HIV-1 replication in human lymphoid tissue.
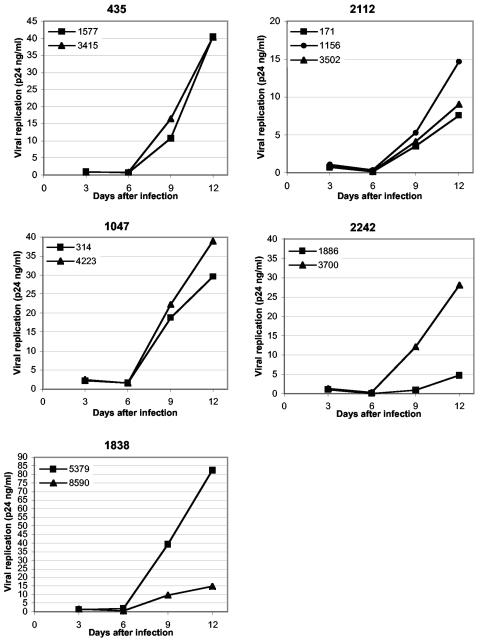
For tissue inoculation, viruses isolated from a given patient were adjusted to the same concentration of p24. The p24 concentration correlated well with the amount of infectious virus, as determined from 50% tissue culture infective dose titration on PBMC (data not shown). A representative experiment for each HIV-1 isolate is shown in Fig. 1, and the levels of replication by day 12 postinfection in five different tissues are shown in Table 2. Replication of these isolates, as monitored from the release of p24, became evident at day 6 postinfection and continued to increase during the course of the experiment, as reported earlier for other HIV-1 variants (16). The absolute levels of viral replication in tissue samples varied from donor to donor (see also reference 40). Because of the limited amount of material in each tissue sample, systematic comparison of isolates from switch and nonswitch virus patients could not be carried out. However, the replication capacities of isolates from one patient, tested in the same tissue, could be compared. We found, however, that the hierarchy of replication capacities of HIV isolates obtained from any one patient remained constant in lymphoid tissues from various donors. For example, in tissues from all tested donors, isolate 5379 from patient 1838 replicated to a higher level than isolate 8590 from the same patient. Similarly, within tissues from all tested donors, isolate 3700 from patient 2242 replicated to a higher level than isolate 1886 from the same patient. Also, in tissues from all tested donors, the isolates from patient 435 replicated to similar levels, and the same was true for isolates from patients 1047 and 2112.
FIG. 1.
Replication of primary R5 HIV-1 isolates in human lymphoid tissue ex vivo. Shown are the replication kinetics of 11 primary R5 HIV-1 isolates from five different patients in ex vivo-infected human lymphoid tissue. One representative experiment (out of five) is shown for each patient. Indicated are the patient numbers and the isolate numbers. Each point represents the p24 concentration accumulated in pooled medium bathing 27 or 54 tissue blocks (9 blocks per 4-ml well) from a single donor over a period of 3 days between medium changes.
TABLE 2.
Virus replication in tissues of five different donors 12 days postinfection
| Patient no. | Isolate no. | Viral replication between days 9 and 12 (p24 ng/ml) for donor no.:
|
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 2112 | 171 | 10 | 14 | 13 | 8 | 7 |
| 1156 | 4 | 13 | 11 | 15 | 6 | |
| 3502 | 6 | 6 | 14 | 9 | 8 | |
| 1838a | 5379 | 11 | 16 | 28 | 13 | 82 |
| 8590 | 2 | 6 | 4 | 3 | 15 | |
| 435 | 1577 | 21 | 9 | 73 | 4 | 40 |
| 3415 | 10 | 5 | 56 | 4 | 40 | |
| 1047 | 314 | 6 | 30 | 15 | 3 | 39 |
| 4223 | 9 | 39 | 12 | 14 | 52 | |
| 2242a | 1886 | 14 | 5 | 1 | 1 | 15 |
| 3700 | 74 | 28 | 7 | 3 | 25 | |
The relative replication capacities of the isolates from patients 1838 and 2242 were significantly different (P = 0.04, Wilcoxon signed rank test).
Cytokine and chemokine production in infected lymphoid tissue.
We studied whether different HIV-1 isolates induce different cytokine/chemokine responses in infected tissue, using a multiplexed fluorescent microsphere immunoassay. We measured the cytokines/chemokines MIP-1α, MIP-1β, RANTES, MIG, IP-10, tumor necrosis factor alpha, SDF-1, IFN-γ, granulocyte-macrophage colony-stimulating factor, IL-1α, IL-1-β, IL-2, IL-4, IL-12, IL-15, and IL-16 in the supernatants of infected and uninfected tissues. There was no difference between the concentrations of any of the cytokines/chemokines in uninfected tissues and in tissues infected with the 11 R5 isolates used in this study (data not shown). IFN-γ, IL-2, IL-4, IL-12, and IL-15 concentrations were below detection levels (14, 41, 123, 41, and 5 pg/ml, respectively) in both infected and uninfected samples.
Loss of CCR5+ CD4+ T lymphocytes.
We evaluated CD4+ T-cell loss by enumerating tissue lymphocytes using flow cytometry. To account for CD4+ downregulation by HIV-1 infection (26, 45), we gated on CD3+ CD8− cells, since the CD3+ CD8− cell subset in uninfected tissue blocks from 16 donors consisted predominantly of CD3+ CD4+ cells (see also reference 23). Infection with the R5 isolates used in this study caused a slight loss (4.1% ± 1.8%; n = 50) of CD3+ CD8− T cells relative to matched uninfected tissues. The natural targets for these viruses, the CCR5+ CD4+ T cells, constituted 4% to 7% of CD3+ CD8− T cells in these tissues, as revealed with flow cytometry (Table 3), and cell loss in this subset (CD3+ CD8− CCR5+) averaged 42.5% ± 3.6% (n = 50) of that in matched uninfected controls (Fig. 2).
TABLE 3.
Distributions of cell populations in lymphoid tissues infected with different primary R5 HIV-1 isolatesa
| Patient no. | Isolate | Percent
|
|||
|---|---|---|---|---|---|
| CD8− of CD3+ | CCR5+ of CD3+ CD8− | p24+ of CD3+ CD8− | CCR5+ of CD3+ CD8− p24+ | ||
| 435 | Uninfected | 86.1 | 5.8 | 0.4 | NAb |
| 1577 | 84.9 | 3.6 | 1.9 | 8.8 | |
| 3415 | 84.0 | 3.0 | 2.1 | 4.6 | |
| 1838 | Uninfected | 82.5 | 5.0 | 0.3 | NA |
| 5379 | 78.9 | 1.9 | 3.6 | 5.3 | |
| 8590 | 74.1 | 1.9 | 1.8 | 17.9 | |
| 1047 | Uninfected | 81.0 | 6.0 | 0.6 | NA |
| 314 | 80.2 | 2.1 | 2.7 | 3.1 | |
| 4223 | 78.5 | 2.3 | 3.5 | 3.2 | |
| 2112 | Uninfected | 82.5 | 6.3 | 0.5 | NA |
| 171 | 82.7 | 5.2 | 2.4 | 1.9 | |
| 1156 | 82.5 | 4.2 | 2.1 | 0.4 | |
| 3502 | 81.1 | 4.0 | 2.7 | 1.2 | |
| 2242 | Uninfected | 80.6 | 5.6 | 0.4 | NA |
| 1886 | 79.2 | 5.4 | 0.2 | NA | |
| 3700 | 78.7 | 4.4 | 1.4 | 7.0 | |
See the text for details. One representative experiment is shown for each patient, corresponding to Fig. 1.
NA, not applicable.
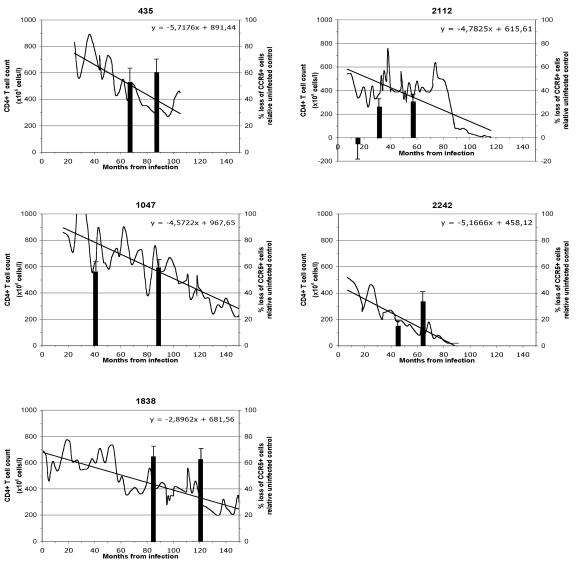
FIG. 2.
T-cell depletion in HIV-1-infected patients and in human lymphoid tissue infected ex vivo with patients' HIV-1 isolates. Shown are the numbers of CD4+ T cells in patients' blood and the loss of CD8− CCR5+ T cells in human lymphoid tissue infected ex vivo by HIV isolates from the same patients. Patients 435, 1047, and 1838 are nonswitch virus patients and yielded viruses of R5 phenotype throughout the study. Patients 2112 and 2242 are switch virus patients who acquired CXCR4-using virus 76 months after infection. The left axis and line show the CD4+ T cell count as the numbers of cells (106) per liter of blood. The regression line and corresponding linear equation are indicated for each patient during the follow-up period. The right axis and bars show the percent loss of CCR5+ CD8− T lymphocytes in ex vivo-infected lymphoid tissue from single donors 12 days postinfection relative to matched uninfected control tissue samples. The data represent the means plus standard errors of the mean of experiments with tissues from at least four donors. For each donor, tissue cell numbers were evaluated in 27 or 54 pooled tissue blocks. The infection date was calculated as the midpoint between the last negative and the first positive samples.
Isolates from nonswitch virus patients 435, 1047, and 1838 caused losses of CD3+ CD8− CCR5+ cells of 57.2% ± 5.3%, 58.6% ± 4.1%, and 60.0% ± 6.4%, respectively (n = 8 to 10). R5 isolates from switch virus patients 2112 and 2242 depleted a smaller fraction of CD3+ CD8− CCR5+ cells in ex vivo-infected lymphoid tissue: the three isolates from patient 2112 depleted on average none (−4.5% ± 12.6%), 26.5% ± 6.7%, and 30.5% ± 6.4% (n = 4) of these cells relative to the matched uninfected controls, and the two tested isolates from patient 2242 depleted 15.8% ± 3.3% and 33.9% ± 7.2% of CD3+ CD8− CCR5+ cells relative to the matched uninfected controls. On average, infection of tissues from 14 donors with six R5 isolates from the three nonswitch virus patients resulted in the loss of 59.6% ± 2.7% of CD3+ CD8− CCR5+ cells, whereas infection of tissues from 9 donors with five R5 isolates from the two switch virus patients resulted in a significantly (P < 0.0001; mixed-model analysis) smaller loss of CD3+ CD8− CCR5+ cells, 20.8% ± 4.2%, relative to matched uninfected controls. Thus, the severity of CD3+ CD8− CCR5+ cell depletion in R5 HIV-infected tissue blocks seemed to depend on whether the virus was isolated from the switch or the nonswitch virus patients. The level of cell depletion did not correlate with the level of replication in the corresponding tissue (data not shown).
Also, for any given patient, the levels of CD8− CCR5+ T-cell depletion in infected tissues were different for sequential R5 isolates. Because of the donor-to-donor variability, we restricted comparison of these sequential isolates to matched tissue blocks. In both switch virus patients (2112 and 2242), the depletion of CD3+CD8− CCR5+ cells was higher for the last than for the first sequential isolate (Fig. 2), while in nonswitch virus patients, the depletion of CD3+CD8− CCR5+ cells was already high with the early isolate and did not increase over time (Fig. 2).
To test to what extent the decrease in the numbers of CD3+ CD8− CCR5+ cells in infected tissues is due to cell depletion and to what extent it is due to downregulation of CCR5 following HIV-1 infection of tissues, we compared the decrease in the number of CD3+ CD8− CCR5+ cells with that in the total number of CD3+ CD8− cells. We assumed that the death of a CD3+ CD8− CCR5+ cell should be reflected by the loss of a CD3+ CD8− cell, whereas a decrease in the number of CD3+ CD8− CCR5+ cells due to downregulation would not be reflected in a decrease in the total number of CD3+ CD8− cells. However, the levels of depletion in the R5-infected tissues were too small to make this comparison statistically sound. Nevertheless, gating on productively infected cells, we found that on average only 5.3% ± 0.8% of the CD3+ CD8− p24+ cells expressed CCR5 (19 infections by 10 different isolates in tissues from 10 different donors), indicating that in productively infected cells CCR5 has been downregulated (Table 3). Therefore, CD3+ CD8− p24+ cells are the counterparts of CCR5+ CD3+ CD4+ cells in the noninfected population.
T cells of different activation status support productive infection of R5 HIV-1 isolates.
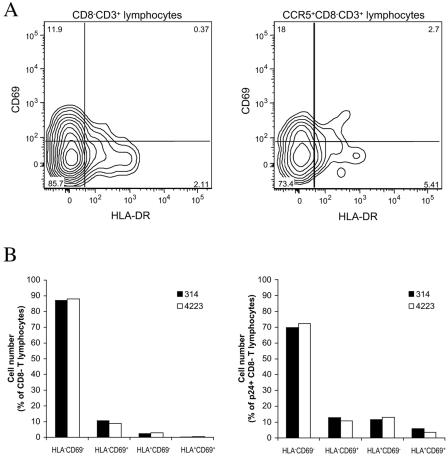
We investigated whether the tested HIV isolates differentially infect and deplete activated and nonactivated cells. There were no differences between the two patient categories, switch and nonswitch virus patients (data not shown), and the data below are therefore pooled. In this study, we defined activation as expression of CD69 and HLA-DR. The former is considered an early activation marker, and the latter is considered a late one (11, 24). First, we compared the distribution of activation markers among CD3+ CD8− cells with that among cells of the CD3+ CD8− CCR5+ subset (Fig. 3A shows a representative experiment). On average, 82% ± 1% (n = 6) of the CD3+ CD8− cells were CD69− HLA-DR−, thus exhibiting a nonactivated phenotype. Single-positive CD69+ cells, single-positive HLA-DR+ cells, and double-positive CD69+ HLA-DR+ cells constituted on average 13% ± 1%, 4% ± 0.4%, and 1% ± 0.1% of the CD3+ CD8− cells, respectively. In the CCR5-expressing subsets of CD3+ CD8− cells, CD69− HLA-DR−, CD69+ HLA-DR−, CD69− HLA-DR+, and CD69+ HLA-DR+ cells constituted 71% ± 5%, 20% ± 4%, 6% ± 1%, and 3% ± 0.8% of the CD3+ CD8− CCR5+ cells, respectively, thus representing a significant increase (P = 0.004; Mann-Whitney test) in the frequency of double-positive activated cells in this population. Does HIV-1 infection of lymphoid tissue result in activation of the general T-lymphocyte population and/or of infected T cells? Analysis of tissues from six different donors infected with 11 isolates revealed no significant difference between the distributions of activation markers among CD3+ CD8− cells and in matched uninfected tissues: the proportions of CD69− HLA-DR−, CD69+ HLA-DR−, CD69− HLA-DR+, and CD69+ HLA-DR+ cells were 83% ± 1%, 12% ± 0.9%, 4% ± 0.3%, and 1% ± 0.1%, respectively (n = 14; P = 0.937; Mann-Whitney test) (Fig. 3 shows a representative experiment). In contrast, in productively infected T cells (CD3+ CD8− p24+), activation marker expression was significantly increased compared with that in the total population of CD3+ CD8− cells: CD69− HLA-DR−, CD69+ HLA-DR−, CD69− HLA-DR+, and CD69+ HLA-DR+ cells in the CD8− p24+ T-cell subset constituted 66% ± 3%, 19% ± 3%, 10% ± 1%, and 5% ± 1% of the total number of cells, respectively (n = 13; P = 0.002; Mann-Whitney test) (Fig. 3B shows a representative experiment). However, the frequencies of activated cells among p24+ CD8− T lymphocytes did not significantly (P = 0.078; Mann-Whitney test) exceed that in the general population of CCR5+ CD8− T lymphocytes, which are potential targets for R5 HIV-1.
FIG. 3.
Activation status of lymphocytes in HIV-1-infected human lymphoid tissues ex vivo; comparison of the distributions of activation markers among different cell populations in uninfected lymphoid tissue and in ex vivo-infected tissue after 12 days in culture. The data were obtained in experiments using 54 pooled tissue blocks per condition from a single donor. (A) Distributions of CD69 and HLA-DR among CD8− CD3+ lymphocytes and CCR5+ CD8− CD3+ lymphocytes in uninfected tissue; one representative experiment out of six is shown. The contour plots are at log 50% probability. (B) Distributions of CD69 and HLA-DR among CD8− CD3+ lymphocytes (left) and p24+ CD8− CD3+ lymphocytes (right) in tissue infected with isolates 314 and 4223 from patient 1047; one representative experiment out of six matching that presented in panel A is shown.
In summary, in tissues, the proportion of cells with an activated phenotype was higher among CD8− T lymphocytes expressing CCR5 than in the total CD8− T-lymphocyte population. However, the activation status of the host cell does not seem to be a determinant for productive HIV-1 infection.
HIV-1 infects CD25+ T cells.
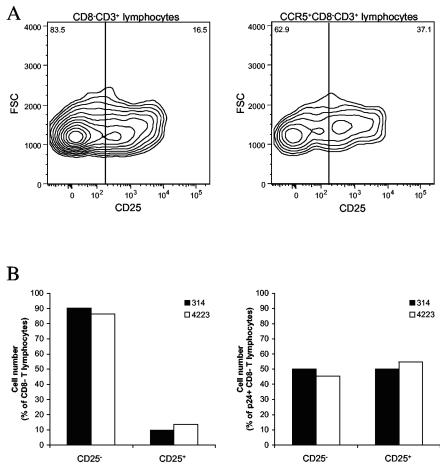
We investigated whether the R5 HIV-1 isolates used in this study infect CD4+ T cells that express CD25, a marker which is present on both activated and regulatory CD4+ T cells (53, 54, 56). As in our studies of activation markers, we have pooled the data from switch and nonswitch virus patients. In the tonsillar tissues from six donors used for these experiments, CD25+ cells constituted 19% ± 1% of the CD3+ CD8− cells. Of these CD25+ cells, 16% ± 4% were CD69+, 14% ± 4% were HLA-DR+, and 1.4% ± 0.3% were CD69+ HLA-DR+. Further analysis showed that the CD3+ CD8− CCR5+ subset was significantly enriched in CD25+ cells, which constituted 30% ± 2% (n = 6; P = 0.004; Mann-Whitney test) of this subset (Fig. 4A shows a representative experiment). Infection of tissues with HIV-1 did not change the fraction of CD25+ CD3+ CD8− cells (17% ± 1% in infected tissues versus 19% ± 1% in matched controls; P = 0.132; n = 14; Mann-Whitney test). In contrast, the fraction of productively infected (CD3+ CD8− p24+) T cells was significantly enriched in CD25+ cells relative to that in the total CD3+ CD8− subset (Fig. 4B shows a representative experiment): CD25+ CD3+ CD8− p24+ cells constituted 48% ± 3% of the CD3+ CD8− p24+ T cells. In summary, CD4+ CD25+ T cells efficiently support productive infection by HIV-1 of the R5 phenotype.
FIG. 4.
Expression of CD25 on T cells in HIV-1-infected human lymphoid tissue ex vivo; comparison of the distributions of CD25 among different cell populations in uninfected and ex vivo-infected lymphoid tissue after 12 days in culture. The data were obtained in experiments using 54 pooled tissue blocks per condition from a single donor. (A) Distributions of CD25 among CD8− CD3+ lymphocytes (left) and among CCR5+ CD8− CD3+ lymphocytes (right) in uninfected tissue. The contour plots are at log 50% probability. Presented is one representative experiment out of six. (B) The distributions of CD25 among CD8− CD3+ lymphocytes (left) and among p24+ CD8− CD3+ lymphocytes in tissue infected with isolates 314 and 4223 from patient 1047; one representative experiment out of six matching that presented in panel A is shown.
DISCUSSION
Recent studies of HIV pathogenesis in vivo have emphasized that critical events in HIV disease occur in lymphoid tissue and are not necessarily reflected by changes in blood (6, 35). Here, we used a system of ex vivo tonsillar tissue to study the tissue pathogeneses of 11 HIV primary isolates, all of which utilized CCR5 for cell entry but which were obtained from patients at different stages of disease progression. We showed that these patients' HIV-1 isolates efficiently replicate in ex vivo-infected human lymphoid tissue. These isolates deplete their natural targets, i.e., CCR5+ CD4+ T cells, and such depletion seems to be related to the modes of disease progression in the patients that harbored them.
It is widely accepted that HIV disease progression is determined by a complex and as yet poorly understood combination of host and viral factors (15). By infecting lymphoid tissue from one donor with a panel of different isolates, and by infecting a panel of lymphoid tissues from different donors with one particular HIV-1 isolate, we were able to separate which parameters are controlled by host and viral factors in HIV tissue pathogenesis. We have found that although the absolute levels of viral replication varied as much as 30-fold between tissues obtained from different donors and were different for different isolates, the viral hierarchy among sequential isolates remained constant, emphasizing viral factors as major determinants of the relative replication capacities of these isolates in human lymphoid tissues ex vivo. In contrast, the absolute replicative capacity of HIV-1 isolates is controlled by host (tissue) factors that seem to enhance or suppress all replicating HIV-1 variants.
Replication of HIV isolates in this ex vivo system resulted in depletion of CD4+ T cells, but only those expressing CCR5 (see also reference 22). These T cells constitute a minority of CD4+ T cells (5, 22), and therefore, the 50% depletion of the cells observed in our experiments did not translate into a significant depletion of the total numbers of CD4+ T lymphocytes. We found that depletion of CD4+ CCR5+ cells was accompanied not only by downregulation of CD4, observed earlier in other systems (26, 45), but also by downregulation of CCR5. Coreceptor downregulation was reported earlier for CXCR4 (14, 57, 59) and has recently been reported for CCR5 (8, 59) also.
It should be pointed out that the levels of CCR5+ CD4+ T-cell depletion caused by a given isolate in tissues from different donors were similar in spite of the large variation in the levels of replication. Thus, together with relative replicative capacity, the absolute levels of CD4+ T-cell depletion by the R5 isolates used in the present work seemed to be largely controlled by viral factors, whereas the absolute replication levels were greatly affected by a tissue (host) factor(s).
We made attempts to identify host factors by measuring tissue production of cytokines and chemokines, since these host factors are known to affect HIV-1 pathogenesis (1). However, in our ex vivo tissues, no infection with any of the primary R5 HIV-1 isolates affected the levels of the 16 measured cytokines/chemokines. Earlier, similar results were reported for an R5 laboratory strain and for recombinant viruses carrying R5 Envs, whereas an X4 strain significantly changed chemokine release (9, 27). However, one recent study (8) has suggested that R5 HIV-1 infection of fetal thymic organ cultures induces IL-10 and transforming growth factor β, cytokines not studied here, and thereby upregulates the expression of CCR5.
Another tissue factor that may control the absolute level of viral replication is the activation status of cell targets. The use of ex vivo human lymphoid tissues, which do not require exogenous stimulation to support productive HIV-1 infection, allowed us to address this question. In vitro infection of PBMC requires activated or mature cells (7, 25, 42, 47, 52). In contrast, in the context of lymphoid tissue, nonactivated CD4+ T cells support productive infection as well (13, 19, 20). Recently, Kinter et al. (31) provided further evidence for the role of the lymphoid tissue microenvironment in controlling HIV infection by demonstrating that HIV-1 productively infected nonactivated CD4+ T cells in tissue ex vivo, while the same cells could not be infected if isolated from this tissue. Our present results confirm that in tissues the majority of the productively infected cells are of the nonactivated phenotype, as evidenced by the lack of CD69 and HLA-DR expression. These results reflect the situation in vivo, where HIV-1 gene expression is detected in nonactivated and naïve cells (4, 37, 60).
To further characterize tissue cell targets for primary R5 isolates, we analyzed the expression of CD25, a marker of activated CD4+ T cells (56) that is also expressed on regulatory CD4+ T cells (53, 54). Tonsils are thought to harbor a larger proportion of regulatory CD4+ CD25+ T cells than peripheral blood, because of constant antigen exposure and the need to control inflammation and tissue destruction (50). We found that only a small number of T cells coexpress the activation markers CD69, HLA-DR, and CD25. This finding supports the notion that a fraction of CD4+ CD25+ cells in tonsil tissue have a regulatory function and may not be activated. This may be an important host factor affecting HIV infection, in view of a recently reported suppression of HIV-specific responses in vitro by CD25+ regulatory T cells isolated from HIV-infected donors (32). Infection and depletion of T-cell subsets, as shown by our experiments, may include the regulatory T cells and could be an important host factor affecting the efficiency of HIV replication ex vivo.
As discussed above, the relative replication level and the ability to deplete CD4+ T cells are largely determined by viral factors. Although several viral gene products, including Nef, Vpu, Vpr, and Vif, have been reported to determine viral replication capacity in various systems, including the one used for the current study (17, 18, 36, 44, 51), most of our knowledge regarding differential pathogenesis of HIV-1 in tissues is related to coreceptor usage. Rapid progression of HIV-1 disease has been shown to be associated with evolution of virus coreceptor use from CCR5 to CXCR4. One explanation for the higher virulence of X4 viruses is the abundance of their target cells (CD4+ CXCR4+) in lymphoid tissue (22). However, it has been an enigma that about 50% of patients progress to AIDS without apparent R5-to-X4 evolution (12, 28).
Here, we studied viral isolates, all of which were of the R5 phenotype. Whatever the viral factors that determine differential pathogenesis of these R5 isolates in tissues are, we provide here the first published evidence that the evolution of these factors is consistent with the pattern of disease progression. The degree of depletion of CCR5+ CD4+ T cells by a given viral isolate may have predictive value and reflects whether the individual from whom the virus was obtained eventually became a switch virus patient. Indeed, R5 viruses isolated from nonswitch virus patients depleted more target cells than isolates from switch virus patients. Conversely, the patients' viral load, expressed as the level of HIV-1 p24 antigen in serum, was undetectable in nonswitch virus patients, while it was detectable in switch virus patients. It is tempting to speculate that the highly cytopathic R5 virus in nonswitch patients eliminates the CD4+ CCR5+ target cells and thereby limits its own replication. The less cytopathic R5 virus from switch virus patients leaves more target cells intact and therefore replicates to higher titers in vivo. A high viral load in vivo, in combination with the eventual appearance of CXCR4-using virus in the switch virus patient, results in an increased severity of HIV-1 infection, with early clinical symptoms, and in low CD4 T-cell counts. Also, the appearance of X4 HIV-1 in switch virus patients seems to be preceded by an evolution of R5 HIV-1, since our experiments demonstrated that sequential isolates from such patients increase their ability to deplete CCR5+ CD4+ T cells during the course of the patient's infection.
In conclusion, various host factors seem to enhance or inhibit replication of all viral isolates, whereas viral factors determine which isolate has a higher or lower relative capacity to replicate. R5 isolates from patients with progressive HIV-1 disease can efficiently infect, replicate, and deplete CCR5+ CD4+ T cells in human lymphoid tissue ex vivo. In the course of disease progression leading to the switch to X4 dominance, R5 HIV-1 variants appear to undergo evolution associated with an increase of their cytopathicity. R5 HIV-1 isolates from nonswitch virus patients are more cytopathic than R5 variants from switch virus patients, and this difference may explain the steady decline of CD4+ T cells in patients with continuous dominance of R5 HIV-1.
Acknowledgments
We thank M. R. Santi and the Department of Pathology of Children's Hospital (Washington, D.C.) for their kind assistance in providing tonsillar tissue and Bengt Johansson-Lindblom, William Agace, and Håkan Lövkvist for expert advice.
Grants were received from the Swedish Research Council, the Swedish International Development Cooperation Agency/Department for Research Cooperation (SIDA/SAREC), and the Crafoord Foundation.
REFERENCES
- 1.Alfano, M., and G. Poli. 2002. The cytokine network in HIV infection. Curr. Mol. Med. 2:677-689. [DOI] [PubMed] [Google Scholar]
- 2.Berger, E. A., R. W. Doms, E. M. Fenyo, B. T. Korber, D. R. Littman, J. P. Moore, Q. J. Sattentau, H. Schuitemaker, J. Sodroski, and R. A. Weiss. 1998. A new classification for HIV-1. Nature 391:240. [DOI] [PubMed] [Google Scholar]
- 3.Bjorndal, A., H. Deng, M. Jansson, J. R. Fiore, C. Colognesi, A. Karlsson, J. Albert, G. Scarlatti, D. R. Littman, and E. M. Fenyo. 1997. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 71:7478-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaak, H., A. B. van't Wout, M. Brouwer, B. Hooibrink, E. Hovenkamp, and H. Schuitemaker. 2000. In vivo HIV-1 infection of CD45RA+CD4+ T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4+ T cell decline. Proc. Natl. Acad. Sci. USA 97:1269-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleul, C. C., L. Wu, J. A. Hoxie, T. A. Springer, and C. R. Mackay. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou, C. S., O. Ramilo, and E. S. Vitetta. 1997. Highly purified CD25− resting T cells cannot be infected de novo with HIV-1. Proc. Natl. Acad. Sci. USA 94:1361-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhary, S. K., N. R. Choudhary, K. C. Kimbrell, J. Colasanti, A. Ziogas, D. Kwa, H. Schuitemaker, and D. Camerini. 2005. R5 human immunodeficiency virus type 1 infection of fetal thymic organ culture induces cytokine and CCR5 expression. J. Virol. 79:458-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciuffi, A., G. Bleiber, M. Munoz, R. Martinez, C. Loeuillet, M. Rehr, M. Fischer, H. F. Gunthard, A. Oxenius, P. Meylan, S. Bonhoeffer, D. Trono, and A. Telenti. 2004. Entry and transcription as key determinants of differences in CD4 T-cell permissiveness to human immunodeficiency virus type 1 infection. J. Virol. 78:10747-10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotner, T., J. M. Williams, L. Christenson, H. M. Shapiro, T. B. Strom, and J. Strominger. 1983. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J. Exp. Med. 157:461-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Roda Husman, A. M., R. P. van Rij, H. Blaak, S. Broersen, and H. Schuitemaker. 1999. Adaptation to promiscuous usage of chemokine receptors is not a prerequisite for human immunodeficiency virus type 1 disease progression. J. Infect. Dis. 180:1106-1115. [DOI] [PubMed] [Google Scholar]
- 13.Eckstein, D. A., M. L. Penn, Y. D. Korin, D. D. Scripture-Adams, J. A. Zack, J. F. Kreisberg, M. Roederer, M. P. Sherman, P. S. Chin, and M. A. Goldsmith. 2001. HIV-1 actively replicates in naive CD4+ T cells residing within human lymphoid tissues. Immunity 15:671-682. [DOI] [PubMed] [Google Scholar]
- 14.Endres, M. J., P. R. Clapham, M. Marsh, M. Ahuja, J. D. Turner, A. McKnight, J. F. Thomas, B. Stoebenau-Haggarty, S. Choe, P. J. Vance, T. N. Wells, C. A. Power, S. S. Sutterwala, R. W. Doms, N. R. Landau, and J. A. Hoxie. 1996. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell 87:745-756. [DOI] [PubMed] [Google Scholar]
- 15.Fauci, A. S. 1996. Host factors in the pathogenesis of HIV disease. Antibiot. Chemother. 48:4-12. [DOI] [PubMed] [Google Scholar]
- 16.Glushakova, S., B. Baibakov, L. B. Margolis, and J. Zimmerberg. 1995. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat. Med. 1:1320-1322. [DOI] [PubMed] [Google Scholar]
- 17.Glushakova, S., J. C. Grivel, K. Suryanarayana, P. Meylan, J. D. Lifson, R. Desrosiers, and L. Margolis. 1999. Nef enhances human immunodeficiency virus replication and responsiveness to interleukin-2 in human lymphoid tissue ex vivo. J. Virol. 73:3968-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glushakova, S., Y. Yi, J. C. Grivel, A. Singh, D. Schols, E. De Clercq, R. G. Collman, and L. Margolis. 1999. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J. Clin. Investig. 104:R7-R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gondois-Rey, F., J. C. Grivel, A. Biancotto, M. Pion, R. Vigne, L. B. Margolis, and I. Hirsch. 2002. Segregation of R5 and X4 HIV-1 variants to memory T cell subsets differentially expressing CD62L in ex vivo infected human lymphoid tissue. AIDS 16:1245-1249. [DOI] [PubMed] [Google Scholar]
- 21.Grivel, J. C., N. Malkevitch, and L. Margolis. 2000. Human immunodeficiency virus type 1 induces apoptosis in CD4+ but not in CD8+ T cells in ex vivo-infected human lymphoid tissue. J. Virol. 74:8077-8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grivel, J. C., and L. B. Margolis. 1999. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat. Med. 5:344-346. [DOI] [PubMed] [Google Scholar]
- 23.Grivel, J. C., F. Santoro, S. Chen, G. Faga, M. S. Malnati, Y. Ito, L. Margolis, and P. Lusso. 2003. Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex vivo. J. Virol. 77:8280-8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara, T., L. K. Jung, J. M. Bjorndahl, and S. M. Fu. 1986. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J. Exp. Med. 164:1988-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helbert, M. R., J. Walter, J. L'Age, and P. C. Beverley. 1997. HIV infection of CD45RA+ and CD45RO+ CD4+ T cells. Clin. Exp. Immunol. 107:300-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoxie, J. A., J. D. Alpers, J. L. Rackowski, K. Huebner, B. S. Haggarty, A. J. Cedarbaum, and J. C. Reed. 1986. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science 234:1123-1127. [DOI] [PubMed] [Google Scholar]
- 27.Ito, Y., J. C. Grivel, and L. Margolis. 2003. Real-time PCR assay of individual human immunodeficiency virus type 1 variants in coinfected human lymphoid tissues. J. Clin. Microbiol. 41:2126-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson, A., K. Parsmyr, E. Sandstrom, E. M. Fenyo, and J. Albert. 1994. MT-2 cell tropism as prognostic marker for disease progression in human immunodeficiency virus type 1 infection. J. Clin. Microbiol. 32:364-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlsson, I., L. Antonsson, Y. Shi, A. Karlsson, J. Albert, T. Leitner, B. Olde, C. Owman, and E. M. Fenyo. 2003. HIV biological variability unveiled: frequent isolations and chimeric receptors reveal unprecedented variation of coreceptor use. AIDS 17:2561-2569. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson, I., L. Antonsson, Y. Shi, A. Karlsson, J. Albert, B. Olde, M. Jansson, C. Owman, and E. M. Fenyo. 2004. Coevolution of RANTES sensitivity and mode of CCR5 receptor use by human immunodeficiency virus type 1 of the R5 phenotype. J. Virol. 78:11807-11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinter, A., A. Moorthy, R. Jackson, and A. S. Fauci. 2003. Productive HIV infection of resting CD4+ T cells: role of lymphoid tissue microenvironment and effect of immunomodulating agents. AIDS Res. Hum. Retrovir. 19:847-856. [DOI] [PubMed] [Google Scholar]
- 32.Kinter, A. L., M. Hennessey, A. Bell, S. Kern, Y. Lin, M. Daucher, M. Planta, M. McGlaughlin, R. Jackson, S. F. Ziegler, and A. S. Fauci. 2004. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 200:331-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koot, M., I. P. Keet, A. H. Vos, R. E. de Goede, M. T. Roos, R. A. Coutinho, F. Miedema, P. T. Schellekens, and M. Tersmette. 1993. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann. Intern. Med. 118:681-688. [DOI] [PubMed] [Google Scholar]
- 34.Leitner, T., G. Korovina, S. Marquina, T. Smolskaya, and J. Albert. 1996. Molecular epidemiology and MT-2 cell tropism of Russian HIV type 1 variant. AIDS Res. Hum. Retrovir. 12:1595-1603. [DOI] [PubMed] [Google Scholar]
- 35.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrowski, M. A., T. W. Chun, S. J. Justement, I. Motola, M. A. Spinelli, J. Adelsberger, L. A. Ehler, S. B. Mizell, C. W. Hallahan, and A. S. Fauci. 1999. Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J. Virol. 73:6430-6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantaleo, G., C. Graziosi, J. F. Demarest, L. Butini, M. Montroni, C. H. Fox, J. M. Orenstein, D. P. Kotler, and A. S. Fauci. 1993. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362:355-358. [DOI] [PubMed] [Google Scholar]
- 39.Pantaleo, G., C. Graziosi, and A. S. Fauci. 1993. The role of lymphoid organs in the pathogenesis of HIV infection. Semin. Immunol. 5:157-163. [DOI] [PubMed] [Google Scholar]
- 40.Penn, M. L., J. C. Grivel, B. Schramm, M. A. Goldsmith, and L. Margolis. 1999. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc. Natl. Acad. Sci. USA 96:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penn, M. L., M. Myers, D. A. Eckstein, T. J. Liegler, M. Hayden, F. Mammano, F. Clavel, S. G. Deeks, R. M. Grant, and M. A. Goldsmith. 2001. Primary and recombinant HIV type 1 strains resistant to protease inhibitors are pathogenic in mature human lymphoid tissues. AIDS Res. Hum. Retrovir. 17:517-523. [DOI] [PubMed] [Google Scholar]
- 42.Roederer, M., P. A. Raju, D. K. Mitra, and L. A. Herzenberg. 1997. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J. Clin. Investig. 99:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosok, B., J. E. Brinchmann, P. Voltersvik, J. Olofsson, L. Bostad, and B. Asjo. 1997. Correlates of latent and productive HIV type-1 infection in tonsillar CD4+ T cells. Proc. Natl. Acad. Sci. USA 94:9332-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rucker, E., J. Munch, S. Wildum, M. Brenner, J. Eisemann, L. Margolis, and F. Kirchhoff. 2004. A naturally occurring variation in the proline-rich region does not attenuate human immunodeficiency virus type 1 nef function. J. Virol. 78:10197-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salmon, P., R. Olivier, Y. Riviere, E. Brisson, J. C. Gluckman, M. P. Kieny, L. Montagnier, and D. Klatzmann. 1988. Loss of CD4 membrane expression and CD4 mRNA during acute human immunodeficiency virus replication. J. Exp. Med. 168:1953-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scarlatti, G., V. Lombardi, A. Plebani, N. Principi, C. Vegni, G. Ferraris, A. Bucceri, E. M. Fenyo, H. Wigzell, P. Rossi, et al. 1991. Polymerase chain reaction, virus isolation and antigen assay in HIV-1-antibody-positive mothers and their children. AIDS 5:1173-1178. [DOI] [PubMed] [Google Scholar]
- 47.Schnittman, S. M., H. C. Lane, J. Greenhouse, J. S. Justement, M. Baseler, and A. S. Fauci. 1990. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc. Natl. Acad. Sci. USA 87:6058-6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schols, D., S. Struyf, J. Van Damme, J. A. Este, G. Henson, and E. De Clercq. 1997. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 186:1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shankarappa, R., J. B. Margolick, S. J. Gange, A. G. Rodrigo, D. Upchurch, H. Farzadegan, P. Gupta, C. R. Rinaldo, G. H. Learn, X. He, X. L. Huang, and J. I. Mullins. 1999. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 73:10489-10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simark-Mattsson, C., U. Dahlgren, and K. Roos. 2002. CD4+CD25+ T lymphocytes in human tonsils suppress the proliferation of CD4+CD25− tonsil cells. Scand. J. Immunol. 55:606-611. [DOI] [PubMed] [Google Scholar]
- 51.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spina, C. A., H. E. Prince, and D. D. Richman. 1997. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J. Clin. Investig. 99:1774-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stephens, L. A., C. Mottet, D. Mason, and F. Powrie. 2001. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31:1247-1254. [DOI] [PubMed] [Google Scholar]
- 54.Taams, L. S., J. Smith, M. H. Rustin, M. Salmon, L. W. Poulter, and A. N. Akbar. 2001. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 31:1122-1131. [DOI] [PubMed] [Google Scholar]
- 55.Tersmette, M., R. A. Gruters, F. de Wolf, R. E. de Goede, J. M. Lange, P. T. Schellekens, J. Goudsmit, H. G. Huisman, and F. Miedema. 1989. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J. Virol. 63:2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uchiyama, T., S. Broder, and T. A. Waldmann. 1981. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac+ cells. J. Immunol. 126:1393-1397. [PubMed] [Google Scholar]
- 57.Valente, S. T., C. Chanel, J. Dumonceaux, R. Olivier, S. Marullo, P. Briand, and U. Hazan. 2001. CXCR4 is down-regulated in cells infected with the CD4-independent X4 human immunodeficiency virus type 1 isolate m7NDK. J. Virol. 75:439-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van't Wout, A. B., N. A. Kootstra, G. A. Mulder-Kampinga, N. Albrecht-van Lent, H. J. Scherpbier, J. Veenstra, K. Boer, R. A. Coutinho, F. Miedema, and H. Schuitemaker. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Investig. 94:2060-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, J. M., H. Ueda, O. M. Howard, M. C. Grimm, O. Chertov, X. Gong, W. Gong, J. H. Resau, C. C. Broder, G. Evans, L. O. Arthur, F. W. Ruscetti, and J. J. Oppenheim. 1998. HIV-1 envelope gp120 inhibits the monocyte response to chemokines through CD4 signal-dependent chemokine receptor down-regulation. J. Immunol. 161:4309-4317. [PubMed] [Google Scholar]
- 60.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]
- 61.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]