Abstract
We report here the generation of transgenic chickens using a retroviral vector for the production of recombinant proteins. It was found that the transgene expression was suppressed when a Moloney murine leukemia virus-based retroviral vector was injected into chicken embryos at the blastodermal stage. When a concentrated viral solution was injected into the heart of developing embryos after 50 to 60 h of incubation, transgene expression was observed throughout the embryo, including the gonads. For practical production, a retroviral vector encoding an expression cassette of antiprion single-chain Fv fused with the Fc region of human immunoglobulin G1 (scFv-Fc) was injected into chicken embryos. The birds that hatched stably produced scFv-Fc in their serum and eggs at high levels (∼5.6 mg/ml). We obtained transgenic progeny from a transgenic chicken generated with this procedure. The transgene was stably integrated into the chromosomes of transgenic progeny. The transgenic progeny also expressed scFv-Fc in the serum and eggs.
Transgenic bioreactors possess great potential for the production of recombinant pharmaceutical proteins (10, 15, 34). Transgenic animals have been generated for the production of recombinant proteins in the milk of livestock species such as goats, sheep, pigs, and cows. A high level of expression, more than 1 mg/ml of milk, has been reported with some proteins, and efforts toward a practical application for pharmaceutical use have continued (15). However, mammalian systems have several drawbacks in that they require a relatively large area for breeding and a long period for sexual maturation. As an alternative transgenic bioreactor, avian species such as chickens and quails have attracted a great deal of attention (11, 37). In particular, chickens have several advantages, including high protein productivity in eggs, ease of and small space requirements for breeding, similarity of the glycosylation pattern of proteins to that of humans (31), and absence of the prion problem.
To date, many approaches have attempted to generate transgenic birds (38). Efforts have mainly involved either the injection of retroviral vectors into embryos at the blastodermal stage (1, 7, 8, 24, 26, 32, 35, 36, 42, 43) or the microinjection of DNA into fertilized eggs at the single-cell stage (21, 39). Recently, lentiviral vectors were also used to generate transgenic chickens (3, 23). In most previous studies, a reporter gene such as lacZ and GPF has been used as the target, and expression was limited within cells. Harvey et al. reported that β-lactamase was produced in the serum and egg white of transgenic chickens generated with an avian leukosis virus-based retroviral vector (7, 8). As a practical model for the production of a pharmaceutical protein, human alpha-2b interferon was produced using the same system (32). However, the expression levels in serum and eggs were not high enough compared with the mammalian transgenic bioreactor systems.
Because of their availability, laid fertilized eggs at the blastodermal stage (stage X) are often utilized for gene transfer, although the embryonic cells have already proliferated to approximately 60,000 during egg formation in the oviduct (14). For blastodermal stage embryos, retroviral vectors have frequently been applied due to efficient integration into the host genome. In our previous study (24), a concentrated Moloney murine leukemia virus (MoMLV)-based replication-defective retroviral vector pseudotyped with vesicular stomatitis virus G protein (VSV-G) was injected into the subgerminal cavity of blastodermal stage quail embryos. The embryos were hatched by the embryonic culture method using surrogate eggshells with high frequency, and the viral vector sequence was detected in the tissues of all quails that hatched (G0). Furthermore, the efficiency of transgenesis was very high; more than 80% of offspring (G1) generated by mating with nontransgenic partners were transgenic. However, the expression of the reporter gene under the control of the internal promoter was very weak in G1 and G2 transgenic quails, although it was detectable. Since expression of the transgene was suppressed even in the G0 quails, and since substantial expression was observed upon viral infection of embryonic fibroblasts obtained at later embryonic stages, we speculated that the inactivation of the transgene using retroviral vectors occurred early in embryonic development.
In the present study, we examine the correlation between chicken embryonic development and retroviral gene expression by injecting a replication-defective retroviral vector into embryos at various developmental stages. Based on this result, transgenic chickens with a maximal level of transgene expression were generated. For the practical production of a recombinant protein, a retroviral vector encoding an expression cassette of antiprion single-chain Fv fused with the Fc region of human immunoglobulin G1 (scFv-Fc) was injected into chicken embryos at the suitable embryonic developmental stage. The birds that hatched stably expressed the recombinant protein at high levels in serum and egg white. We also obtained G1 and G2 transgenic progeny expressing the transgene at a high level derived from a G0 founder generated with this procedure.
MATERIALS AND METHODS
Plasmid construction.
The retroviral vector plasmid pMSCVneo (9) (Clontech, Palo Alto, CA) was digested with BglII and BamHI to remove the expression cassette of the neor gene. Instead, the neor gene derived from pLNHX (Clontech), chicken β-actin promoter derived from pmiwZ (41), and β-galactosidase gene derived from pCMVβ were ligated into BglII-and BamHI-digested pMSCVneo to generate pMSCV/NΔAβ.
The retroviral vector plasmid pLNHX was digested with XhoI to remove the internal promoter. The Rous sarcoma virus promoter derived from pLXRN (Clontech) and the β-galactosidase gene derived from pCMVβ (Clontech) were ligated into XhoI-digested pLNHX to generate pLNRβ.
The construction of plasmid pMSCV/GΔAscFv-Fc, in which an antiprion scFv-Fc gene expressed under the control of the chicken β-actin promoter was introduced, was described in our previous report (28).
Retroviral vector production.
VSV-G-pseudotyped pantropic replication-defective retroviral vectors were produced in a similar manner to that described in our previous report (24). The virus producer cell lines were established by introducing the respective viral vectors into a retroviral packaging cell line GP293 (Clontech) and using the limiting dilution method for cloning. The viral particles were produced by DNA transfection with a VSV-G expression vector plasmid pVSV-G (Clontech) into the virus-producer cells using a lipofection reagent (Lipofectamine 2000; Invitrogen, Carlsbad, CA). The medium containing viral particles was collected and filtered. The viral solution was then concentrated by ultracentrifugation. After the supernatant was carefully removed, the virus pellet was well suspended in 30 to 40 μl of 50 mM Tris-HCl buffer (pH 7.8) containing 130 mM NaCl and 1 mM EDTA. Polybrene (Sigma-Aldrich, St. Louis, MO) was added to the viral solution at a concentration of 8 μg/ml before the injection. The viral titer was determined for NIH 3T3 cells in terms of the expression of the GFP or lacZ gene residing in the viral vectors.
Microinjection of viral vectors into avian embryos and embryo culture.
The embryo culture procedure was based on that of Perry (30) with some modifications essentially described in our previous report (13). Laid fertilized eggs from chickens (White Leghorn) were incubated at 37.8°C under a relative humidity of 65% with rocking through an angle of 90° at 15-min intervals for 0 to 60 h. Before the injection, eggshells were cut horizontally with an electric drill (Minitor, Tokyo, Japan) equipped with a diamond cutter to make an opening of 35 mm in diameter at the sharp end. The viral solution was injected into the embryonic body or heart (1.5 to 3.5 μl per embryo) with a pulled glass micropipette using a microinjector (Transjector 5246; Eppendorf, Hamburg, Germany). Immediately after the injection, the opening of the eggshell was sealed with a polytetrafluorethylene membrane (Milliwrap; Nihon Millipore, Tokyo, Japan) and plastic film (Saran Wrap; Asahi Kasei Kogyo, Tokyo, Japan) using thin albumen as glue. The embryos were incubated at 37.8°C under a relative humidity of 65% with rocking through an angle of 90° at 30-min intervals until the 2- to 3-day stage. Subsequently, the embryos were transferred to surrogate chicken eggshells derived from eggs at least 25 g heavier than the donor eggs.
In the recipient eggshells, an opening 45 mm in diameter was created in the blunt end. After the transfer of the embryos, calcium lactate suspended in thin albumen was added to the embryo (25 mg per embryo) and then the opening in the recipient eggshell was sealed with plastic film. The embryos were incubated at 37.8°C and 65% relative humidity with rocking through an angle of 30° at hourly intervals. Pure oxygen gas was introduced into the incubator, and the aeration rate was incrementally increased. One day before the expected day of hatching, rocking was stopped and the plastic film was punctured to assist embryonic pulmonary respiration.
Detection of β-galactosidase expression.
In situ staining using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was performed to detect β-galactosidase expression in embryos. The embryonic bodies at the 5-day stage were isolated and washed with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4 · 12H2O and 1.5 mM KH2PO4, pH 7.4). They were fixed with 0.4 ml of aldehyde solution containing 0.2% glutaraldehyde and 2% formaldehyde in PBS for 40 min at 4°C, rinsed three times with PBS, and then stained with 0.4 ml of X-Gal solution (5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, and 1 mg/ml X-Gal in PBS) for 3 h at 37°C. More than five embryos for each condition were applied for staining, and the experiment was performed for several batches of virus solution. Thus, the reproducibility of the results was confirmed.
For quantification of β-galactosidase activity, the whole body of the embryos was isolated at the 5-day stage, washed with PBS, and then minced and sonicated in 0.8 ml of reaction buffer (10 mM KCl, 1 mM MgCl2, 0.1% Triton X-100, 5 mM 2-mercaptoethanol, 2 mM phenylmethylsulfonyl fluoride, and 0.1 M sodium phosphate buffer, pH 7.5) to make cell lysates. The β-galactosidase activity was measured using o-nitrophenyl-β-d-galactopyranoside (ONPG) as the substrate (1 unit was defined as the activity producing 1 μmol of o-nitrophenol per minute). The activity was normalized against the protein concentration. The protein concentration of the sample solution was determined by the bicinchoninic acid (Sigma) method (40), using bovine serum albumin as a standard. At least four embryos for each condition were analyzed for β-galactosidase activity.
Detection of transgene in transgenic chickens.
Genomic DNA from the chorioallantoic membranes just after hatching or tissue of mature birds was extracted using a genomic DNA preparation kit (MagExtractor; Toyobo, Osaka, Japan). PCR was performed with 50 ng of the genomic DNA as a template. After an initial denaturation of DNA at 94°C for 2 min, PCR was started: 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s, for 35 cycles. The primers used to amplify the recombinant viral vector sequences were 5′-AGCTCACCCTGAAATTCATCTGCACCACTG-3′ and 5′-GTTGTATTCCAGCTTGTGGCCGAGAATGTT-3′ for GFP (311 bp), and 5′-GTCTTATTAGCGGTGCTGGTAGTAGCACAA-3′ and 5′-GAGACTTCTGCTGGTACCAGCCATA-3′ for scFv-Fc (393 bp).
For identification of the chromosomal location of the transgene in transgenic chickens, fluorescent in situ hybridization (FISH) analysis was performed with a procedure described by Kuroiwa et al. (16). The transgene was detected using a fluorescein isothiocyanate-labeled probe prepared from the pMSCV/GΔAscFv-Fc plasmid by digestion with BamHI to remove the chicken-derived sequences.
SDS-PAGE and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli (18) to check the expression of scFv-Fc in serum and eggs of the transgenic avians. Purified scFv-Fc produced by recombinant CHO-K1 (28) was used as a positive control. Samples were boiled in the SDS-PAGE sample buffer with or without 2-mercaptoethanol for 5 min and then electrophoresed on a 7.5% gel. The serum and egg samples (30 μg or 15 μg protein per lane, respectively) were applied to the wells. The proteins on the gel were detected by protein staining (Simply Blue Safestain; Invitrogen).
For Western blot analysis of scFv-Fc and antigens, samples were boiled in the SDS-PAGE sample buffer with 2-mercaptoethanol for 5 min and then electrophoresed on 12% or 10% polyacrylamide gels (cell lysates of each tissue [20 μg], serum [2 μg], egg white [3 μg], and yolk [18 μg] were applied to the wells), and transferred onto a polyvinylidene difluoride membrane (Hybond-P, Amersham Biosciences). The scFv-Fc was detected with peroxidase-conjugated rabbit anti-human IgG antibodies (Organon Teknica), and the green fluorescent protein (GFP) was detected with peroxidase-conjugated goat anti-GFP antibodies (Santa Cruz Biotechnology, Santa Cruz, CA).
RESULTS
Development stage dependency in transgene expression.
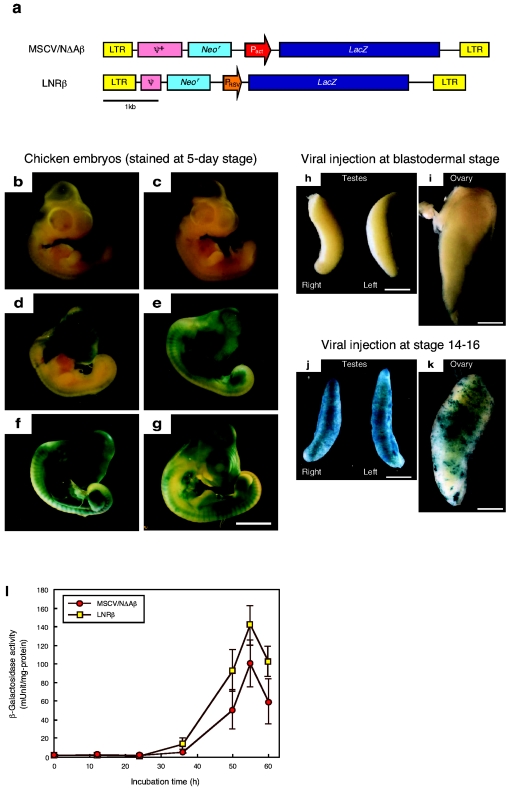
To examine the optimal developmental stage for retroviral injection in transgene expression, viral vectors encoding a bacterial β-galactosidase gene as the reporter (MSCV/NΔAβ and LNRβ; Fig. 1a) with a titer of more than 108 infectious units (IU)/ml were injected into embryos at various developmental stages. The β-galactosidase expression in the embryos injected with the MSCV/NΔAβ viral vector, whose viral base is the mouse stem cell virus (9), was evaluated using in situ X-Gal staining (Fig. 1b to g). No evident β-galactosidase expression was observed in embryos following injection at the blastodermal stage (Fig. 1b), although a vector sequence was detected in the genomic DNA prepared from a whole embryonic body (data not shown). At this stage, since the viral solution was injected into the subgerminal cavity, from which primordial germ cells originate in the central part, the transgene was detected more extensively in the gonads than the embryonic body. Thus, faintly stained cells were sparsely distributed in the embryonic body, and the gonad was also stained very faintly (Fig. 1 h and i). On the other hand, gross staining could be seen in the embryos when the timing of the injection was delayed and the viral solution was injected into embryonic bodies.
FIG. 1.
Developmental stage-dependent expression of the lacZ gene in chicken embryos transduced using retroviral vectors. (a) Structure of the MSCV/NΔAβ and LNRβ retroviral vectors. neor, neomycin resistance gene; lacZ, bacterial β-galactosidase gene; LTR, long terminal repeat from MoMLV (LNRβ) or MSCV (MSCV/NΔAβ); PRSV, Rous sarcoma virus promoter; Pact, chicken β-actin promoter; ψ+ or ψ, virus packaging signal sequence from MSCV (MSCV/NΔAβ) or MoMLV (LNRβ). (b to g) X-Gal staining of chicken embryos injected with the MSCV/NΔAβ viral vector at 0 h (b), 24 h (c), 36 h (d), 50 h (e), 55 h (f), or 60 h (g) of incubation. Scale bar, 5 mm. (h to k) X-Gal staining of gonads obtained from 21-day embryos injected with the MSCV/NΔAβ viral vector at 0 h (h and i) or 55 h (j and k) incubation. Scale bar, 1 mm. (l) β-Galactosidase activity in cell lysates from embryos injected with the MSCV/NΔAβ or LNRβ viral vector after various periods of incubation.
If the heart was developed after 50 h of incubation, the viral solution was injected into the heart of the embryos to deliver the vector efficiently to the whole embryo, including primordial germ cells, using the blood circulation system. Maximal expression was observed when the viral vectors were injected into the embryos after 55 h of incubation, stage 14 to 16 in the staging of Hamburger and Hamilton (6) (Fig. 1f). High-level transgene expression was observed in the gonad as well (Fig. 1j and k). This suggested that the transgene might be transmitted to progeny without gene suppression.
For quantification of the β-galactosidase expression, the enzyme activity was measured for the cell lysate from the embryos (Fig. 1l). The expression pattern was similar to that for in situ X-Gal staining; distinct activity appeared as the embryonic stage proceeded, and maximal activity was observed on viral injection after 55 h of incubation. The activity was very weak in embryonic bodies with injection at the blastodermal stage. The copy number of the transgene introduced into the genome of infected embryos was determined by real-time PCR. The results showed that 48% of cells in the embryos injected after 55 h of incubation possessed a vector sequence on the average, whereas about 1% of embryonic cells were infected after blastodermal injection. The expression level per transgene copy number for blastodermal injection was 40-fold less than that for injection after 55 h of incubation.
In order to compare the difference in viral type and promoter controlling transgene expression, the LNRβ viral vector, which has the same viral base as the original MoMLV, was also injected into chicken embryos. A similar expression pattern was observed for both vectors (Fig. 1l).
The viral titer significantly influenced β-galactosidase expression. Since the expression was weak with a viral titer below 107 IU/ml, at least 108 IU/ml is necessary for high-level expression of the transgene, although injection of more than 5 × 109 IU/ml resulted in a serious toxic effect on embryonic development (data not shown). From these results, it is preferable to inject viral vectors with a titer of more than 108 IU/ml into the heart of embryos after 55 h of incubation to obtain the highest level of transgene expression.
Generation of G0 transgenic chickens expressing scFv-Fc.
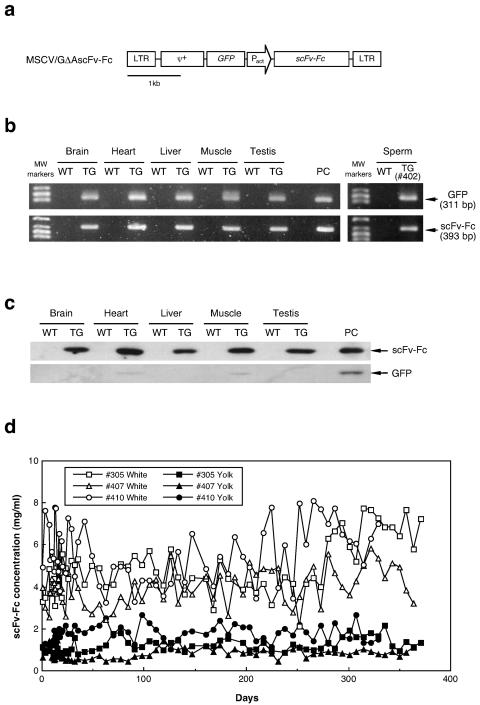
As an example of the production of a useful protein, the retroviral vector MSCV/GΔAscFv-Fc (Fig. 2a), in which an antiprion scFv-Fc gene was introduced under the control of the chicken β-actin promoter, was injected into chicken embryos to generate G0 transgenic birds. The hatchability of the embryos and the G0 chickens used for analysis is summarized in Table 1. A viral solution with a titer of 0.6 × 109 to 1.8 × 109 IU/ml was injected into the heart of embryos after 55 h of incubation. After the viral injection, the embryos were incubated to hatch by surrogate eggshell culture. In four trials, the viral vectors were injected into a total of 51 embryos, 32 of which hatched. The hatchability was 89% at maximum in the third trial and 63% on average. The scFv-Fc sequence was detected in all hatched birds by PCR using genomic DNA isolated from chorioallantoic membranes remaining at hatch and blood cells as a template (data not shown). After sexual maturation, a male G0 transgenic chicken was sacrificed to evaluate transgene insertion by PCR using genomic DNA extracted from several tissues, such as brain, heart, liver, muscle, and testis (Fig. 2b). Both scFv-Fc and GFP sequences encoded in the retroviral vector were detected in all tissues tested. Furthermore, the transgene sequences were also detected in the sperm of G0 roosters (Fig. 2b).
FIG. 2.
Analysis of G0 transgenic chickens expressing scFv-Fc. (a) Structure of the MSCV/GΔAscFv-Fc retroviral vector. GFP, green fluorescent protein gene; scFv-Fc, chicken antiprion single-chain antibody fragment gene joined with the Fc region gene from human immunoglobulin G1. The other abbreviations are the same as those for the MSCV/NΔAβ vector (Fig. 1a). (b) PCR analysis for detection of the transgene. The retroviral vector sequences were amplified by PCR using the primers for GFP (upper) or scFv-Fc (lower) and genomic DNA obtained from the tissues (brain, heart, liver, muscle, and testis) and sperm of a transgenic rooster as a template. WT, wild type; TG, transgenic; PC, positive control (pMSCV/GΔAscFv-Fc plasmid as a template). (c) Western blot analysis for transgene expression (scFv-Fc and GFP) in tissues from an adult male (brain, heart, liver, muscle, and testis). scFv-Fc or GFP produced by recombinant CHO cells was used as a positive control (PC). (d) Long-term production of scFv-Fc protein in eggs laid by G0 transgenic hens.
TABLE 1.
Hatchability of virus-injected embryos
| Expt no. | Viral titer (IU/ml) | No. of embryos
|
|
|---|---|---|---|
| Injected | Hatched (%) | ||
| 1 | 6.0 × 108 | 16 | 12 (75) |
| 2 | 7.5 × 108 | 10 | 5 (50) |
| 3 | 1.2 × 109 | 9 | 8 (89) |
| 4 | 1.8 × 109 | 16 | 7 (44) |
| Total | 51 | 32 (63) | |
The G0 transgenic birds were randomly selected from the low-titer injection (below 109 IU/ml) and high-titer injection (more than 109 IU/ml) groups (Table 2). Since scFv-Fc expression was controlled by a constitutive promoter of a cytoskeletal protein, β-actin, and since the scFv-Fc gene included a secretion signal sequence derived from chicken lysozyme, the scFv-Fc expressed in various tissues and organs was expected to be secreted and/or transferred to the bloodstream. Thus, the scFv-Fc concentration in the serum of the transgenic birds was analyzed by enzyme-linked immunosorbent assay (Table 2). The expression level depended significantly on the viral titer at injection, and high-level expression of scFv-Fc, more than 1 mg/ml, was observed among birds in the high-titer injection group. Expression remained stable for over a year throughout the breeding period.
TABLE 2.
List of representative G0 chickens
| Bird no. | Viral titer (IU/ml) | Injection volume (μl) | Sex (M, male; F, female) | scFv-Fc concna(mg/ml)
|
||
|---|---|---|---|---|---|---|
| Serum | White | Yolk | ||||
| 102 | 6.0 × 108 | 2.5 | F | 0.24 ± 0.13 | 0.06 ± 0.02 | 0.04 ± 0.02 |
| 105 | 2.0 | M | 0.29 ± 0.18 | |||
| 108 | 3.0 | F | 0.40 ± 0.18 | 0.59 ± 0.22 | 0.10 ± 0.04 | |
| 110 | 2.5 | F | 1.72 ± 0.34 | 3.88 ± 1.32 | 0.85 ± 0.23 | |
| 111 | 2.0 | M | 1.74 ± 0.46 | |||
| 116 | 2.5 | M | 0.82 ± 0.35 | |||
| 305 | 1.2 × 109 | 2.5 | F | 2.08 ± 0.44 | 4.85 ± 1.25 | 1.18 ± 0.32 |
| 308 | 3.5 | M | 1.73 ± 0.45 | |||
| 402 | 1.8 × 109 | 1.5 | M | 1.60 ± 0.37 | ||
| 405 | 2.5 | M | 3.50 ± 0.70 | |||
| 407 | 1.5 | F | 1.97 ± 0.64 | 4.00 ± 0.84 | 0.81 ± 0.25 | |
| 410 | 3.0 | F | 3.56 ± 0.93 | 5.57 ± 1.36 | 1.77 ± 0.38 | |
The scFv-Fc concentration was determined by solid-phase enzyme-linked immunosorbent assay using purified scFv-Fc or human Fc fragment as the standard.
Transgene expression in various tissues was also analyzed by Western blotting (Fig. 2c). The scFv-Fc protein was detected at high levels in all tissues tested, such as brain, heart, liver, muscle, and testis. In contrast, GFP expression induced by the viral long terminal repeat promoter was slightly detectable only in heart and muscle, since the promoter activity is very weak in chicken cells. After sexual maturation, the scFv-Fc concentration in eggs was measured (Table 2, Fig. 2d). The scFv-Fc protein was detected in both egg white and yolk, and production was maintained for over a year. The concentration was higher in egg white than in yolk. The transgenic chickens in the high-titer injection group produced the protein in the egg white at a concentration of 4 to 5.6 mg/ml. GFP was not detected in the serum or eggs (data not shown).
Generation of G1 and G2 transgenic progeny.
Transgene sequences were detected in the sperm of G0 transgenic roosters by PCR (Fig. 2b). However, the strength of the signal varied among roosters depending on the viral titer injected. Since the signal for rooster 402 was strong, this chicken was mated with nontransgenic hens to generate G1 transgenic progeny (Table 3). Among 181 G1 progeny subjected to the detection of a transgene sequence by PCR using genomic DNA isolated from blood as a template, six chicks were determined to be transgenic. Furthermore, five of them produced scFv-Fc at a concentration of 0.5 to 1.9 mg/ml in serum.
TABLE 3.
Generation of transgenic progeny expressing antiprion scFv-Fc
| Transgenic chickens | Parental chicken | Bird no. | Sex (M, male; F, female) | scFv-Fc concn (mg/ml)
|
Transgene copy numbera | Chromosomal locationa | Germline transmission efficiency | ||
|---|---|---|---|---|---|---|---|---|---|
| Serum | White | Yolk | |||||||
| G1 | G0 no. 402 | 1 | M | <1 × 10−6 | 1 | Micro | NDb | ||
| 2 | F | 1.12 ± 0.16 | 0.22 ± 0.06 | 0.48 ± 0.15 | 2 | Micro × 2 | 21/27 (78%) | ||
| 3 | M | 0.51 ± 0.10 | 1 | 3q2.6-2.8 | 22/43 (51%) | ||||
| 4 | F | 0.50 ± 0.15 | 0.41 ± 0.13 | 0.22 ± 0.12 | 1 | 3q2.6-2.8 | ND | ||
| 5 | F | 1.94 ± 0.57 | 1.50 ± 0.24 | 0.76 ± 0.20 | 1 | Micro | ND | ||
| 6 | F | 0.47 ± 0.03 | 0.43 ± 0.12 | 0.17 ± 0.05 | 1 | 3q2.6-2.8 | ND | ||
| G2 | G1 no. 2 | 1 | F | 0.68 ± 0.15 | 0.10 ± 0.05 | 0.24 ± 0.14 | 2 | ||
| 2 | F | 0.56 ± 0.09 | 0.05 ± 0.01 | 0.52 ± 0.07 | 2 | ||||
| 3 | F | 0.76 ± 0.14 | ND | ND | 2 | ||||
| 4 | F | 0.30 ± 0.10 | ND | ND | 1 | ||||
| 5 | M | 0.20 ± 0.07 | 1 | ||||||
| 6 | M | 0.29 ± 0.03 | 1 | ||||||
| 7 | M | 0.20 ± 0.08 | 1 | ||||||
| 8 | M | 0.25 ± 0.09 | 1 | ||||||
| G2 | G1 no. 3 | 9 | F | 0.30 ± 0.10 | 0.16 ± 0.05 | 0.18 ± 0.06 | 1 | ||
| 10 | F | 0.41 ± 0.13 | 0.36 ± 0.09 | 0.26 ± 0.10 | 1 | ||||
| 11 | F | 0.30 ± 0.10 | 0.17 ± 0.05 | 0.21 ± 0.06 | 1 | ||||
| 12 | M | 0.38 ± 0.09 | 1 | ||||||
| 13 | M | 0.38 ± 0.09 | 1 | ||||||
| 14 | M | 0.33 ± 0.13 | 1 | ||||||
Transgene copy number and chromosomal location of the transgene were determined by FISH and/or Southern blot analyses.
ND, not determined.
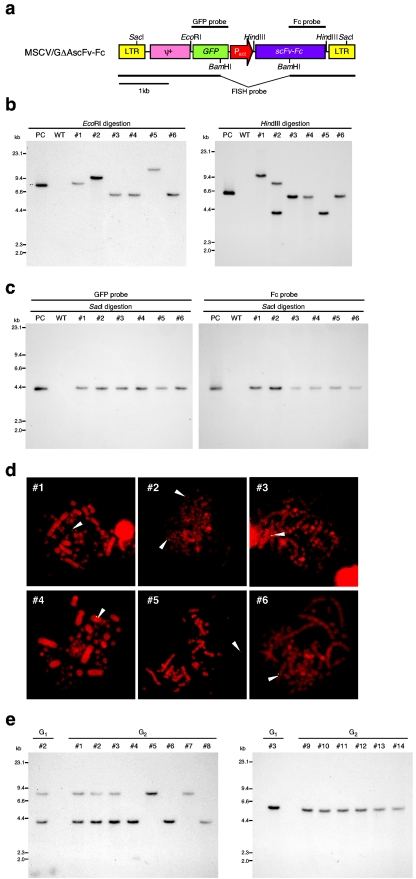
To determine the copy number, intactness, and chromosomal location of the transgene, Southern blot and FISH analyses were performed on the G1 transgenic chickens (Fig. 3b to d). Among the six G1 transgenic chickens, five had one copy and one had two copies of the transgene (Fig. 3b and d). Furthermore, FISH analysis revealed that the transgene was inserted at the same chromosomal site in three G1 transgenic chickens (number 3, 4, and 6), suggesting that these chickens were derived from the same primordial germ cell line. In fact, the expression level of scFv-Fc in the serum of these chickens was also almost the same (Table 3). In the other G1 transgenic chickens, the vector sequence was detected in microchromosomes by FISH analysis. Judging from the difference in size of EcoRI- or HindIII-digested DNA fragments containing the transgene on Southern blotting (Fig. 3b), it was suggested that the transgene was inserted into different sites of microchromosomes in these chickens. In order to confirm whether the vector sequence remained intact in the G1 transgenic chickens, genomic DNA digested with SacI, which cuts at both 5′ and 3′ long terminal repeat sequences of the vector (Fig. 3a), was subjected to Southern blot analysis (Fig. 3c). An intact vector fragment (4.3 kb) was detected with either the GFP or Fc probe in all G1 transgenic chickens tested. This indicated that no recombination or deletion had occurred within the integrated transgene.
FIG.3.
Analysis of G1 and G2 transgenic chickens expressing scFv-Fc. (a) Restriction enzyme map of the MSCV/GΔAscFv-Fc retroviral vector and locations of probes for Southern and FISH analyses. (b) Determination of the copy number of the transgene in the G1 genome by Southern blot analysis. Genomic DNA extracted from the blood of G1 transgenic chickens was digested with EcoRI (left) or HindIII (right), electrophoresed, and hybridized with the GFP probe. WT, wild type; PC, positive control (pMSCV/GΔAscFv-Fc plasmid). (c) Confirmation of the intactness of the vector sequence in the G1 genome by Southern blot analysis. Genomic DNA fragments digested with SacI were electrophoresed and hybridized with the probe for GFP (left) or Fc (right). (d) FISH analysis for determination of the chromosomal location of the transgene for G1 transgenic chickens. The vector sequence was detected using a fluorescein isothiocyanate-labeled probe prepared from the pMSCV/GΔAscFv-Fc plasmid by digestion with BamHI. The arrowheads indicate the location of the transgene. (e) Southern blot analysis for G2 transgenic chickens derived from G1 transgenic chickens 2 (left) and 3 (right). Genomic DNA extracted from the blood of G2 transgenic chickens was digested with HindIII, electrophoresed, and hybridized with the GFP probe.
After sexual maturation, the scFv-Fc concentration in eggs laid by G1 transgenic hens was measured (Table 3). The expression level varied among hens, and one transgenic hen (number 5) produced scFv-Fc at a concentration of 1.5 mg/ml in egg white. The results on expression of the transgene indicated that the expression level in G1 transgenic chickens depended on the chromosomal location of the transgene.
The efficiency of germ line transmission from G1 to G2 progeny was then analyzed in two G1 transgenic chickens (numbers 2 and 3) by mating with nontransgenic chickens (Table 3). The frequency of transgenic appearance was 78% (21/27) and 51% (22/43), respectively, corresponding to the transgene copy number of parental chickens and to Mendel's law. As shown in Fig. 3e, G2 transgenic progeny had one or two copies of the transgene corresponding to those of the parental transgenic chickens, suggesting that the vector sequence was not changed during transgenesis. All the G2 transgenic progeny expressed scFv-Fc. Although the expression level was reduced compared with that of parental G1 chickens, the levels in the serum and eggs were maintained over the breeding period.
Analysis of scFv-Fc produced by transgenic chickens.
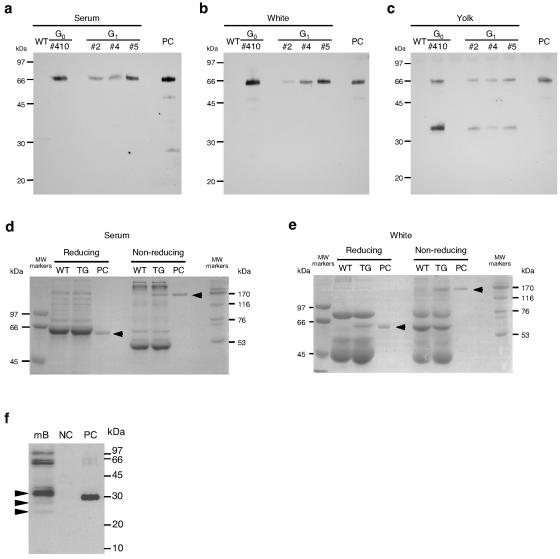
The structure and function of scFv-Fc produced by transgenic chickens were analyzed. In the Western blot analysis using anti-human Fc antibodies for detection, a single band of scFv-Fc was detected in the samples from serum and egg whites of G0 and G1 transgenic chickens (Fig. 4a and b). Although the size of the monomeric scFv-Fc estimated from the amino acid sequence is 52 kDa, that estimated by Western blot analysis was 66 kDa. This is probably due to a posttranslational modification with a polysaccharide chain of the Fc region as observed with regular antibodies. On the other hand, two bands were observed in the yolk samples (Fig. 4c). The size of the lower band corresponded to the Fc region of human immunoglobulin G, indicating that the scFv-Fc produced in yolk was partially digested.
FIG. 4.
Western blot and SDS-PAGE analyses of scFv-Fc produced by transgenic chickens. (a to c) Western blot analysis for scFv-Fc expression in serum (a), egg white (b), and yolk (c) produced by G0 and G1 transgenic hens. WT, wild type; PC, positive control (30 ng of purified scFv-Fc produced by recombinant CHO cells). (d and e) SDS-PAGE analysis of scFv-Fc under reducing and nonreducing conditions in the serum (d) and egg white (e) of a G0 transgenic hen. (f) Antigen recognition of scFv-Fc by Western blot analysis. The natural prion proteins in mouse brain homogenate (mB) were detected by Western blotting using the scFv-Fc purified from the egg white produced by a transgenic chicken. NC, negative control (GST); PC, positive control (GST-PrP) (28).
On the SDS-PAGE gels with protein staining, unique bands corresponding to scFv-Fc could be seen for the egg white and serum samples from a transgenic bird (Fig. 4d and e). Since the Fc region introduced into scFv-Fc in this study contained a hinge region, the scFv-Fc produced by animal cells is expected to form a cysteine-cysteine (S-S)-linked dimer. In SDS-PAGE, the scFv-Fc produced by transgenic chickens showed a band with the size of a monomer under reducing conditions and a dimer under nonreducing conditions, although the band corresponding to scFv-Fc in the serum sample from transgenic chickens overlapped a major serum protein under reducing conditions. These results suggested that the scFv-Fc produced by transgenic chickens was properly assembled.
Then, the antigen recognition ability of scFv-Fc purified from egg white was checked by Western blot analysis (Fig. 4f). Natural prion proteins from mouse brain homogenate and glutathione S-transferase (GST) fused to the epitope peptide sequence of human prion protein for scFv-Fc (GST-PrP) (28) were used as antigens. scFv-Fc recognized several protein bands in the mouse brain homogenate, and the molecular weights of the bands agreed well with those of prion proteins differing in the degree of polysaccharide modification. The banding patterns were very similar to those of previous reports (22, 27, 28). scFv-Fc also detected GST-PrP as a positive control. Thus, the scFv-Fc proteins produced in the egg whites of transgenic chickens have antigen-binding activity similar to that of the original monoclonal antibody.
DISCUSSION
For the manipulation of avian embryos, it is preferable to use fertilized eggs after oviposition for gene transfer, since a large number are available compared with single-cell-stage fertilized eggs obtained by sacrificing hens. In order to introduce a transgene efficiently into the embryonic cells, retroviral vectors have often been used to generate transgenic chickens. We have used MoMLV-based replication-defective retroviral vectors for the generation of transgenic birds. The vector system has been well developed and applied to human gene therapy. The vector has been also employed for the generation of transgenic animals. However, MoMLV-based vectors are highly susceptible to gene silencing (2, 12, 20), and high-level expression of the transgene in transgenic progeny has not been reported.
In the present study, through retroviral injection at the later stages of embryonic development, we achieved stable and high-level expression of the transgene in serum and eggs not only in G0 birds, but also in some transgenic progeny. Since an MoMLV-derived mouse stem cell virus (MSCV) was used as the viral base in this study, this alteration might partially contribute to the stable and high-level expression of the transgene. In fact, the expression of GFP driven by the long terminal repeat promoter was detected in muscular tissues of transgenic chickens using the MSCV-based vector, although it was not detected using the original MoMLV-based vector in the previous study (24). This indicates that the MSCV-based vector may be more advantageous than the original MoMLV-based vector for the generation of transgenic animals expressing exogenous genes.
An additional reason for the achievement of high-level expression even with injection at the later embryonic stage is that the viral vector could be delivered to the developing embryonic cells of almost all tissues, including the oviduct and gonad, through the bloodstream by injection of a concentrated viral solution into the heart. Maximal expression was observed with a viral injection after 55 h of incubation. At this embryonic stage, primordial germ cells circulate through the bloodstream from the germinal crescent to migrate into the gonadal anlage (5, 17). When a G0 transgenic rooster was mated with nontransgenic hens, G1 transgenic progeny, which are uniform for the transgene, appeared with a frequency of 3.3%. Among them, five G1 transgenic chickens expressed scFv-Fc at 0.5 to 1.9 mg/ml in their serum, and G1 transgenic hens produced the protein at 0.2 to 1.5 mg/ml in egg white. These expression levels were lower than those of G0 chickens generated with a high viral titer. One possible explanation for this may be that transgenic chickens with extremely high-level expression of the transgene died during embryonic development, since a chicken β-actin promoter was used to induce the target gene ubiquitously in the whole body. Alternatively, since the viral vector was injected at the later stage in which an embryonic body was steadily developed, G0 birds must be mosaic for the transgene. The mosaic insertion and/or expression might allow high-level expression of the transgene in the tissues of G0 birds. However, further study is necessary to clear up this point.
The expression levels of scFv-Fc varied among the G1 transgenic chickens, and were related to the chromosomal location of the transgene inserted. The position effect of a chromosomal insertion on transgene expression has been reported in transgenic mice (4, 33). Therefore, selection of high-producer chickens in terms of productivity in serum and eggs will be necessary for commercial production using transgenic progeny. However, expression was somewhat reduced in the G2 transgenic chickens compared with the parental chickens. Thus, some kind of transgene suppression occurred during germ line transmission. Since the suppression was not severe, this mechanism may be different from that of retroviral gene silencing caused by injection into early-stage embryos. A reduction of transgene expression in progeny has been observed even in transgenic animals generated by the microinjection of DNA into a fertilized oocyte (29). The expression level in transgenic progeny could be enhanced through mating between transgenic chickens for the accumulation of transgene copies based on the chromosomal location of the transgene (data not shown).
The scFv-Fc protein was also detected in yolk, but some portion was partially digested. It is known that chicken antibodies are transferred to yolk from blood through Fc receptors on the cell surface (19). Mohammed et al. reported that human immunoglobulin G antibodies accumulated in the yolk when they were injected into the bloodstream (25). We found that the scFv-Fc produced by recombinant CHO cells was also transferred into the yolk from the bloodstream, but most of the transferred proteins were digested upstream of Fc and the hinge region (unpublished data), possibly by a sequence-specific protease during the transfer. The scFv-Fc protein produced in the serum of transgenic chickens could be transferred to the yolk being subjected to digestion, and hence a low-molecular-weight protein corresponding to Fc was detected in the yolk samples.
In order to collect intact scFv-Fc in yolk as well as egg white, it is necessary to genetically change the amino acid sequence in the region where scFv and Fc join. When full antibodies and other Fc-fused proteins were produced in transgenic hens, they were not digested in the yolk (unpublished data). Therefore, the phenomenon of digestion during transfer is specific for the scFv-Fc used in the present study, and antibodies and Fc-fused proteins produced in the serum of transgenic hens can be collected from the yolk.
In conclusion, we stably generated G0 transgenic chickens expressing a foreign gene in serum and egg white at a commercially feasible level. G1 transgenic chickens generated from a G0 founder also expressed the transgene at a high level, and transmission of the transgene to the next generation accompanied the expression. We obtained transgenic chickens producing other proteins such as full antibodies and human erythropoietin in serum and egg white with the range of several μg/ml to mg/ml. This system is promising for the commercial production of recombinant proteins using transgenic chicken bioreactors.
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research (nos. 14350434 and 17360396) from the Japan Society for the Promotion of Science (JSPS) and a grant from the Science and Technology Incubation Program in Advanced Region by the Japan Science and Technology Agency (JST).
We thank H. Matsuda, Hiroshima University, for the generous gift of the antiprion scFv gene.
REFERENCES
- 1.Bosselman, R. A., R. Y. Hsu, T. Boggs, S. Hu, J. Bruszewski, S. Ou, L. Kozar, F. Martin, C. Green, F. Jacobsen, M. Nicolson, J. A. Schultz, K. M. Seman, W. Rishell, and R. G. Stewart. 1989. Germline transmission of exogenous genes in the chicken. Science 243:533-535. [DOI] [PubMed] [Google Scholar]
- 2.Challita, P. M., and D. B. Kohn. 1994. Lack of expression from a retroviral vector after transduction of murine hematopoietic stem cells is associated with methylation in vivo. Proc. Natl. Acad. Sci. USA 91:2567-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman, S. C., A. Lawson, W. C. Macarthur, R. J. Wiese, R. H. Loechel, M. Burgos-Trinidad, J. K. Wakefield, R. Ramabhadran, T. J. Mauch, and G. C. Schoenwolf. 2005. Ubiquitous GFP expression in transgenic chickens using a lentiviral vector. Development 132:935-940. [DOI] [PubMed] [Google Scholar]
- 4.Dobie, K. W., M. Lee, J. A. Fantes, E. Graham, A. J. Clark, A. Springbett, R. Lathe, and M. McClenaghan. 1996. Variegated transgene expression in mouse mammary gland is determined by the transgene integration locus. Proc. Natl. Acad. Sci. USA 93:6659-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujimoto, T., T. Ninomiya, and A. Ukeshima. 1976. Observations of the primordial germ cells in blood samples from the chick embryo. Dev. Biol. 49:278-282. [DOI] [PubMed] [Google Scholar]
- 6.Hamburger, V., and H. L. Hamilton. 1951. A series of normal stages in the development of the chick. J. Morphol. 88:49-92. [PubMed] [Google Scholar]
- 7.Harvey, A. J., G. Speksnijder, L. R. Baugh, J. A. Morris, and R. Ivarie. 2002. Expression of exogenous protein in the egg white of transgenic chickens. Nat. Biotechnol. 20:396-399. [DOI] [PubMed] [Google Scholar]
- 8.Harvey, A. J., G. Speksnijder, L. R. Baugh, J. A. Morris, and R. Ivarie. 2002. Consistent production of transgenic chickens using replication deficient retroviral vectors and high-throughput screening procedures. Poult. Sci. 81:202-212. [DOI] [PubMed] [Google Scholar]
- 9.Hawley, R. G., F. H. L. Lieu, A. Z. C. Fong, and T. S. Hawley. 1994. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1:136-138. [PubMed] [Google Scholar]
- 10.Houdebine, L. M. 2000. Transgenic animal bioreactors. Transgenic Res. 9:305-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivarie, R. 2003. Avian transgenesis: progress towards the promise. Trends Biotechnol. 21:14-19. [DOI] [PubMed] [Google Scholar]
- 12.Jahner, D., H. Stuhlmann, C. L. Stewart, K. Harbers, J. Lohler, I. Simon, and R. Jaenisch. 1982. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature 298:623-628. [DOI] [PubMed] [Google Scholar]
- 13.Kamihira, M., S. Oguchi, A. Tachibana, Y. Kitagawa, and S. Iijima. 1998. Improved hatching for in vitro quail embryo culture using surrogate eggshell and artificial vessel. Dev. Growth Differ. 40:449-455. [DOI] [PubMed] [Google Scholar]
- 14.Kochav, S., M. Ginsberg, and H. Eyal-Giladi. 1980. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of development of the chick. II. Microscopic anatomy and cell population dynamics. Dev. Biol. 79:296-308. [DOI] [PubMed] [Google Scholar]
- 15.Kues, W. A., and H. Niemann. 2004. The contribution of farm animals to human health. Trends Biotechnol. 22:286-294. [DOI] [PubMed] [Google Scholar]
- 16.Kuroiwa, A., M. Uchikawa, Y. Kamachi, H. Kondoh, C. Nishida-Umehara, J. Masabanda, D. K. Griffin, and Y. Matsuda. 2002. Chromosome assignment of eight SOX family genes in chicken. Cytogenet. Genome Res. 98:189-193. [DOI] [PubMed] [Google Scholar]
- 17.Kuwana, T. 1993. Migration of avian primordial germ cells toward the gonadal anlage. Dev. Growth Differ. 35:237-243. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Loeken, M. R., and T. F. Roth. 1983. Analysis of maternal IgG subpopulations which are transported into the chicken oocyte. Immunology 49:21-28. [PMC free article] [PubMed] [Google Scholar]
- 20.Lois, C., E. J. Hong, S. Pease, E. J. Brown, and D. Baltimore. 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295:868-872. [DOI] [PubMed] [Google Scholar]
- 21.Love, J., C. Gribbin, C. Mather, and H. Sang. 1994. Transgenic birds by DNA microinjection. Biotechnology 12:60-63. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda, H., H. Mitsuda, N. Nakamura, S. Furusawa, S. Mohri, and T. Kitamoto. 1999. A chicken monoclonal antibody with specificity for the N-terminal of human prion protein. FEMS Immunol. Med. Microbiol. 23:189-194. [DOI] [PubMed] [Google Scholar]
- 23.McGrew, M. J., A, Sherman, F. M. Ellard, S. G. Lillico, H. J. Gilhooley, A. J. Kingsman, K. A. Mitrophanous, and H. Sang. 2004. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 5:728-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizuarai, S., K. Ono, K. Yamaguchi, K. Nishijima, M. Kamihira, and S. Iijima. 2001. Production of transgenic quails with high frequency of germ-line transmission using VSV-G psedotyped retroviral vector. Biochem. Biophys. Res. Commun. 286:456-463. [DOI] [PubMed] [Google Scholar]
- 25.Mohammed, S. M., S. Morrison, L. Wims, K. R. Trinh, A. G. Wildeman, J. Bonselaar, and R. J. Etches. 1998. Deposition of genetically engineered human antibodies into the egg yolk of hens. Immunotechnology 4:115-125. [DOI] [PubMed] [Google Scholar]
- 26.Mozdziak, P. E., S. Borwornpinyo, D. W. McCoy, and J. N. Petitte. 2003. Development of transgenic chickens expressing bacterial β-galactosidase. Dev. Dyn. 226:439-445. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura, N., Y. Aoki, H. Horiuchi, S. Furusawa, H. I. Yamanaka, T. Kitamoto, and H. Matsuda. 2000. Construction of recombinant monoclonal antibodies from a chicken hybridoma line secreting specific antibody. Cytotechnology 32:191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ono, K., M. Kamihira, Y. Kuga, H. Matsumoto, A. Hotta, T. Itoh, K. Nishijima, N. Nakamura, H. Matsuda, and S. Iijima. 2003. Production of anti-prion scFv-Fc fusion proteins by recombinant animal cells. J. Biosci. Bioeng. 95:231-238. [PubMed] [Google Scholar]
- 29.Opsahl, M. L., M. McClenaghan, A. Springbett, S. Reid, R. Lathe, A. Colman, and C. B. Whitelaw. 2002. Multiple effects of genetic background on variegated transgene expression in mice. Genetics 160:1107-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry, M. M. 1988. A complete culture system for the chick embryo. Nature 331:70-72. [DOI] [PubMed] [Google Scholar]
- 31.Raju, T. S., J. B. Briggs, S. M. Borge, and A. J. S. Jones. 2000. Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 10:477-486. [DOI] [PubMed] [Google Scholar]
- 32.Rapp, J. C., A. J. Harvey, G. L. Speksnijder, W. Hu, and R. Ivarie. 2003. Biologically active human interferon alpha-2b produced in the egg white of transgenic hens. Transgenic Res. 12:569-575. [DOI] [PubMed] [Google Scholar]
- 33.Robertson, G., D. Garrick, W. Wu, M. Kearns, D. Martin, and E. Whitelaw. 1995. Position-dependent variegation of globin transgene expression in mice. Proc. Natl. Acad. Sci. USA 92:5371-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudolph, N. S. 1999. Biopharmaceutical production in transgenic livestock. Trends Biotechnol. 17:367-374. [DOI] [PubMed] [Google Scholar]
- 35.Salter, D. W., E. J. Smith, S. H. Hughes, S. E. Wright, A. M. Fadly, R. L. Witter, and L. B. Crittenden. 1986. Gene insertion into chicken germ line by retroviruses. Poult. Sci. 65:1445-1458. [DOI] [PubMed] [Google Scholar]
- 36.Salter, D. W., E. J. Smith, S. H. Hughes, S. E. Wright, and L. B. Crittenden. 1987. Transgenic chickens: insertion of retroviral genes into the chicken germ line. Virology 157:236-240. [DOI] [PubMed] [Google Scholar]
- 37.Sang, H. 1994. Transgenic chicken -methods and potential applications. Trends Biotechnol. 12:415-420. [DOI] [PubMed] [Google Scholar]
- 38.Sang, H. 2004. Prospects for transgenesis in the chick. Mech. Dev. 121:1179-1186. [DOI] [PubMed] [Google Scholar]
- 39.Sherman, A., A. Dawson, C. Mather, H. Gilhooley, Y. Li, R. Mitchell, D. Finnegan, and H. Sang. 1998. Transposition of the Drosophila element mariner into the chicken germ line. Nat. Biotechnol. 16:1050-1053. [DOI] [PubMed] [Google Scholar]
- 40.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 41.Suemori, H., Y. Kadokawa, K. Goto, I. Araki, H. Kondoh, and N. Nakatsuji. 1990. A mouse embryonic stem cell line showing pluripotency of differentiation in early embryos and ubiquitous β-galactosidase expression. Cell Differ. Dev. 29:181-186. [DOI] [PubMed] [Google Scholar]
- 42.Thoraval, P., M. Afanassieff, F. L. Cosset, F. Lasserre, G. Verdier, F. Coudert, and G. Dambrine. 1995. Germline transmission of exogenous genes in chickens using helper-free ecotropic avian leucosis virus-based vectors. Transgenic Res. 4:369-377. [DOI] [PubMed] [Google Scholar]
- 43.Vick, L., Y. Li, and K. Simkiss. 1993. Transgenic birds from transformed primordial germ cells. Proc. R. Soc. Lond. B Biol. Sci. 251:179-182. [DOI] [PubMed] [Google Scholar]