Abstract
The p5 promoter region of adeno-associated virus type 2 (AAV-2) is a multifunctional element involved in rep gene expression, Rep-dependent replication, and site-specific integration. We initially characterized a 350-bp p5 region by its ability to behave like a cis-acting replication element in the presence of Rep proteins and adenoviral factors. The objective of this study was to define the minimal elements within the p5 region required for Rep-dependent replication. Assays performed in transfected cells (in vivo) indicated that the minimal p5 element was composed by a 55-bp sequence (nucleotides 250 to 304 of wild-type AAV-2) containing the TATA box, the Rep binding site, the terminal resolution site present at the transcription initiation site (trs+1), and a downstream 17-bp region that could potentially form a hairpin structure localizing the trs+1 at the top of the loop. Interestingly, the TATA box was absolutely required for in vivo but dispensable for in vitro, i.e., cell-free, replication. We also demonstrated that Rep binding and nicking at the trs+1 was enhanced in the presence of the cellular TATA binding protein, and that overexpression of this cellular factor increased in vivo replication of the minimal p5 element. Together, these studies identified the minimal replication origin present within the AAV-2 p5 promoter region and demonstrated for the first time the involvement of the TATA box, in cis, and of the TATA binding protein, in trans, for Rep-dependent replication of this viral element.
The productive life cycle of adeno-associated virus type 2 (AAV-2) depends upon the presence of a helper virus such as adenovirus or herpes simplex virus that provide essential factors required for both AAV-2 DNA replication and gene expression (1). The viral genome, composed of a 4.7-kb single-stranded DNA molecule, contains two open reading frames (ORFs), rep and cap, encoding the regulatory (Rep78, Rep68, Rep52, and Rep40) and structural (VP1, VP2, and VP3) proteins, respectively. The genome is flanked by 145-base inverted terminal repeats (ITRs) that constitute the essential cis-acting elements required for AAV DNA replication.
The current model for AAV-2 DNA replication predicts that viral DNA replicates by a self-priming displacement mechanism that is initiated from the ITR and requires cellular polymerases, helper virus-derived factors and AAV-2 Rep proteins (17, 34, 59, 62). In particular, two AAV-2 regulatory proteins, Rep78 and Rep68, are essential for the replication process. Both of these proteins possess DNA binding, ATPase, helicase, and endonuclease activities. They were shown to bind the ITR at a specific site, called the Rep binding site (RBS), and to cleave it at the terminal resolution site (trs) between two thymidine residues (19, 48, 49). This process is essential for the completion of the synthesis of a double-stranded monomer form, which is then used as the template for the reinitiation of DNA synthesis (17, 59).
Previous studies have demonstrated that efficient nicking at the trs required, besides the Rep-binding site, the contact of Rep with a five-base sequence called RBE′, present at the tip of one of the ITR arms (5, 9, 30, 31, 42, 65). In addition, the sequence surrounding the trs was shown to be important for the formation of a stem-loop structure that exposed the trs on the single-stranded loop (3, 4). Recent crystallographic data have confirmed that the N-terminal domain of Rep interacted with RBE′ and suggested that this interaction was implicated in the orientation of the Rep molecules bound at the ITR as well as in the stimulation of the extrusion of the trs by the Rep helicase activity (5). Two of these elements, the Rep-binding site and the trs, are also present within the chromosome 19 AAVS1 locus, where wild-type AAV-2 was shown to site-specifically integrate (21, 26, 43). More recently a secondary structure was also described near the trs of AAVS1 (20). Besides its role during AAV-2 site-specific integration, this chromosomal element was also shown to be able to replicate in the presence of Rep (55).
The observation that recombinant AAV vectors containing only the viral ITR replicated less efficiently than wild-type virus led several groups to postulate the presence within the AAV-2 genome of an additional cis-acting replication element, besides the ITRs, and to demonstrate its presence within the 5′ portion of the rep gene (33, 37, 54). We showed that a 350-bp sequence (previously named CARE) encompassing the p5 promoter and the 5′ portion of the rep ORF (nucleotides 190 to 540 of wild-type AAV-2) was able to promote Rep-dependent replication of a plasmid containing the ITR-deleted rep-cap genome and the encapsidation of these sequences during recombinant AAV production (36, 37). This element contained the binding sites for transcription factors such as YY1, and the major late transcription factor, a TATA box, a Rep-binding site, and a trs-like motif (8, 30, 58). Binding of Rep78 and Rep68 to the p5 Rep-binding site was previously shown to mediate the transcriptional repression of the p5 promoter observed in both the absence and presence of adenovirus (22, 38). The p5 trs-like motif was identified because it enabled Rep-dependent AAV-2 replication in the absence of the left ITR (58). In addition to its ability to behave as a cis-acting replication origin, the p5 region (nucleotides 151 to 289 of wild-type AAV-2) was recently shown to enhance Rep-mediated site-specific integration of plasmid DNA into the human chromosome 19 AAVS1 site (39, 40).
The objective of this study was to define the minimal elements within the p5 region required for Rep-dependent replication. Replication assays performed in transfected cells (in vivo) indicated that a minimal functional p5 element was composed of a 55-bp region (nucleotides 250 to 304 of wild-type AAV-2) containing the TATA box, the Rep-binding site, the trs present at the transcription initiation site (trs+1), and a downstream 17-bp region that could potentially form a hairpin structure localizing the trs+1 at the top of the loop. Interestingly, the TATA box was absolutely required for in vivo but dispensable for in vitro, i.e., cell-free, replication. Finally, we demonstrated that Rep binding and nicking at the trs+1 was enhanced in the presence of cellular TATA binding protein (TBP), and that the overexpression of this cellular factor increased the in vivo replication of the minimal p5 element. Together, these studies characterized the minimal AAV replication origin present within the p5 promoter region and demonstrated for the first time the involvement of the TATA box, in cis, and of the TBP, in trans, for the Rep-dependent replication of this element.
MATERIALS AND METHODS
Cells and viruses.
HeLa, HeRC32 (7), and 293 cells were maintained in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal bovine serum (Sigma) and 1% penicillin/streptomycin (Gibco BRL, 5,000 U/ml). Wild type adenovirus type 5 (ATCC VR-5) was produced and titrated using standard procedures (12), and used at a multiplicity of infection of 50 on HeLa or HeRC32 cells and 10 on 293 cells
Plasmids.
The p5-containing plasmids were constructed by blunt-end ligation of the various p5 versions into a HincII site in plasmid pSP72 (Promega). The wild-type p5 (nucleotides 190 to 353 of wild-type AAV-2, GenBank no. NC 001401), the p5mtrs, and the p5mRBS fragments were obtained by PpuMI digestion of plasmids pCARE.LZ, pCAREmtrs.LZ, and pCAREmRBS.LZ (37), respectively, and blunted with T4 DNA polymerase (New England Biolabs). The deletion mutant p5D3 was obtained by DraIII digestion of the PpuMI-PpuMI wild-type p5 fragment, and mutant p5D5 was obtained by BstUI digestion of the p5D3 fragment version. The p5 mutants D7, D10, D10mTATA, D12, D14, D15, and D16 (Table 1) were generated by PCR using Platinum Pfx DNA polymerase (Invitrogen) and plasmid pAV2 as a substrate (24). For the p5D10mTATA mutant, we used a PCR primer that changed 2 bases of the p5 TATA box to introduce a SnaBI site (TACGTA). The p5D6 mutant was obtained by annealing two complementary 33-bp oligonucleotides corresponding to nucleotides 261 to 293 of wild-type AAV-2. For the in vivo replication assays, a cytomegalovirus-enhanced green fluorescent protein (EGFP)-simian virus 40 polyadenylation site cassette, derived from AseI-AflIII digestion of plasmid pEGFP-N1 (Clontech), was inserted downstream of the p5 element by blunt-end ligation into the SmaI site of pSP72 to obtain the various p5-GFP plasmids.
TABLE 1.
Description of the p5 mutant versions used in in vivo and in vitro replication assays
| p5 version | Length (bp) | Positionsa | TATA box | Rep binding site | trs+1 | Hairpinb |
|---|---|---|---|---|---|---|
| p5wt | 163 | 190-353 | + | + | + | + |
| p5mtrs | 163 | 190-353 | + | + | − | − |
| p5mRBS | 162 | 190-353 | + | − | + | + |
| p5D3 | 116 | 237-353 | + | + | + | + |
| p5D5 | 73 | 237-310 | + | + | + | + |
| p5D7 | 72 | 233-304 | + | + | + | + |
| p5D10 | 55 | 250-304 | + | + | + | + |
| p5D10mTATA | 56 | 249-304 | − | + | + | + |
| p5D12 | 45 | 260-304 | − | + | + | + |
| p5D14 | 44 | 250-293 | + | + | + | − |
| p5D6 | 33 | 261-293 | − | + | + | − |
Plasmid pXJ41-hTBP contained the cDNA encoding human TBP under control of the cytomegalovirus promoter (a kind gift from Làszlò Tora, Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France). Plasmid pCMVRep contained the complete rep coding sequence under the control of the cytomegalovirus promoter.
Production and purification of recombinant Rep68.
The sequence encoding the AAV-2 Rep68 protein was cloned between the NdeI and BamHI sites of plasmid pET-19b (Novagen). N-terminally His10-tagged protein was expressed in Escherichia coli BL21(DE3)pLysS cells and purified under native conditions by nickel-nitrilotriacetic acid-agarose chromatography (QIAGEN). After elution in 250 mM imidazole, the purified protein was desalted over PD-10 columns (Amersham Pharmacia Biotech) into a buffer containing 25 mM Tris-HCl (pH 7.5), 200 mM NaCl, 1 mM dithiothreitol, 0.1 mM EDTA, 0.1% NP-40, and 20% glycerol. A His10-tagged β-galactosidase protein was expressed and purified under the same conditions to be used as a negative control. Purified proteins were verified by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) followed by Coomassie staining and Western blotting. Mutant Rep68 Y156F was also purified from E. coli as a His-tagged protein and was a kind gift of M. Yoon-Robarts and R. M. Linden (Mount Sinaï School of Medicine, New York).
Nicking assay.
The DNA substrates for the Rep endonuclease (nicking) assay were obtained by PpuMI digestion of plasmids pCARE.LZ and pCAREmtrs.LZ (37). The 160-bp restriction fragment was dephosphorylated and 5′ end labeled at 37°C in a 30-μl reaction volume containing 50 μCi of [γ-32P]ATP (5,000 Ci/mmol, Amersham Pharmacia Biotech) and 10 U of T4 polynucleotide kinase (New England Biolabs). After 20 min. at 65°C, unincorporated nucleotides were removed by passing through a Sephadex G-25 column (Amersham Pharmacia Biotech), and radiolabeled DNA was divided in two tubes for digestion by either BbrPI or MspI. The BbrPI-PpuMI (144 bp) and PpuMI-MspI (133 bp) substrates were then PAGE purified, eluted from the gel in 10 mM Tris, 1 mM EDTA (TE) buffer, ethanol precipitated, and resuspended in 10 μl of water.
Nicking assays were performed in a 20-μl reaction volume containing 25 mM HEPES-KOH (pH 7.5), 6.25 mM MgCl2, 1 mM ATP, 1 mM dithiothreitol, 1 μg poly(dI-dC), 0.2 ng of bovine serum albumin, 2,000 cpm of the radiolabeled DNA substrate, and 1 pmol of purified Rep68. After 1 h at 37°C, reaction products were digested with proteinase K, phenol-chloroform extracted, ethanol precipitated, and separated along with sequencing reactions on 6% denaturing polyacrylamide gels containing 50% urea. Gels were then dried and subjected to autoradiography at −80°C. Sequencing was performed on the same fragments using the Sequenase version 2.0 kit (U.S. Biochemicals) and [α-35S]dATP (1,000 Ci/mmol, Amersham Pharmacia Biotech).
To study the effect of TATA binding protein on Rep endonuclease activity, nicking assays were performed in the same conditions as electrophoretic mobility shift assay (EMSA) except that the reactions contained 6.25 mM MgCl2 and 1 mM ATP and they were incubated for 1 h at 37°C after addition of Rep68. Also, in these experiments, the final concentration of proteins was kept to 200 ng by adding purified β-galactosidase where necessary. Reaction products were then processed as described above and were separated on 8% denaturing polyacrylamide gels.
In vivo replication assay.
Subconfluent monolayers of HeRC32 cells (7) seeded in a six-well plate were cotransfected with 2 μg of each p5-GFP plasmid and pcDNA3.1/Hygro/lacZ (Invitrogen) by standard calcium phosphate precipitation. After 6 h, cells were infected (or not) with adenovirus type 5 and collected at 48 h postinfection. Total cellular DNA was extracted from the cell pellet and analyzed by Southern blot after digestion with either DpnI or MboI (37). Membranes were hybridized with a 757-bp GFP probe that was obtained by NotI and Eco47III digestion of plasmid pEGFP-N1. Transfection efficiency was monitored by standard dot blot hybridization using a 722-bp lacZ probe, obtained by Eco47III and EcoRV digestion of plasmid pcDNA3.1/Hygro/lacZ. To study the effect of TBP overexpression on p5 activity, subconfluent 293 cells seeded in six-well plates were cotransfected with 0.5 μg of p5-GFP, 2 μg of pCMVRep, and 2 μg of either pXJ41-hTBP-Neo or pCI-Neo (Promega). Cells were then infected (or not) with wild-type adenovirus type 5 and processed 48 h postinfection for Southern blot analysis as described above.
In vitro replication assay.
Cellular extracts from adenovirus type 5-infected HeLa cells were prepared as previously described (60). DNA substrates were prepared by digestion of the p5-containing plasmids with AflIII and XmnI, followed by phenol-chloroform extraction, ethanol precipitation, and resuspension of the digested DNA in TE buffer. Replication assays were performed in a 15-μl reaction volume containing 50 ng of the DNA substrate, 75 μg cell extracts, 4% glycerol, 40 mM HEPES (pH 7.7), 40 mM creatine phosphate (pH 7.7), 7 mM MgCl2, 4 mM ATP, 200 μM each of CTP, GTP, and UTP, 100 μM each of dATP, dGTP,and dTTP, 10 μM dCTP, 2 mM dithiothreitol, 6 mM potassium glutamate, and 2 μg creatine phosphokinase. After 3 h incubation at 35°C, the reaction mixture was completed by adding 300 ng of purified Rep68 or control β-galactosidase and 10 μCi of [α-32P]dCTP (6,000 Ci/mmol, Amersham-Pharmacia Biotech) and incubated for 15 h at 35°C. Unincorporated nucleotides were then removed by Sephadex G-25 chromatography and reactions were digested with proteinase K, phenol-chloroform extracted, ethanol precipitated and resuspended in 20 μl of water.
Reaction products were separated on 1% agarose gels in 1× Tris-borate-EDTA buffer, transferred on nylon membranes (Positive TM Membrane, Q-Biogene), and analyzed by PhosphorImager (Molecular Dynamics). The incorporation of 32P into each DNA fragment was quantified using IPLab Gel 1.5 software (Signal Analytics Corp.) and expressed as the ratio between the level of incorporation into the lower p5-containing fragment and the upper non-p5-containing fragment.
Western blot analysis.
Cells were lysed in radioimmunoprecipitation assay buffer (20 mM Tris-HCl, pH 7.4, 50 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 0.5% deoxycholate, 0.5% sodium dodecyl sulfate) in the presence of a cocktail of protease inhibitors (Roche). Total cell protein concentration was determined for each sample using a Bradford assay (Bio-Rad). Proteins (25 μg) were loaded on a 7.5% SDS-polyacrylamide gel and then transferred to a nitrocellulose membrane according to the manufacturer's recommendations (Bio-Rad). The membrane was blocked overnight at 4°C, in phosphate-buffered saline containing 0.1% Tween and 5% dried milk, and then incubated for 1 h at room temperature with the anti-Rep 303.9 antibody (63, 64) (kindly provided by J. A. Kleinschmidt, German Cancer Center, Heidelberg, Germany) diluted in phosphate-buffered saline, 0.1% Tween, 1% dried milk. After washing, a horseradish peroxidase-conjugated anti-rabbit antibody (Sigma) was applied at a 1/5,000 dilution for 1 h at room temperature. Finally, after extensive washes in phosphate-buffered saline-0.1% Tween, the membrane was soaked in ECL reagent (Amersham Biosciences) and exposed to a film. The membrane was then reprobed with the anti-hTBP SI-1 (Santa Cruz Biotechnologies) and an antitubulin (SIGMA) antibody.
Electromobility shift assay.
DNA substrates for EMSA were obtained by digestion of p5-containing plasmids with HindIII and BamHI. An unrelated DNA fragment was prepared by digestion of plasmid pSP72 with XhoI and EcoRI. The restriction fragments (89 bp for p5D10, 79 bp for p5D12) were purified by agarose gel extraction and labeled for 15 min at 37°C in a 30 -μl reaction volume containing 50 μCi of [α-32P]dCTP (6,000 Ci/mmol, Amersham Pharmacia Biotech), 166 μM each of dGTP, dATP, and dTTP, and 5 U of Klenow (Roche). After 20 min. at 70°C, unincorporated nucleotides were removed by Sephadex G-25 chromatography. Radiolabeled DNA was PAGE purified, eluted from gel in TE buffer, ethanol-precipitated, and resuspended in 15 μl of water.
EMSA was performed in a 20-μl reaction volume containing 4 μl of 5× binding buffer (50 mM HEPES, pH 7.9, 20 mM MgCl2, 300 mM KCl, 25 mM [NH4]2SO4, 25 mM dithiothreitol, 40% glycerol, and 100 μg/μl polyethylene glycol 6000), 2 μl of BC100 buffer (20 mM Tris-Cl, pH 7.9, 100 mM KCl, 1 mM dithiothreitol, 0.2 mM EDTA, 0.5 μg/μl bovine serum albumin, and 20% glycerol), 0.5 μg of poly(dG-dC), and 5,000 cpm (∼1 ng) of the radiolabeled DNA substrate, in the presence or not of 100 ng of human TBP (ProteinOne, MD). After 30 min at 30°C, 100 ng of purified Rep68 was added where indicated. After an additional 30 min incubation at 30°C, reactions were loaded on 5% native polyacrylamide (50:1) gels containing 4 mM MgCl2 and 0.5 mM dithiothreitol and were subjected to electrophoresis at 120 V in 1× Tris-glycine-EDTA buffer containing 4 mM MgCl2.
For competition EMSA, a 0- to 300-fold excess (as indicated on Fig. 5B) of unlabeled p5D10 fragment was added to the binding reactions 30 min after addition of Rep68, and the reactions were kept at 30°C for an additional 30 min before electrophoresis. To get the same DNA concentration in all reactions, dilutions of the unlabeled p5D10 competitor fragment were made with a solution of an equal DNA concentration of the XhoI-EcoRI fragment of pSP72. Gels were dried and analyzed by autoradiography and PhopshorImager scanning.
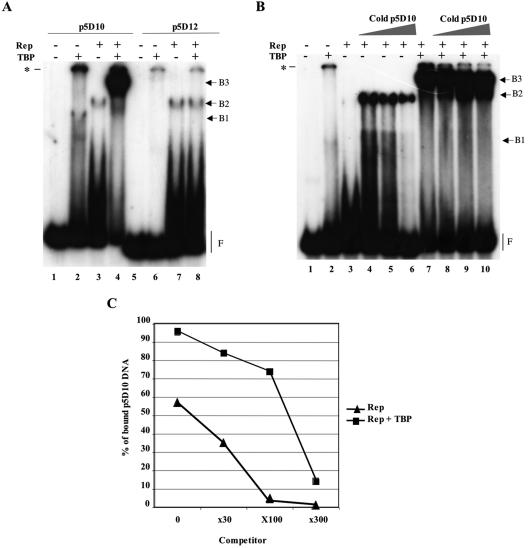
FIG. 5.
Evidence for the formation of Rep and TBP complexes on the minimal p5D10 promoter element. (A) Enhanced binding of Rep68 to the p5D10 element in the presence of human TBP. EMSA of radiolabeled p5D10 (lanes 1 to 4) or p5D12 (lanes 5 to 8) DNA fragments incubated with either purified human TBP, Rep68, or both proteins. (B) Competition EMSA. The binding was performed using radiolabeled p5D10 DNA substrate incubated either alone or with the indicated proteins. The binding reactions were then completed with an excess (0-, 30-, 100-, or 300-fold) of unlabeled p5D10 competitor DNA and kept at 30°C for an additional 30 min. (C) Quantification of the amount of bound p5D10 labeled DNA. The amount of free DNA (F) in each lane was quantified by PhosphorImager analysis of the gel presented in panel B and the % of bound p5D10 DNA was calculated by subtracting each value from that obtained in the absence of any protein (lane 1). The asterisk in panels A and B indicates the position of the wells in the gel.
RESULTS
Identification of Rep nicking sites within the AAV-2 p5 promoter element.
Our initial studies identified a cis-acting replication element within a 350-bp region (nucleotides 190 to 540 of wild-type AAV-2) covering the p5 promoter and the 5′ portion of the rep ORF (37). Further analysis indicated that this functional element could be restricted to a 163-bp region (nucleotides 190 to 353) that contained most of the p5 promoter and extended 65 bp after the transcription initiation site (data not shown) (Fig. 1A). This region contained the binding sites for several transcription factors such as major late transcription factor and YY1, the TATA box, the Rep-binding site, and the trs previously identified by Wang et al. (58). In that study, the trs was localized by a primer extension method at the p5 transcription initiation site without, however, indicating which DNA strand was cut. Subsequently, the finding by Wu et al. of no detectable nicking near the p5 Rep-binding site questioned the presence of this trs (66).
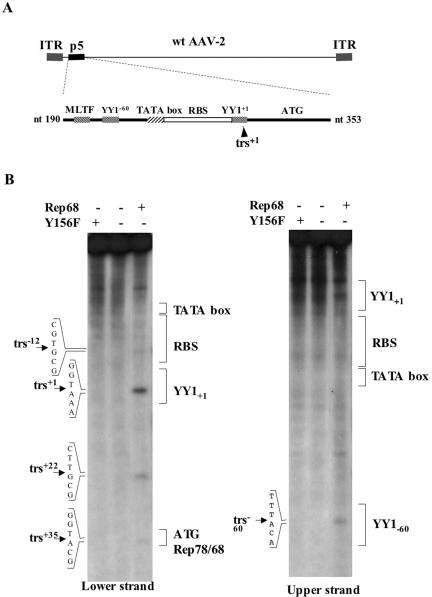
FIG. 1.
(A) Schematic view of the wild-type AAV-2 genome and of the wild-type p5 element. (B) Mapping of the Rep68 nicking sites on the p5 element. Nicking reactions were performed on a p5 substrate (nucleotides 190 to 353 of wild-type AAV-2) labeled on either strand in the presence of 1 pmol of Rep68 as described in the Materials and Methods section. The reaction products were resolved on 6% denaturing polyacrylamide gels along with a sequencing reaction to precisely map the nicking sites. The five identified nicking sites are indicated, as well as the position of the outstanding p5 elements.
To confirm the presence of a trs and to identify the DNA strand cut by Rep, a nicking assay was performed on a double-stranded p5 promoter element (nucleotides 190 to 353 of wild-type AAV-2). The result of this assay confirmed the presence of a major trs+1 at the p5 transcription initiation site, between nucleotides 287 and 288 of wild-type AAV-2, and localized it on the noncoding (lower) strand (trs+1, Fig. 1B). In addition, other minor nicking sites were also identified. Three of them localized on the noncoding strand, between nucleotides 274 and 275 (trs−12), 308 and 309 (trs+22), and 321 and 322 (trs+35). The last one, trs−60, was localized at nucleotides 226 to 227 on the upper coding strand within the YY1−60 site. The specificity of these nicking sites was demonstrated using a recombinant Rep protein (Y156F) mutated in its endonuclease domain (57, 67) or, as an additional control, a recombinant β-galactosidase protein that was produced and purified using the same methods (Fig. 1B).
Definition of the minimal p5 element sufficient for in vivo replication.
The above results confirmed the presence of a major trs at the p5 transcription initiation site. This finding, together with our previous observation indicating that replication of the 350-bp p5 element was abrogated by mutating either the Rep-binding site or the trs+1 (37), strongly suggested that the minimal p5 element could be restricted to a short fragment containing only these two elements. However, the presence of at least four other minor nicking sites within the p5 element raised the question about their potential involvement in the Rep-dependent replication of the p5 region. In addition, we noticed that the sequence surrounding the trs+1 could potentially form a hairpin (Fig. 2B) that localized the trs+1 at the top of the loop resembling the structure formed on the viral ITR (3, 4).
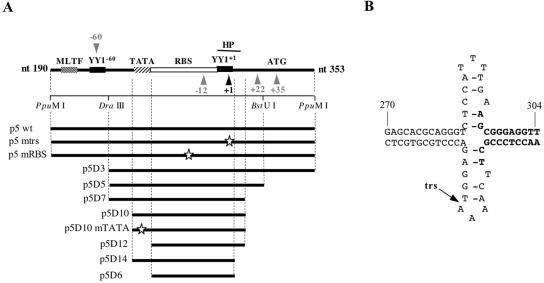
FIG. 2.
(A) Presentation of the wild-type and mutated p5 elements. The arrowheads indicate the position of the Rep68 nicking sites (black: major cleavage site at trs+1; gray: other minor trs sites). The putative hairpin (HP) structure overlapping trs+1 is represented by a thick black line. White stars show the positions of the point mutations that were introduced within the p5 sequence (37). (B) Putative hairpin secondary structure present at the p5 transcription initiation site (trs+1). Bold nucleotides indicate the deletion of the hairpin structure present in mutants p5D14 and p5D6.
To investigate the role of each of these elements, the 163-bp p5 element (p5 wild-type) was progressively deleted to eliminate each trs (mutants p5D3, p5D5, and p5D7) (Fig. 2A and Table 1). In addition, a deletion was generated to prevent the formation of the hairpin structure while retaining both the trs+1 and the YY1+1 site (mutant p5D14). Finally, the TATA box flanking the Rep-binding site was also deleted or mutated (mutants p5 D10mTATA, p5D12, and p5D6). Each of these mutated p5 versions was introduced upstream from a GFP-expression cassette in plasmid pCMV-GFP and evaluated for the ability to direct Rep-dependent replication of the plasmid upon transfection into adenovirus-infected and Rep-expressing cells (Fig. 3A). The replication of these different p5-containing plasmids was analyzed by Southern blot after digestion of DNA with either DpnI or MboI.
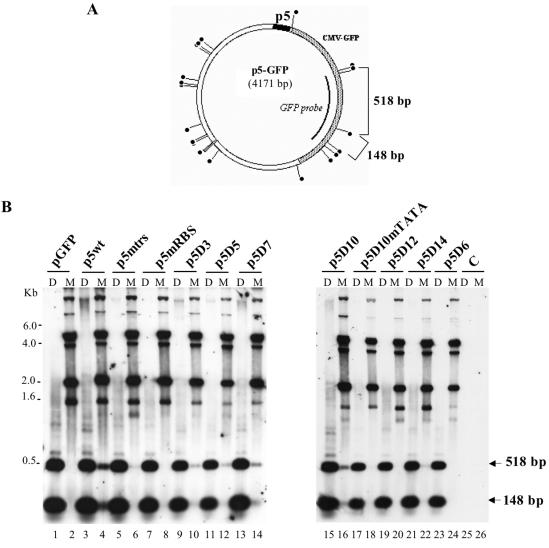
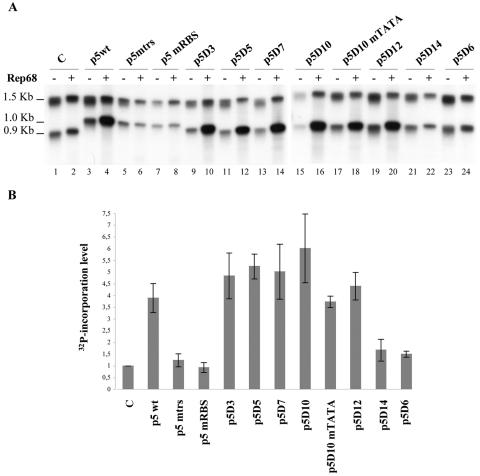
FIG. 3.
Identification of a minimal p5 element (p5D10) able to replicate in adenovirus-infected and Rep-expressing cells. (A) Map of the p5-GFP plasmid indicating the position of the DpnI/MboI sites and the GFP fragment used as a probe. (B) In vivo replication assay of the p5-containing plasmids. Total DNA extracted 48 h posttransfection of adenovirus type 5-infected HeRC32 cells, was digested with either DpnI (D) or MboI (M) and analyzed by Southern blot using a GFP probe. The two expected MboI or DpnI digestion products (518 and 148 bp) hybridizing to the GFP probe are indicated by an arrow. The upper bands visible in the MboI-digested samples represent input plasmid DNA. The amount of sample loaded in each lane was normalized using an internal control measuring the transfection efficiency (see Materials and Methods).
As previously demonstrated (37), transfection of a plasmid containing the entire p5 element (p5wt) resulted in the generation of faint DpnI-resistant forms that migrated as a smear whereas incubation with MboI resulted in the appearance of digested bands. Both these results indicated that at least part of the input plasmid had replicated on both DNA strands (Fig. 3B, lanes 3 and 4). In this context, mutation of either the trs+1 (p5mtrs) or the Rep-binding site (p5mRBS) abrogated the replication of the 163-bp p5 element (Fig. 3B, lanes 6 and 8), confirming our previous results (37). The analysis conducted with the p5 deletion mutants indicated that the minimal p5 element that was able to replicate was the mutant p5D10, which contained the Rep-binding site, trs+1, trs−12, the potential hairpin, and the TATA box (Fig. 3B, lanes 15 and 16). Even though the level of replication of the deletion mutants up to p5D10 was always lower compared to that obtained with the wild-type p5 element, this result indicated that the other nicking sites found on p5 were not strictly required. In contrast, mutation or deletion of the TATA box flanking the Rep-binding site completely abrogated replication of this minimal p5 element (Fig. 3B, lanes 17 to 20). Also, the deletion of the nucleotides encompassing the hairpin structure (p5D14) severely affected replication, reducing it to a background level (Fig. 3B, lanes 21 and 22). Finally, as expected from the previous results, a p5 element containing only the Rep-binding site and the trs+1 (p5D6) was unable to replicate.
Altogether these results indicated that a minimal p5 element able to drive Rep-dependent replication of a plasmid was composed by a 55-bp fragment containing the TATA box, the Rep-binding site, the trs+1, and a potential hairpin; the Rep-binding site, the trs+1, and the TATA box were essential for the replication of this element; and mutation of the potential hairpin structure severely reduced Rep-dependent replication to background levels.
Definition of the minimal p5 element sufficient for in vitro replication.
AAV replication has been extensively studied using in vitro replication assays performed in the presence of extracts from adenovirus-infected cells and recombinant Rep proteins (17, 34, 59-61). Similarly, we previously demonstrated that the p5 element could also replicate in a cell-free assay (37). To confirm the above results, the various p5-containing plasmids were evaluated for their ability to replicate in vitro, after digestion with enzymes generating two fragments, the smaller one containing the p5 element. The incorporation of labeled nucleotides in the presence of Rep68 was quantified for each p5-containing fragment and expressed as the fold increase with respect to a control plasmid with no p5 element. Importantly, each construct was also incubated in the presence of recombinant β-galactosidase to assess the specificity of Rep-dependent replication.
The results presented in Fig. 4 showed that, as previously reported, the wild-type p5 element was able to drive in vitro replication at a significant level compared to a control plasmid with no p5. In this context, mutation of either the Rep-binding site or the trs+1 site (p5mRBS and p5mtrs, respectively) completely inhibited replication. The p5 deletion mutants up to p5D10 replicated as efficiently as the wild-type element. In this assay, deletion or mutation of the TATA box only slightly affected the ability of the p5 to replicate and the observed reductions were not statistically significant (compare mutant p5D10 with p5D10mTATA and p5D12). In contrast, deletion of the hairpin structure (mutant p5D14) eventually associated with a deletion of the TATA box (mutant p5D6) reduced replication to a background level that was not significantly different from that measured using a control plasmid (P > 0.05). These data confirmed the role of the Rep-binding site, the trs+1, and the potential hairpin for Rep-dependent replication of the p5 element. However, in contrast to what was observed using in vivo replication assays (Fig. 3B), the TATA box was dispensable for in vitro replication of p5-containing DNA substrates. The reason for this difference is for the moment unclear (see Discussion), but this discrepancy interestingly pointed to a different requirement for cellular factors between the two assays.
FIG. 4.
Identification of a minimal p5 (p5D12) element able to replicate in a cell-free assay. (A) In vitro replication assay of the p5-containing plasmids. Plasmids were digested with AflIII and XmnI and incubated with cell extracts from adenovirus type 5-infected HeLa cells (75 μg) and 300 ng of either Rep68 (+) or β-galactosidase (−) protein in the presence of [α-32P]dCTP. The reaction products were visualized by autoradiography of the membrane. The p5 element is contained in the lower DNA band. C, control pSP72 plasmid with no p5 element. (B) Quantification of the in vitro replication assays. The level of Rep-dependent replication of each individual p5-containing fragment was expressed as 32P incorporation calculated as described in Materials and Methods. The mean and standard deviation were calculated from three independent experiments.
Effect of TBP on Rep binding and nicking within the p5 element.
Rep was previously demonstrated to interact in vitro and in vivo with TBP (16). This finding, together with the requirement for the TATA box for in vivo replication of the p5 element, led us to investigate the effect of TBP on Rep activities further. The interaction between Rep and TBP on the p5 promoter was first analyzed by EMSA using either the p5D10 or the p5D12 deletion mutant as the substrate. As expected, purified TBP bound to the p5D10 substrate but not to the p5D12 TATA box-deleted mutant (Fig. 5A, complex B1, lanes 2 and 6). In contrast, purified Rep68 bound to both the p5D10 and the p5D12 substrates with apparently the same efficiency (Fig. 5A, complex B2, lanes 3 and 7). The presence of a smear migrating more slowly than the free DNA probe also suggested that most of the Rep-DNA complexes were unstable in the gel.
When Rep68 was added to a preformed p5D10-TBP complex, a shifted band of higher molecular weight was produced (Fig. 5A, lane 4). The formation of this complex was associated with an increased amount of bound DNA, as judged by the intensity of the band and the amount of free DNA probe. This binding product also appeared when radiolabeled DNA was added after preincubation of Rep68 with TBP or when TBP was added after incubation of Rep68 with DNA (data not shown). However, this complex was not formed when p5D12 was used as a substrate (Fig. 5A, lane 8) or when purified β-galactosidase was added instead of TBP (data not shown). This result indicated that binding of TBP to the TATA box was necessary for the formation of this complex and further suggested that this high-molecular-weight band contained both Rep68 and TBP bound to the same substrate.
To determine if the stability of the protein-DNA complexes bound at the p5 was increased in the presence of TBP, a competition EMSA was performed using an excess of unlabeled p5D10 substrate in the reaction (Fig. 5B and C). Surprisingly, we found that addition of a 30-fold excess of unlabeled p5D10 substrate considerably increased the level of Rep-DNA complexes (Fig. 5B, lanes 3 and 4). Importantly, the fact that the total amount of DNA was kept constant in each reaction by adding unrelated DNA indicated that this effect was specific to substrates containing the Rep-binding site. As previously suggested by Smith et al., purified Rep proteins can form in solution high-molecular-weight complexes that are rearranged into hexameric forms upon incubation with AAV ITR sequences (46). As such, it is possible that, in our case, the addition of an excess of p5 substrate initially displaced the equilibrium toward the formation of Rep-DNA complexes. However, as previously observed with Rep alone, most of these complexes were unstable in the gel as indicated by the smear of labeled DNA (Fig. 5B, lanes 3 and 4). In contrast, in the presence of TBP, no effect on the formation of Rep-DNA complexes was observed upon addition of a 30-fold excess of unlabeled p5D10 substrate (Fig. 5B, lanes 7 and 8). Starting from this state, the addition of a 100- to a 300-fold excess of unlabeled p5D10 DNA decreased the level of bound material in the presence of Rep alone (Fig. 5B, lanes 5 and 6). In the presence of Rep and TBP, the same doses of competitor slightly decreased the size of the protein-DNA complex to a band with a mobility similar to that observed with the Rep proteins alone (Fig. 5B, lane 10).
Because the apparent stability in the gel of each protein-DNA complex was different depending on the presence or absence of TBP, bound DNA was quantified on the basis of the amount of free probe found in each lane. Figure 5C clearly confirmed that a larger amount of bound DNA was observed upon addition of TBP. In addition, these results showed that the dissociation rate of the DNA-protein complex formed in the presence of TBP was lower than that observed for the complex constituted in the absence of TBP. Globally, these data indicated that the addition of TBP increased the amount of bound p5 DNA and generated a protein-DNA complex that was more stable than in the absence of this cellular factor.
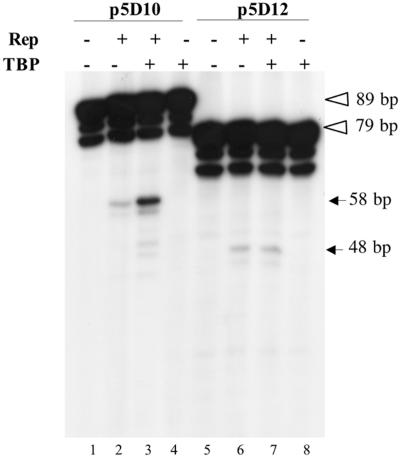
We next examined the effect of TBP on Rep68 endonuclease activity using either the p5D10 or the p5D12 substrate labeled on both strands. Reactions were first incubated at 30°C in the same conditions as EMSA to ensure binding of TBP to the TATA box and then 1 h at 37°C in the presence of ATP to allow Rep helicase and endonuclease activity. As shown in Fig. 6 (lanes 2 and 6), the efficiency of Rep68 nicking at the p5 trs+1 was identical on both p5D10 and p5D12 substrates. When TBP was added to the reaction, nicking of p5D10 substrate was clearly enhanced, whereas, no effect of TBP was observed on the p5D12 template (Fig. 6, compare lanes 3 and 7). This result indicated that the increased Rep binding at the p5 Rep-binding site observed in the presence of TBP correlated with enhanced nicking at the trs+1, providing a link with the requirement for this cellular factor observed using in vivo replication assays.
FIG. 6.
Effect of TBP on Rep endonuclease activity on the minimal p5D10 element. Nicking assays were conducted on either p5D10 or p5D12 DNA substrates in the presence (+) or absence (−) of purified TBP and Rep68. The final amount of recombinant protein in the reaction was kept constant by adding purified β-galactosidase. Reactions were performed in the same conditions as those described for EMSA except that they contained 1 mM ATP, 6.25 mM MgCl2 and that they were incubated for 1 h at 37°C after addition of Rep68. Reaction products were resolved on 8% denaturing polyacrylamide gels. The open arrowheads indicate the position of the uncut p5D10 and p5D12 fragments labeled at both termini whereas the solid arrows indicate the position of the product expected after nicking at the trs+1 (see Fig. 2).
Effect of TBP overexpression on p5-dependent replication.
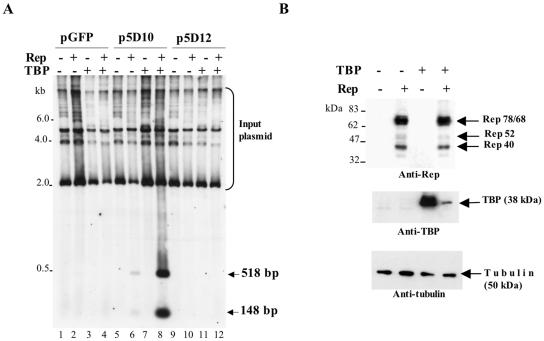
We finally asked whether overexpression of this cellular factor could enhance the Rep-dependent replication of the minimal p5 element. For this, adenovirus-infected 293 cells were cotransfected with the p5D10 and p5D12 plasmids together with a Rep-expressing construct and with (or without) a plasmid encoding human TBP. As previously observed, only the p5D10 element could replicate in the presence of adenovirus and Rep, whereas plasmid p5D12 and a control plasmid were unable to do so (Fig. 7A, compare lane 6 with lanes 10 and 2). Overexpression of TBP led to a substantial increase of p5D10 replication in the presence of Rep (Fig. 7A, lane 8) but had no effect either when expressed alone or when assayed on the p5D12 and control plasmids. Importantly, the effect of TBP on p5D10 replication was not due to an increased synthesis of Rep proteins, as verified by Western blot analysis of the transfected cells (Fig. 7B). Conversely, synthesis of TBP was reduced in the presence of Rep proteins, probably because of a translational inhibition exerted by the Rep proteins (53). Together, these results indicated that TBP was able to substantially enhance Rep-dependent replication of the minimal p5 element when the TATA box was present.
FIG. 7.
Effect of TBP overexpression on in vivo replication of the minimal p5D10 element. (A) Southern blot analysis of in vivo replication products obtained by transfecting adenovirus type 5-infected 293 cells with plasmids pGFP, p5D10-GFP, and p5D12-GFP in the presence (+) or absence (−) of plasmids pCMV-rep (Rep) and pXJ41-TBP (TBP). Total DNA was digested with MboI and hybridized to a GFP probe. (B) Analysis of Rep and TBP expression in transfected cells. Adenovirus-infected 293 cells were transfected with plasmid p5D10-GFP and either plasmid pCMV-rep (Rep) or plasmid pXJ41-hTBP (TBP) or both. Total proteins were analyzed by Western blot using an anti-Rep antibody (303.9). The membrane was then stripped and reprobed with an anti-TBP antibody and then with an antitubulin antibody. Note that endogenous TBP was visible only after prolonged exposure of the blot.
DISCUSSION
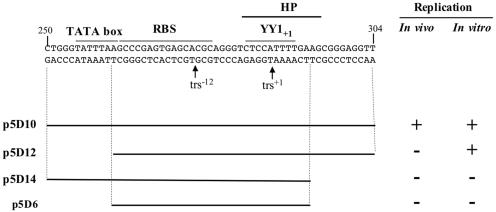
Previous reports demonstrated that the AAV-2 p5 promoter region contained a multifunctional element that was involved in rep gene expression, Rep-dependent replication, and site-specific integration (8, 33, 36-40, 54). The aim of this study was to characterize a minimal p5 element on the basis of its ability to replicate in the presence of adenoviral factors and Rep proteins. The minimal p5 element that was functional in both in vivo and in vitro replication assays was composed of a 55-bp region that included the TATA box, the Rep-binding site, the trs+1, and a potential hairpin structure surrounding the trs (Fig. 8). The Rep-binding site, the trs+1, and the hairpin structure were absolutely required for replication in both assays, whereas the TATA box was dispensable for in vitro replication.
FIG. 8.
Summary of the results using in vivo and in vitro replication assays performed with the minimal p5 elements (p5D10, p5D12, p5D14, and p5D6). +, replication; −, no replication.
A critical step for the characterization of the minimal p5 element consisted in the identification of the Rep nicking sites within the p5 region. Indeed, a previous study by Wang et al. had indicated the presence of a cryptic trs at the transcription initiation site, without indicating the strand that was cleaved (58). The subsequent finding by Wu et al. of no detectable nicking near the p5 Rep-binding site further put in question the presence of this trs (66).
Using nicking assays, we confirmed the presence of a major trs at the transcription initiation site and localized it on the lower DNA strand between nucleotides 287 and 288 (Fig. 1). In addition, we showed that at least four other minor trs sites could be detected within the p5 region. Most of these nicking sites were not required for p5 replication and the minimal element characterized in this study (p5D10) contained only two trs sites, trs+1 and trs−12. The requirement for the former trs was demonstrated by the direct mutation of this site (p5mtrs) (Fig. 3). The role of the second trs (trs−12) is presently unknown and difficult to evaluate since it localized within the Rep-binding site. Cleavage at this site could be explained by a nicking reaction occurring in trans by Rep protein bound on a DNA substrate, nicking a second DNA template.
In both the viral ITR and the chromosome 19 AAVS1 region, the Rep proteins have been shown to cleave the DNA between two thymidine residues, resulting in the covalent attachment of Rep to the nicking site through a phosphotyrosyl linkage (19, 27, 47, 49, 55). It is interesting that none of the trs sites identified in the p5 resembled those found in the ITR or the AAVS1 region except for the presence of a thymidine residue 5′ to the nicking site. Also, great variability was observed in the spacer sequence separating the Rep-binding site from each trs within the p5, further distinguishing this substrate from the AAVS1 locus and the ITR (32).
These observations, together with the experimental evidence demonstrating that most of these nicking sites, beside the trs+1, were not required for p5 replication, raise questions about their biological relevance. Interestingly, an additional trs site was recently described within the ITR (25). In that study, nicking was observed only using a partially purified Rep preparation, further suggesting that a cellular factor was involved in this cleavage. In our case, a highly purified Rep preparation was used and cleavage at the p5 trs was not observed using either a mutated Rep protein or an unrelated β-galactosidase protein. Nevertheless, it remains possible that these cleavage sites, even if Rep dependent, represent artificial reaction products due to the conditions under which the nicking assay is performed.
The sequence surrounding the major trs+1 was also important for replication of the p5 element. This domain can potentially form a hairpin structure that localizes the trs+1 on the extruded loop. Previous analyses on the viral ITR demonstrated that a potential stem-loop was present at the trs and that the extrusion of this structure was mediated by the ATP-dependent helicase activity of Rep (3, 4). A secondary structure was also recently described near the trs within the AAVS1 locus (20). In the present study, we demonstrated that deletion of the nucleotides forming the stem of the hairpin impaired p5 replication. This result strongly supports the existence of secondary structure at the trs+1 and also suggests that cleavage at this site required the separation of the DNA strands through the helicase activity of Rep.
Interestingly, in the case of the p5, the hairpin and the trs+1 localize within the YY1+1 recognition site, and binding of this factor to this site was previously shown to mediate TBP-independent initiation of transcription in vitro and DNA strand separation (18, 44, 56). It is tempting to speculate that formation of the hairpin structure at the p5 trs+1 is also favored by YY1 binding, further putting into question the relationships between replication and transcription initiation. Additional mutations affecting the YY1 binding site but not the trs+1 or the hairpin structure should help define the role of this cellular factor for Rep-dependent replication of the p5.
The major finding of this study was the demonstration of the involvement of the TATA box and of the TBP for the replication of the p5 element. It was previously reported that TBP could bind to Rep 78 in vitro and in vivo without, however, demonstrating any biological effect associated with this interaction (16). Our results indicated that the TATA box was absolutely required for in vivo replication of a minimal (Fig. 3) or a wild-type p5 element (data not shown). In addition, we demonstrated that when the TATA box was present, TBP stimulated Rep binding at the Rep-binding site (Fig. 5) and nicking at the trs+1 (Fig. 6). The biological relevance of these observations was further supported by enhancement of p5 replication observed in cells overexpressing TBP (Fig. 7).
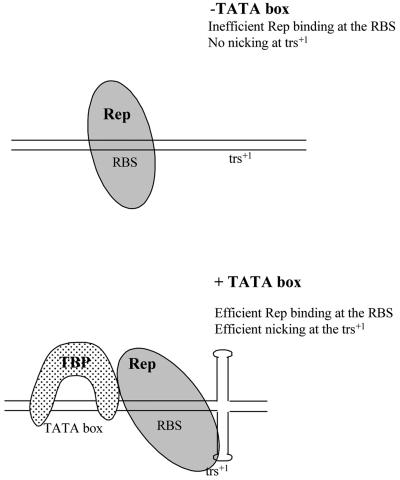
On the basis of these findings and of previous observations concerning the Rep-ITR interactions, the following model can be proposed for the p5 element (Fig. 9). In vivo, i.e., in live cells, in the absence of the TATA box, Rep proteins may not be able to bind the p5 Rep-binding site. If Rep binding occurs, it must not be very efficient and in any case, not sufficient to induce cleavage at the trs+1 since no replicative activity of the TATA-less p5 element was observed. In the presence of the TATA box, TBP increases the efficiency of Rep binding at the Rep-binding site, and also nicking at the trs+1, thus stimulating replication of the p5 element.
FIG. 9.
Theoretical model for Rep-TBP interaction on the p5 element. See text for details.
Recent studies on the ITR have suggested that the interaction of Rep with the RBE′ induced a change in DNA conformation that resulted in the stimulation of the extrusion of the trs by Rep helicase activity (3-5). Similarly, in the case of the p5, an attractive hypothesis is that the interaction of Rep with TBP induces a conformational change that enhances Rep recognition of the Rep-binding site and favors the extrusion of the trs. Indeed, TBP is known to bend DNA once bound to the TATA box (35). This hypothesis is also supported by previous studies demonstrating that nicking at the AAVS1 trs by Rep was dependent upon topological constraints (23).
Finally, an additional argument comes from the observation that high mobility group chromosomal protein 1 (HMG-1) increased Rep binding and nicking at the trs in the ITR. This protein, which influences DNA flexibility, also interacts directly with Rep (10). A similar effect on Rep activities was also demonstrated with some viral and cellular single-stranded DNA binding proteins (52). Several additional experiments should be performed to validate this hypothesis. Also, it is worth noting that the model presented does not take into account the complex interaction between TBP and the other factors that constitute the transcription initiation complex (41) or the observation that Rep can form multimeric complexes upon binding its recognition site (5, 6, 25, 46).
It is interesting that the TATA box was completely dispensable for in vitro replication of the p5 element (Fig. 5), and this despite TBP's being present in the cell extracts used in this assay (data not shown). This observation points out a different requirement for cellular factors between in vivo and in vitro replication assays and further strengthens the hypothesis according to which the conformation of DNA is an essential parameter. Indeed, the template for in vivo replication is supercoiled plasmid DNA eventually wrapped by cellular histones, whereas in vitro replication is performed using naked linear double-stranded DNA molecules.
The TBP was previously shown to interact with numerous cellular and viral proteins that are transcriptional regulators (2, 11, 13, 28, 41, 45, 50). However, only a few reports describe an effect of TBP on the replication of viral origins. In particular, TBP was shown to inhibit the replication of viruses such as simian virus 40 and human papillomavirus by interacting with viral regulatory proteins or by preventing their binding to the origin of replication (14, 15). Our results demonstrated for the first time a positive effect of TBP on the replication of a viral element. Interestingly, it was previously reported that TBP could enhance eukaryotic DNA replication by binding to the replication origin in an Saccharomyces cerevisiae model (29, 51). As such, our findings further underline the fundamental difference between transforming viruses such as simian virus 40 or human papillomavirus and AAV.
In conclusion, these studies have contributed to the definition of a minimal replication-competent p5 region. It remains to be defined whether this minimal p5 element is also able to direct site-specific integration of plasmid DNA as previously demonstrated with a wild-type p5 region (39, 40) and to determine if p5 replication is required for this effect. Indeed, previous studies have shown that replication of the viral ITR was not required for site-specific integration (68). More importantly, future studies should address the question of the role of this element in the context of the ITRs.
It is possible, as previously suggested by Tullis et al. (54), that this element is required for optimal DNA replication. Our preliminary in vivo assays performed by transfection of plasmid DNA suggest that replication of an AAV vector is not enhanced when the p5 element is present (data not shown). However, it is possible that, in this context, the effect of the p5 is masked by the strength of the ITRs. Alternatively, the presence of the p5 may be required to improve other steps in the virus life cycle such as DNA encapsidation (36) or integration (39, 40). These studies will be critical to determine the potential requirement for this element for the generation of a novel class of recombinant AAV vectors with improved biological properties.
Acknowledgments
We thank Juergen Kleinschmidt for providing the 303.9 antibodies and Làszlò Tora for plasmid pXJ41-hTBP. We also thank Michael Linden and Peter Ward for their help with the nicking and in vitro replication assays. Finally, we thank Mark Haskins for critically reading the manuscript.
This work was supported by the Association Française contre les Myopathies (AFM), Vaincre les Maladies Lysosomales (VML), Association Nantaise de Thérapie Génique (ANTG), the Fondation pour la Thérapie Génique en Pays de la Loire, and the INSERM. A.F. was sponsored by a fellowship from the Conseil Général des Pays de la Loire and Univaloire.
REFERENCES
- 1.Berns, K. I., and C. Giraud. 1996. Biology of adeno-associated virus. Curr. Top. Microbiol. Immunol. 218:1-23. [DOI] [PubMed] [Google Scholar]
- 2.Boyd, J. M., P. M. Loewenstein, Q.-Q. Tang, L. Yu, and M. Green. 2002. Adenovirus E1A N-terminal amino acid sequence requirements for repression of transcription in vitro and in vivo correlate with those required for E1A interference with TBP-TATA complex formation. J. Virol. 76:1461-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brister, J. R., and N. Muzyczka. 2000. Mechanism of Rep-mediated adeno-associated virus origin nicking. J. Virol. 74:7762-7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brister, J. R., and N. Muzyczka. 1999. Rep-mediated nicking of the adeno-associated virus origin requires two biochemical activities, DNA helicase activity and transesterification. J. Virol. 73:9325-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess Hickman, A., D. R. Ronning, Z. N. Perez, R. M. Kotin, and F. Dyda. 2004. The nuclease domain of adeno-associated virus Rep coordinates replication initiation using two distinct DNA recognition interfaces. Mol. Cell 13:403-414. [DOI] [PubMed] [Google Scholar]
- 6.Cathomen, T., D. Collete, and M. D. Weitzman. 2000. A chimeric protein containing the N terminus of the adeno-associated virus Rep protein recognizes its target site in an in vivo assay. J. Virol. 74:2372-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chadeuf, G., D. Favre, J. Tessier, N. Provost, P. Nony, J. Kleinschmidt, P. Moullier, and A. Salvetti. 2000. Efficient recombinant adeno-associated virus production by a stable rep-cap HeLa cell line correlates with adenovirus-induced amplification of the integrated rep-cap genome. J. Gene Med. 2:260-268. [DOI] [PubMed] [Google Scholar]
- 8.Chang, L.-S., Y. Shi, and T. Shenk. 1989. Adeno-associated virus p5 promoter contains an adenovirus E1A-inducible element and a binding site for the major late transcription factor. J. Virol. 63:3479-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiorini, J. A., S. M. Wiener, R. O. Owens, S. R. M. Kyöstiö, R. M. Kotin, and B. Safer. 1994. Sequence requirements for stable binding and function of Rep68 on the adeno-associated virus type 2 inverted terminal repeats. J. Virol. 68:7448-7457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello, E., P. Saudan, E. Winocour, L. Pizer, and P. Beard. 1997. High mobility group chromosomal protein 1 binds to the adeno-associated virus replication protein (Rep) and promotes Rep-mediated site-specific cleavage of DNA, ATPase activity and transcriptional repression. EMBO J. 16:5943-5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das, D., and W. M. Scovell. 2001. The binding interaction of HMG-1 with the TATA-binding protein/TATA complex. J. Biol. Chem. 276:32597-32605. [DOI] [PubMed] [Google Scholar]
- 12.Graham, F. L., and L. Prevec. 1991. Manipulation of adenovirus vectors, p. 109-128. In E. J. Murray (ed.), Gene transfer and protocol, vol. 7. The Humana Press, Inc., Clifton, N.J. [DOI] [PubMed] [Google Scholar]
- 13.Hall, D. B., and K. Struhl. 2002. The VP16 activation domain interacts with multiple transcriptional components as determined by protein-protein cross-linking in vivo. J. Biol. Chem. 277:46043-46050. [DOI] [PubMed] [Google Scholar]
- 14.Hartley, K. A., and K. A. Alexander. 2002. Human TATA binding protein inhibits human papillomavirus type 11 DNA replication by antagonizins E1-E2 protein complex formation on the viral origin of replication. J. Virol. 76:5014-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbig, U., K. Weisshart, P. Taneja, and E. Fanning. 1999. Interaction of the transcription factor TFIID with simian virus 40 (SV40) large T antigen interferes with replication of SV40 DNA in vitro. J. Virol. 73:1099-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermonat, P. L., A. D. Santin, R. B. Batchu, and D. Zhan. 1998. The adeno-associated virus Rep78 major regulatory protein binds the cellular TATA-binding protein in vitro and in vivo. Virology 245:120-127. [DOI] [PubMed] [Google Scholar]
- 17.Hong, G., P. Ward, and K. I. Berns. 1994. Intermediates of adeno-associated virus DNA replication in vitro. J. Virol. 68:2011-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houbaviy, H. B., A. Usheva, T. Shenk, and S. K. Burley. 1996. Cocrystal structure of YY1 bound to the adeno-associated virus p5 initiator. Proc. Natl. Acad. Sci. USA 93:13577-13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Im, D. S., and N. Muzyczka. 1990. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell 61:447-457. [DOI] [PubMed] [Google Scholar]
- 20.Jang, M. Y., O. H. Yarborough, G. B. Conyers, P. McPhie, and R. A. Owens. 2005. Stable secondary structure near the nicking site for adeno-associated virus type 2 Rep proteins on human chromosome 19. J. Virol. 79:3544-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotin, R. M., R. M. Linden, and K. I. Berns. 1992. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 11:5071-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyöstiö, S. R., R. S. Wonderling, and R. A. Owens. 1995. Negative regulation of the adeno-associated virus (AAV) P5 promoter involves both the P5 rep binding site and the consensus ATP-binding motif of the AAV Rep68 protein. J. Virol. 69:6787-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamartina, S., S. Ciliberto, and C. Toniatti. 2000. Selective cleavage of AAVS1 substrates by the adeno-associated virus type 2 Rep68 protein is dependent on topological and sequence constraints. J. Virol. 74:8831-8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laughlin, C. A., J. D. Tratschin, H. Coon, and B. J. Carter. 1983. Cloning of infectious adeno-associated virus genomes in bacterial plasmids. Gene 23:65-73. [DOI] [PubMed] [Google Scholar]
- 25.Li, Z. L., R. Brister, D.-S. Im, and N. Muzyczka. 2003. Characterization of the adenoassociated virus Rep protein complex formed on the viral origin of DNA replication. Virology 313:364-376. [DOI] [PubMed] [Google Scholar]
- 26.Linden, M. R., P. Ward, C. Giraud, E. Winocour, and K. I. Berns. 1996. Site-specific integration by adeno-associated virus. Proc. Natl. Acad. Sci. USA 93:11288-11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linden, R. M., E. Winocour, and K. I. Berns. 1996. The recombination signals for adeno-associated virus site-specific integration. Proc. Natl. Acad. Sci. USA 93:7966-7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu, W., R. Peterson, A. Dasgupta, and W. M. Scovell. 2000. Influence of HMG-1 and adenovirus oncoprotein E1A on early stages of transcriptional preinitiation complex assembly. J. Biol. Chem. 275:35006-35012. [DOI] [PubMed] [Google Scholar]
- 29.Lue, N. F., and R. D. Kornberg. 1993. A possible role for the yeast TATA-element-binding protein in DNA replication. Proc. Natl. Acad. Sci. USA 90:8018-8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarty, D. M., D. J. Pereira, I. Zolotukhin, X. Zhou, J. H. Ryan, and N. Muzyczka. 1994. Identification of linear DNA sequences that specifically bind the adeno-associated virus Rep protein. J. Virol. 68:4988-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarty, D. M., J. H. Ryan, S. Zolotukhin, X. Zhou, and N. Muzyczka. 1994. Interaction of the adeno-associated virus Rep protein with a sequence within the A palindrome of the viral terminal repeat. J. Virol. 68:4998-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meneses, P., K. I. Berns, and E. Winocour. 2000. DNA sequence motifs which direct adeno-associated virus site-specific integration in a model system. J. Virol. 74:6213-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musatov, S., J. Roberts, D. Pfaff, and M. Kaplitt. 2002. A cis-acting element that directs circular adeno-associated virus replication and packaging. J. Virol. 76:12792-12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni, T.-H., W. F. McDonald, I. Zolothukin, T. Melendy, S. Waga, B. Stillman, and N. Muzyczka. 1998. Cellular proteins required for adeno-associated virus DNA replication in the absence of adenovirus coinfection. J. Virol. 72:2777-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikolov, D. B., H. Chen, E. D. Halay, A. Hoffmann, R. G. Roeder, and S. K. Burley. 1996. Crystal structure of a human TATA box-binding protein/TATA element complex. Proc. Natl. Acad. Sci. USA 93:4862-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nony, P., G. Chadeuf, J. Tessier, P. Moullier, and A. Salvetti. 2003. Evidence for packaging rep-cap sequences into AAV-2 capsids in the absence of the inverted terminal repeat: a model for the generation of rep+ AAV particles. J. Virol. 77:776-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nony, P., J. Tessier, G. Chadeuf, P. Ward, A. Giraud, M. Dugast, R. M. Linden, P. Moullier, and A. Salvetti. 2001. Novel cis-acting replication element in the adeno-associated virus type 2 genome is involved in amplification of integrated rep-cap sequences. J. Virol. 75:9991-9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira, D. J., D. M. McCarty, and N. Muzyczka. 1997. The adeno-associated virus (AAV) Rep protein acts as both a repressor and an activator to regulate AAV transcription during a productive infection. J. Virol. 71:1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Philpott, N. J., C. Giraud-Wali, C. Dupuis, J. Gomos, H. Hamilton, K. I. Berns, and E. Falck-Pedersen. 2002. Efficient integration of recombinant adeno-associated virus DNA vectors requires a p5-rep sequence in cis. J. Virol. 76:5411-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philpott, N. J., J. Gomos, K. I. Berns, and E. Falck-Pedersen. 2002. A p5 integration efficiency element mediates Rep-dependent integration into AAVS1 at chromosome 19. Proc. Natl. Acad. Sci. USA 99:12381-12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pugh, B. F. 2000. Contorl of gene expression through regulation of the TATA-binding protein. Gene 255:1-14. [DOI] [PubMed] [Google Scholar]
- 42.Ryan, J. H., S. Zolotukhin, and N. Muzyczka. 1996. Sequence requirements for binding of Rep68 to the adeno-associated virus terminal repeats. J. Virol. 70:1542-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samulski, R. J., X. Zhu, X. Xiao, J. D. Brook, D. E. Housman, N. Epstein, and L. A. Hunter. 1991. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 10:3941-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seto, E., Y. Shi, and T. Shenk. 1991. YY1 is an initiator sequence-binding protein that directs and activates transcription in vitro. Nature 354:241-245. [DOI] [PubMed] [Google Scholar]
- 45.Seto, E., A. Usheva, G. P. Zambetti, J. Momand, N. Horikoshi, R. Weinmann, A. J. Levine, and T. Shenk. 1992. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc. Natl. Acad. Sci. USA 89:12028-12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, R. H., A. J. Spano, and R. M. Kotin. 1997. The Rep78 gene product of adeno-associated virus (AAV) self-associates to form a hexameric complexes in the presence of AAV ori sequences. J. Virol. 71:4461-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder, R. O., D. S. Im, and N. Muzyczka. 1990. Evidence for covalent attachment of the adeno-associated virus (AAV) Rep protein to the ends of the AAV genome. J. Virol. 64:6204-6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder, R. O., D. S. Im, T. Ni, X. Xiao, R. J. Samulski, and N. Muzyczka. 1993. Features of the adeno-associated virus origin involved in substrate recognition by the viral Rep protein. J. Virol. 67:6096-6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snyder, R. O., R. J. Samulski, and N. Muzyczka. 1990. In vitro resolution of covalently joined AAV chromosome ends. Cell 60:105-113. [DOI] [PubMed] [Google Scholar]
- 50.Song, C.-Z., P. M. Loewenstein, K. Toth, and M. Green. 1995. Transcription factor TFIID is a direct functional target of the adenovirus E1A transcription-repression domain. Proc. Natl. Acad. Sci. USA 92:10330-10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stagljar, I., U. Hübscher, and A. Barberis. 1999. Activation of DNA replication in yeast by recruitment of the RNA polymerase II transrciption complex. Biol. Chem. 380:525-530. [DOI] [PubMed] [Google Scholar]
- 52.Stracker, T. H., G. D. Cassell, P. Ward, Y. M. Loo, B. van Breukelen, S. D. Carrington-Lawrence, R. K. Hamatake, P. C. van der Vliet, S. K. Weller, T. Melendy, and M. D. Weitzmann. 2004. The Rep protein of adeno-associated virus type 2 interacts with single-stranded DNA-binding proteins that enhance viral replication. J. Virol. 78:441-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeuchi, T., T. Kozuka, K. Nakagawa, Y. Aoki, K. Ohtomo, K. Yoshiike, and T. Kanda. 2000. Adeno-associated virus type 2 nonstructural protein Rep78 suppresses translation in vitro. Virology 266:196-202. [DOI] [PubMed] [Google Scholar]
- 54.Tullis, G. E., and T. Shenk. 2000. Efficient replication of adeno-associated virus type 2 vectors: a cis-acting element outside of the terminal repeats and a minimal size. J. Virol. 74:11511-11521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urcelay, E., P. Ward, S. M. Wiener, B. Safer, and R. M. Kotin. 1995. Asymmetric replication in vitro from a human sequence element is dependent on adeno-associated virus Rep protein. J. Virol. 69:2038-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Usheva, A., and T. Shenk. 1994. TATA-binding protein-independent initiation: YY1, TFIIB, and RNA polymerase II direct basal transcription on supercoiled template DNA. Cell 76:1115-1121. [DOI] [PubMed] [Google Scholar]
- 57.Walker, S. L., R. S. Wonderling, and R. A. Owens. 1997. Mutational analysis of the adeno-associated virus Rep68 protein: identification of critical residues necessary for site-specific endonuclease activity. J. Virol. 71:2722-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, X.-S., and A. Srivastava. 1997. A novel terminal resolution-like site in the adeno-associated virus type 2 genome. J. Virol. 71:1140-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ward, P., and K. I. Berns. 1996. In vitro replication of adeno-associated virus DNA: enhancement by extracts from adenovirus-infected HeLa cells. J. Virol. 70:4495-4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward, P., and K. I. Berns. 1991. In vitro rescue of an integrated hybrid adeno-associated virus/simian virus 40 genome. J. Mol. Biol. 218:791-804. [DOI] [PubMed] [Google Scholar]
- 61.Ward, P., and K. I. Berns. 1995. Minimum origin requirements for linear duplex AAV DNA replication in vitro. Virology 209:692-695. [DOI] [PubMed] [Google Scholar]
- 62.Ward, P., F. B. Dean, M. E. O'Donnell, and K. I. Berns. 1998. Role of the adenovirus DNA-binding protein in in vitro adeno-associated virus DNA replication. J. Virol. 72:420-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wistuba, A., A. Kern, S. Weger, D. Grimm, and J. A. Kleinschmidt. 1997. Subcellular compartmentalization of adeno-associated virus type 2 assembly. J. Virol. 71:1341-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wistuba, A., S. Weger, A. Kern, and J. A. Kleinschmidt. 1995. Intermediates of adeno-associated virus type 2 assembly: identification of soluble complexes containing Rep and Cap proteins. J. Virol. 69:5311-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu, J., M. D. Davis, and R. A. Owens. 1999. Factors affecting the terminal reslution site endonuclease, helicase, and ATPase activities of adeno-associated virus type 2 Rep proteins. J. Virol. 73:8235-8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu, J., M. D. Davis, and R. A. Owens. 2001. A Rep recognition sequence is necessary but not sufficient for nicking of DNA by adeno-associated virus type-2 Rep proteins. Arch. Biochem. Biophys. 389:271-277. [DOI] [PubMed] [Google Scholar]
- 67.Yoon-Robarts, M., and R. M. Linden. 2003. Identification of active site residues of the adeno-associated virus type 2 Rep endonuclease. J. Biol. Chem. 278:4912-4918. [DOI] [PubMed] [Google Scholar]
- 68.Young, S. M., and R. J. Samulski. 2001. Adeno-associated virus (AAV) site-specific recombination does not require a Rep-depedent origin of replication within the AAV terminal repeat. Proc. Natl. Acad. Sci. USA 98:13525-13530. [DOI] [PMC free article] [PubMed] [Google Scholar]